Research Article Open Access
Rapid Simultaneous Determination of Sumatriptan Succinate and Naproxen Sodium in Combined Tablets by Validated Ultra Performance Liquid Chromatographic Method
Yarram Ramakoti Reddy1*, Kakumani Kishore Kumar1, MRP Reddy2 and K. Mukkanti11Centre for Chemical Science and Technology, Institute of science and Technology, Jawaharlal Nehru Technological University, Hyderabad 500085, India
2Centre for Meterials for Electronics Technology IDA, HCL post, Cherlapally, Hyderabad, India
- *Corresponding Author:
- Dr. Yarram Ramakoti Reddy
Centre for Chemical Science and Technology
Institute of science and Technology
Jawaharlal Nehru Technological University, Hyderabad 500085, India
Tel: (+)91 9849392039
E-mail: rramakoti48@yahoo.com, yrkccst@gmail.com
Received date: April 18, 2011; Accepted date: May 28, 2011; Published date: May 30, 2011
Citation: Reddy YR, Kumar KK, Reddy MRP, Mukkanti K (2011) Rapid Simultaneous Determination of Sumatriptan Succinate and Naproxen Sodium in Combined Tablets by Validated Ultra Performance Liquid Chromatographic Method. J Anal Bioanal Tech 2:121. doi: 10.4172/2155-9872.1000121
Copyright: © 2011 Reddy YR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A stability- indicating ultra perfomance liquid chromatography (UPLC) method was developed for the simultaneous determination of sumatriptan succinate and Naproxen sodium in pharmaceutical dosage forms. The chromatographic separation was achieved on C18, 50 mm × 4.8 mm, 1.8-μm particle size column. The mobile phase contains a mixture of 0.2% ortho phosphoric acid and acetonitrile as the mobile phase in gradient elution technique. The retention time of Sumatriptan and Naproxen was found to be 1.7 and 2.7 min respectively. The total runtime was 5 min within which two active compounds and degradation products were separated. This method allows the determination of 850-2565 μg mL -1 of sumatriptan succinate and 5000-15000 μg mL -1 of Naproxen sodium. The flow rate was 1.0 mL min -1 and the detection wavelength was 225 nm. The limit of detection (LOD) for sumatriptan succinate and Naproxen sodium was 1.9 and 1.5 μg mL -1 , respectively. The limit of quantification (LOQ) for sumatriptan succinate and Naproxen sodium was 6.3 and 4.8 μg mL -1 , respectively. This method was validated for accuracy, precision, linearity and robustness. sumatriptan succinate and Naproxen sodium were subjected to different ICH prescribed stress conditions of oxidative, acid, base, hydrolytic, thermal and photolytic degradation and the method was also found to be stability indicating.
Keywords
Method development and validation; Simultaneous sumatriptan succinate; Naproxen sodium stability-indicating.
Introduction
Sumatriptan succinate (SS) is a selective 5-hydroxytryptamine (5-HT) receptor subtype agonist. Chemically it is known as 3-[2-(dimethylamino) ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1). The empirical formula is C14H21N3O2S•C4H6O4, representing a molecular weight of 413.5. It is indicated for indicated for the acute treatment of migraine attacks with or without aura in adults.
Naproxen sodium (NS) is a member of arylacetic acid group of nonsteroidal anti-inflammatory drugs (NSAIDS). Chemically it is (S)-6-methoxy-α-methyl-2-naphthaleneacetic acid, sodium salt. The empirical formula is C14H13NaO3, representing a molecular weight of 252.23.
A tablet formulation containing 85 mg of Sumatriptan succinate and 500 mg of Naproxen sodium has recently been approved for the acute treatment of migraine. The combination product was proved to have superior efficacy compared to its individual components for the acute treatment of migraine. Sumatriptan, work early in the migraine process at the trigeminovascular unit as agonists of the serotonin receptors (5-HT receptors) 1B and 1D. They block vasoconstriction and block transmission of signals to the trigeminal nucleus and thus prevent peripheral sensitization. The analgesic effect of Naproxen sodium helps relieve the headache, while the anti-inflammatory effect decreases the neurogenic inflammation in the trigeminal ganglion, thus preventing the development of central sensitization.
So far, several liquid chromatography procedures have been described for the determination of SS and NS [1-19]. But, these procedures were developed to estimate either SS or NS individually and in combination with other drugs from formulation, plasma, urine, intestinal perfusion samples and in bulk drugs. For simultaneous determination of SS and NS in formulation, there are two spectrometric methods [20-21] and an HPTLC [22] method was reported. Whereas no single liquid chromatographic (LC) method has been reported for their simultaneous estimation from the combined tablets. Hence, it is necessary to develop a rapid, accurate and validated LC method for the simultaneous determination of SS and NS from combined dosage form.
Ultra performance liquid chromatography (UPLC) is a recent technique in liquid chromatography, which enables significant reductions in separation time and solvent consumption. Literature indicates that UPLC system allows about ninefold decrease in analysis time as compared to the conventional HPLC system using 5μm particle size analytical columns, and about threefold decrease in analysis time in comparison with 3μm particle size analytical columns without compromise on overall separation [23-27].
Hence, a rapid, accurate stability indicating UPLC method for the simultaneous determination of SS and NS from combined dosage form was developed and validated.
Experimental
Instrumentation and chromatographic conditions
The Waters UPLC Acquity system we used consists of a binary solvent manager, a sample manager and a UV detector. Zorbax SB C-18, 50 mm x 4.6 mm i.d with 1.8μm particles was used as stationary phase. 0.2% ortho phosphoric acid in water (pH 2.2) as solvent A and acetonitrile and water in the ratio 90:10; v/v, was as solvent B used for mobile phase. Prior to use, the mobile phase was mixed thoroughly and degassed. The gradient program was set as B: 0/5, 2/95, 4/95, 4.1/5, 5.0/5. The mobile phase pumped at 1.0 mL min-1. The eluants were monitored at 225nm. The injection volume for samples and standards were 2μL. Methanol, acetonitrile and water in the ratio, 40:40:20; v/v/v, respectively was used as diluent.
Reagents
Standards were supplied by D.C.O. Hyderabad, India. HPLC grade acetonitrile, analytical grade ortho phosphoric acids were purchased from Merck (Mumbai, India). Water was prepared by Millipore MilliQ Plus water purification system. Commercial pharmaceutical preparation of Triximet combined tablets were purchased from the market. The declared content of tablets was Sumatriptan 85 mg and Naproxen 500 mg per tablet.
Preparation of solutions
Standard solutions: A standard solution containing 68μg/mL-1 of SS and 400μg/mL-1 of NS were prepared by dissolving appropriate amount of SS and NS in diluent. All the solutions were covered with aluminium foil to prevent photolytic reaction until the time of analysis.
Sample preparation: Ten tablets, each containing 85 mg of SS and 500 mg of NS were dissolved in 500 mL diluents to get 1710μg mL-1 of SS and 10000μg mL-1 of NS. 4 mL of above solution was diluted to 100 mL to get 68μg mL-1 of SS and 400μg mL-1 of NS. The solution was filtered through 0.45 μm Millipore PVDF filter. Then 2μL of these solutions were injected in the column and chromatogram was. The retention times of SS and NS were found to be 1.8 min and 2.6 min, respectively.
System suitability solution criteria
The system suitability was assessed by five replicate analyses of the drugs at concentrations of 68μg mL-1 of SS and 400μg mL-1 of NS. The acceptance criteria was not more than 2.0% for the RSD for the peak areas and not more than 2.0 for tailing factor for the peaks of the both the drugs.
Method validation
Method validation was performed as per ICH guidance [28-29] for simultaneous determination of SS and NS in the formulations. The following validation characteristics were addressed: linearity, detection limit, quantification limit, precision, accuracy and specificity.
System suitability criteria
The system suitability test solution was injected and the chromatographic parameters like relative standard deviation for replicate injections of both SS and NS and the tailing factor for SS and NS peaks were evaluated for proving the system suitability.
Specificity–forced degradation studies
Forced degradation studies were performed on SS and NS combined tablets to prove the stability indicating property of the method. The stress conditions employed for degradation study of SS and NS include light exposure [29], heat (105°C), acid hydrolysis (1 N HCl at 50°C), base hydrolysis (1N NaOH at 50°C), water hydrolysis at 50°C and oxidation (1% H2O2 at 30°C). For light studies, the monitoring period was 10 days whereas for heat, acid, base and water hydrolysis it was 48 h. Oxidation was carried out for 24h. Peak purity of the principal peak in the chromatogram of stressed samples of SS and NS tablets was checked using photo diode array detector.
Linearity of response
Linearity solutions were prepared from stock solution at five concentration levels from 34μg mL-1 to 102μg mL-1 for SS and from 200μg mL-1 to 600μg mL-1 for NS. The slope, Y-intercept and correlation coefficient were calculated.
Precision
Repeatability (intra-day): The precision of the assay method was evaluated by carrying out six independent assays of SS and NS (1.71 mg mL-1 of SS and 10.0 mg mL-1 of NS) test samples against qualified reference standard. The percentage of RSD of six assay values was calculated.
Intermediate precision (inter-day): Different analyst from the same laboratory evaluated the intermediate precision of the method. This was performed by assaying the six samples of SS and NS tablets against qualified reference standard. The percentage of RSD of six assay values was calculated.
Accuracy (Recovery study)
Recovery of the assay method for SS and NS was established by three determinations of test sample using tablets at 50%, 100% and 150% of analyte concentration (1.71 mg mL-1 of SS and 10.0 mg mL-1 of NS). Each solution was injected trice (n=3) into HPLC system and the average peak area of S and NS peaks was calculated.
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The LOD and LOQ for SS and NS were estimated at a signal-to noise ratio of 3:1 and 10:1, respectively, by injecting a series of dilute solutions with known concentration.
Robustness
To determine the robustness of the method the experimental conditions were deliberately changed and the resolution of SS and NS, tailing factor and % RSD for five replicate injections was evaluated. The mobile phase flow rate was 1.0 mL min−1; to study the effect of flow rate on resolution it was changed to 0.9 and 1.1 mL min−1. The effect of pH was studied at pH 2.1 and 2.3 (instead of pH 2.2). The effect of column temperature was studied at 25 and 35°C (instead of 30°C). In all these experiments the mobile phase components were not changed.
Solution stability and mobile phase stability
The stability of SS and NS in solution was determined by leaving test solutions of the sample and reference standard in tightly capped volumetric flasks at room temperature for 48h during which they were assayed at 24h intervals. Stability in the mobile phase was determined by analysis of freshly prepared sample solutions at 24h intervals for 48h and comparing the results with those obtained from freshly prepared reference standard solutions. The mobile phase was prepared at the beginning of the study period and not changed during the experiment. The RSD (%) of the results was calculated for both the mobile phase and solution-stability experiments.
Method development and optimization of stability indicating assay method
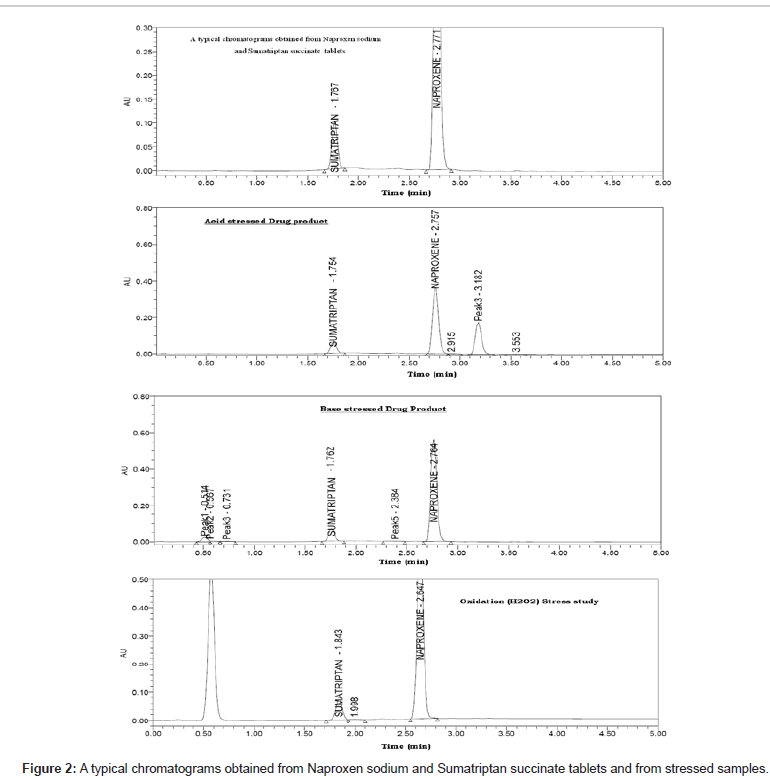
The method was optimized to separate major degradation products formed under varies stress conditions from SS and NS. The main target of the chromatographic method is to get the separation for closely eluting degradation products, mainly for the degradation product at 3.18 RT, which is eluting very closely to the NS. The degradation samples were run using different stationary phases like C18, C8 and Mobile phases containing buffers like phosphate and acetate with different pH (2-7) and using organic modifiers like acetonitrile and methanol in the mobile phase. But the separation was satisfactory in the adopted chromatographic conditions only. It indicated that the gradient elution with 0.2% ortho phosphoric acid in water as solvent A and acetonitrile and water in the ratio 90:10; v/v, was as solvent B for mobile phase was successful in separating drugs and all chromatographic degradation products (Figure 2). The detailed experimentation is reported in Table 1.
| Trial no. | UPLC Conditions |
Remark | |
| 1 | Column | Inertsil ODS-3, 50 mm × 2.1 mm, 2 μm particles | Separation of unknown impurity (RT 3.18) from NS is poor |
| Mobile phase | Solvent A: 0.2% ortho phosphoric acid in water (pH 2.2) Solvent B: Acetonitrile and water in the ratio 90:10 v/v | ||
| Flow rate | 1.0 mL min-1ww | ||
| Gradient | Time (min) / % solution B: 0/5, 2/95, 4/95, 4.1/5, 5.0/5 | ||
| 2 | Column | Waters AQUITY BEH C8, 50 mm × 2.1 mm, 1.7 μm particles | Separation of unknown impurity (RT 3.18) from NS is poor |
| Mobile phase | Solvent A: 0.2% ortho phosphoric acid in water (pH 2.2) Solvent B: Acetonitrile and water in the ratio 90:10 v/v |
||
| Flow rate | 1.0 mL min-1 | ||
| Gradient | Time (min) / % solution B: 0/5, 2/95, 4/95, 4.1/5, 5.0/5 | ||
| 3 | Column Mobile phase Flow rate Gradient |
Zorbax SB C-18 column, 50 mm × 2.1 mm, 1.8 μm particles | NS Peak is strongly retained and tailing of NS is more (1.8) |
| Solvent A: 0.01 M KH2PO4 buffer, tetra hydro furan and methanol in the ratio 67: 23: 10 (v/v/v), pH adjusted to 4.0 with ortho phosphoric acid. Solvent B: Acetonitrile and water in the ratio 90:10 v/v |
|||
| 0.6 mL min-1 | |||
| Time (min) / % solution B: 0/5, 2/95, 4/95, 4.1/5, 5.0/5 | NS Peak is strongly retained and tailing of NS is more (1.9) | ||
| 4 | Column | Zorbax SB C-18 column, 50 mm × 2.1 mm, 1.8 μm particles | |
| Mobile phase | Solvent A: 0.01 M ammonium acetate buffer pH adjusted to 7.0 with aqueous ammonia solution Solvent B: Acetonitrile and water in the ratio 90:10 v/v |
||
| Flow rate | 1.0 mL min-1 | ||
| Gradient program | Time (min) / % solution B: 0/5, 2/95, 4/95, 4.1/5, 5.0/5 | ||
| 5 | Column | Zorbax SB C-18 column, 50 mm × 2.1 mm, 1.8 μm particles | Separation of unknown impurity (RT 3.18) from NS is satisfactory and tailing for both SS and NS peaks were less than 1.3 |
| Mobile phase | Solvent A: 0.2% ortho phosphoric acid in water (pH 2.2) Solvent B: Acetonitrile and water in the ratio 90:10 v/v |
||
| Flow rate | 1.0 mL min-1 | ||
| Gradient program | Time (min) / % solution B: 0/5, 2/95, 4/95, 4.1/5, 5.0/5 |
Table 1: Results from different method development trials.
Results and Discussion
Method validation
Validation of an analytical procedure is the process by which it is established, by laboratories studies, that the performance characteristics of the procedure meet the requirements for the intended analytical applications [28].
System suitability
The system suitability test solution was injected and the chromatographic parameters like relative standard deviation for replicate injections of I and DC and the tailing factor for both SS and NS peaks are evaluated. The relative standard deviation for replicate injections of both SS and NS was 0.5% and 0.3% respectively. The tailing factor for both SS and NS peaks was 1.2% and 1.3%, respectively. This indicates the suitability of the system.
Linearity of response
Calibration curve obtained by least square regression analysis between average peak area and the concentration showed (Table 2a and Table 2b) linear relationship with a regression coefficient of 0.999. The best fit linear equation obtained was Y =8620 Con - 5872 for SS and Y=11070 Con - 13451 for NS. Analysis of residuals indicated that the residuals were normally distributed around the mean with uniform variance across all concentrations suggesting the homoscedastic nature of data. Selected linear model with univariant regression showed minimum % bias indicating goodness of fit which was further supported by the low standard error of estimate and mean sum of residual squares.
| Concentration in µg/ml |
Mean area Response achieved | Response calculated thru Trend line equation | Residual (Response practical -Response theoretical) | Residual square |
| 34.2 | 294426 | 288932.80 | -5493.2 | 98899047.0 |
| 51.3 | 430896 | 436335.20 | 5439.2 | 1133160.3 |
| 68.4 | 580852 | 583737.60 | 2885.6 | 207613517.4 |
| 85.5 | 731256 | 731140.00 | -116.0 | 200366856.0 |
| 102.6 | 881258 | 878542.40 | -2715.6 | 308163981.2 |
| Residual sum of squares | 75474769.6 | |||
| Correlation coefficient 0.999 | ||||
| Trend line equation y = 8620x - 5872 | ||||
Table 2a: Residual summary of Linearity results of Sumatriptan succinate
| Concentration in µg/ml | Mean area Response achieved | Response calculated thru Trend line equation | Residual (Response practical -Response theoretical) | Residual square |
| 203.2 | 2245986 | 2236041.20 | -9944.8 | 98899047.0 |
| 304.8 | 3361852 | 3360787.50 | -1064.5 | 1133160.3 |
| 406.4 | 4471125 | 4485533.80 | 14408.8 | 207613517.4 |
| 508 | 5596125 | 5610280.10 | 14155.1 | 200366856.0 |
| 609.6 | 6752581 | 6735026.40 | -17554.6 | 308163981.2 |
| Residual sum of squares | 816176561.9 | |||
| Correlation coefficient 0.999 | ||||
| Trend line equation y = 11070x - 13451 | ||||
Table 2b: Residual summary of Linearity results of Naproxen sodium
Precision
The precision of an analytical method gives information on the random error. It expresses of agreement between a series of measurements obtained from multiple sampling of the same homogeneous sample under prescribed conditions. The percentage RSD values for the precision study was 0.7%, 0.4% (inter-day precision) and 0.6%, 0.7% (intra-day precision) for SS and NS, respectively. This is confirming good precision of the method (Table 3).
| S.No | Parameter | Variation | % RSD for Assay |
|
| Sumatriptan succinate | Naproxen sodium | |||
| 1 | Repeatability(inter-day) | (a) Analyst-1(b) Waters Acquity UPLC system with PDA detector. (c) Day-1 |
0.7 | 0.4 |
| 2 | Intermediate precision (intra-day) |
(a) Analyst-2(b) Waters Acquity UPLC system with tunable UV detector. (c) Day-2 |
0.6 | 0.4 |
Table 3: Precision results
Accuracy-recovery test
The percentage recovery of SS was ranged from 98.2 to 100.2 and NS was ranged from 99.8 to 101.6. Excellent recoveries were made at each added concentration (Table 4).
| S.No | Concentration (%) | Mean recovery (%) (n = 3) | % RSD | ||
| SS | NS | SS | NS | ||
| 1 | 50 | 99.8 | 99.9 | 0.31 | 0.22 |
| 2 | 100 | 99.3 | 101.0 | 0.20 | 0.28 |
| 3 | 150 | 100.3 | 100.2 | 0.25 | 0.26 |
Table 4: Recovery study
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The limit of detection of SS and NS was 1.9 and 1.5μg /mL-1, respectively for 2μL injection volume. The limit of quantification of SS and NS was 6.3 and 4.8μg /mL-1, respectively for 2μL injection volume.
Robustness
When mobile phase flow rate, pH and column temperature were deliberately varied resolution between SS and NS was greater than 3.0, tailing factor and % RSD for five replicate injections of SS and NS was less than 1.5, illustrating the robustness of the method (Table 5).
| Parameter | Temperature (± 5°C of set temperature) |
Flow rate (± 0.1 ml/min of the set flow) |
pH (± 1 unit) |
||||
| Variation | 20°C | 30°C | 0.6 ml/min | 0.8 ml/min | 95% | 105% | |
| % RSD for 5 replicate injections |
SS | 1.3 | 1.2 | 1.4 | 1.1 | 1.2 | 1.2 |
| NS | 1.2 | 1.2 | 1.2 | 1.1 | 1.3 | 1.2 | |
| USP Resolution between | SS & NS | 5.2 | 5.8 | 5.5 | 5.7 | 5.0 | 5.9 |
Table 5: Robustness Study
Stability in solution and in the mobile phase
RSD (%) for assay of SS and NS during solution stability and mobile phase stability experiments was within 0.9%. No significant changes in the amounts of the two drugs were observed during solution stability and mobile phase experiments. The results from solution stability and mobile phase stability experiments confirmed that standard solutions and e mobile phase were stable for up to 48 h during assay determination (Table 6).
| S.No | Interval | % Assay Solution stability |
% Assay Mobile phase stability |
| 1 | 0 h | 99.4 | 98.2 |
| 3 | 24 h | 99.0 | 98.1 |
| 5 | 48 h | 98.2 | 98.3 |
| % RSD | 0.6 | 0.1 | |
Table 6: Solution and mobile phase stability results
Specificity–forced degradation studies
Degradation was not observed in SS and NS stressed samples that were subjected to light, heat, water and oxidation. However, the degradation was observed under base hydrolysis and acid hydrolysis. The peak purity test results derived from PDA (Photo Diode Array detector) confirmed that the SS and NS peaks were pure and homogeneous in all the analyzed stress conditions. This indicates that the method is specific and stability indicating (Figure 2 and Table 7).
| Stress condition | Time | ~% Degradation |
| Acid hydrolysis (1N HCl,50°C ) |
24 h | 11.2 % |
| Base hydrolysis (1N NaOH, 50°C ) |
24 h | 5.3 % |
| Oxidation (1% H202) | 24 h | 0.4% |
| Water hydrolysis (50°C) | 24 h | 0.5% |
| Thermal (105°C) | 24 h | 0.3% |
| Light (photolytic degradation) |
10 days | 0.2% |
Table 7: Forced degradation results.
Conclusion
A simple specific stability indicating liquid chromatographic method is developed for the quantification of SS and NS simultaneously in combined dosage forms. This method is validated and it is found to be specific, precise, accurate, robust and linear for the detection and quantification of SS and NS. The method is stability-indicating and can be used for routine analysis of production samples and to check the stability of samples of SS and NS simultaneously in combined dosage forms.
Acknowledgement
The authors are very grateful to C-MET for their continuous support for this research work.
References
- Ekpe A, Tong JH, Rodriguez L (2001) High-performance liquid Chromatographic method development and validation for the simultaneous quantitation of naproxen sodium and pseudoephedrine hydrochloride impurities. J Chromatogr Sci 39: 81.
- Dinc A, Ozdemir E, Aksoy H, Ustundag O, Baleanu D (2006) Chemometric determination of naproxen sodium and pseudoephedrine hydrochloridein tablets by HPLC. Chem Pharm Bull 54: 415.
- Monser L, Darghouth F (2003) Simultaneous determination of naproxen and related compounds by HPLC using porous graphitic carbon column. J Pharm Biomed Anal 32:1087-1092.
- Mitakos A, Panderi I (2002) A validated LC method for the determination of clopidogrel in pharmaceutical preparations. J Pharm Biomed Anal 28:431-438.
- Tashtoush BM, Al-Taani BM (2003) HPLC determination of naproxen in Plasma. Pharmazie 58: 614-615.
- Nielsen-Kudsk F (1980) HPLC-determination of some antiinflammatory, weak analgesic and uricosuric drugs in human blood plasma and its application to Pharmacokinetics. Acta Pharmacol Toxicol 47: 267-273.
- Phillips TM, Wellner EF (2006) Measurement of naproxen in human plasma by chip-based immunoaffinity capillary electrophoresis. Biomed Chromatogr 20: 662-667.
- Mikami E, Goto T, Ohno T, Matsumoto H, Nishida M (2000) Simultaneous Analysis of naproxen, nabumetone and its major metabolite 6-methoxy-2- naphthylacetic acid in pharmaceuticals and human urine by HPLC. J Pharm Biomed Anal 23: 917-925.
- Zakeri-Milani P, Barzegar-Jalali M, Tajerzadeh H, Azarmi Y, Valizadeh H (2005) Simultaneous determination of naproxen, ketoprofen and phenol red in samples from rat intestinal permeability studies: HPLC method development and validation. J Pharm Biomed Anal 39: 624-630.
- Hsu Y, Liou Y, Lee J, Chen C, Wu A (2006) Assay of naproxen by highperformance liquid chromatography and identification of its photoproducts by LC-ESI MS. Biomed Chromatogr 20: 787-793.
- USP (2006) The United States Pharmacopeia, 24th Edn The United States Pharmacopeial Convention Inc., Rockville, MD, naproxen sodium monograph.
- EP (2008) European Pharmacopoeia, 6th Edn, 2: 3005.
- USP (2008) The United States Pharmacopoeia, 31st Revision. US Pharmacopoeial Convention Inc. Rockville, MD 3: 3310.
- Nozal MJ, Bernal JL, Toribio L, Martín MT, Diez FJ (2002) Development and validation of an LC assay for sumatriptan succinate residues on surfacesin the manufacture of pharmaceuticals. J Pharm Biomed Anal 30: 285.-291.
- Ge Z, Tessier E, Neirinck L, Zhu Z (2004) High performance liquid chromatographic Method for the determination of sumatriptan with fluorescence detection in human plasma. J Chromatogr B 806: 299-303.
- Vishwanathan K , Bartlett MG, Stewart JT (2000) Determination of antimigraine compounds rizatriptan, zolmitriptan, naratriptan and sumatriptan in human serum by liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 14: 168-172.
- Xu X, Bartlett MG, Stewart JT (2001) Determination of degradation products of sumatriptan. J Pharm Biomed Anal 26: 367-377.
- Cheng KN, Redrup MJ, Barrow A, Williams PN (1998) Validation of a liquid chromatographic tandem mass spectrometric method for the determination of sumatriptan in human biological fluids. J Pharm Biomed Anal 17: 399-408.
- Boulton DW, Duncan GF, Vachharajani NN (2003) Validation and application of a high-performance liquid chromatography/tandem mass spectrometry assay for sumatriptan in human plasma. Biomed Chromatogr 17: 48-52.
- Trinath M, Saurabh K, Banerjee D, Hari Hara Teja, Bonde CG (2010) Development and validation of spectrophotometric method for simultaneous estimation of Sumatriptan and Naproxen sodium in tablet dosage form. Der Pharmacia Sinica 1: 36.
- Gondalia RP, Dhramasi AP, (2010) Spectrophotometric simultaneous estimation of naproxen sodium and sumatriptan succinate in tablet dosage form. Int J Pharm Biomed Sci 1: 24-26.
- Riddhi Gondalia, Abhay Dharamsi (2011) HPTLC Method For Simultaneous Determination of Naproxen Sodium and Sumatriptan Succinate in Pharmaceutical Dosage Form. Int J Pharm Sci research 2: 130.
- Russo R, Guillarme D, Nguyen TTD, Bicchi C, Rudaz S, et al. (2008) Pharmaceutical applications on columns packed with sub-2µm particles. J Chromatogr Sci 46: 199.
- Nguyen DT, Guillarme D, Rudaz S, Veuthey JL (2006) Fast analysis in liquid chromatography using small particle size and high pressure. J Sep Sci 29: 1836-1848.
- Mazzeo JR, Neue UV, Marianna K, Plumb RS (2005) Advancing LC performance with smaller particles and higher pressure. Anal Chem 77: 460- 467.
- Villiers AD, Lestremau F, Szucs R, Gelebart S, David F, et al. (2006) Evaluation of ultra performance liquid chromatography. Par I. Possibilities and limitations. J Chromatogr A 1127: 60-69.
- Wren SAC, Tchelitcheff P (2006) Use of ultra-performance liquid chromatography in pharmaceutical development. J Chromatogr A 1119(1-2): 140-146.
- International Conference on Harmonization (1995) ICH guidelines on validation of analytical procedures: text and methodology Q2 (R1): FDA. Federal Register 60: 11260.
- International Conference on Harmonization (1996) ICH guidelines on stability testing: Photo stability testing of New drug substances and products Q1B.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16209
- [From(publication date):
July-2011 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11335
- PDF downloads : 4874