Research Article Open Access
Quantitative Assessment of Metabolic Changes in the Developing Brain of C57BL/6 Mice by In Vivo Proton Magnetic Resonance Spectroscopy
Benjamin Schmitt1, Ingo vonBoth2, Catherine E Amara3and Andreas Schulze4*
1Department of Radiology, Medical University of Vienna Centre for High-Field MR, Austria
2Department of Laboratory Medicine and Pathobiology, University of Toronto, Canada
3Faculty of Kinesiology & Physical Education, University of Toronto, Canada
4The Hospital for Sick Children, The Research Institute, Genetics and Genome Biology (Work was conducted here), University of Toronto, Canada
- Corresponding Author:
- Andreas Schulze
The Hospital for Sick Children
The Research Institute, Genetics and Genome Biology
University of Toronto, 555 University Avenue
Toronto, ON, M5G 1X8, Canada
Tel: +1 (416) 813 7654
Fax: +1 (416) 813 5345
E-mail: andreas.schulze@sickkids.ca
Received date: August 27, 2013; Accepted date: November 09, 2013; Published date: November 13, 2013
Citation: Schmitt B, vonBoth I, Amara CE, Schulze A (2013) Quantitative Assessment of Metabolic Changes in the Developing Brain of C57BL/6 Mice by In Vivo Proton Magnetic Resonance Spectroscopy. J Alzheimers Dis Parkinsonism 3:129. doi: 10.4172/2161-0460.1000129
Copyright: © 2013 Schmitt B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Localized proton MRS was used to quantify cerebral metabolite concentrations in the thalamus of mice to assess the variation of major metabolites during brain development. Three sets of C57BL/6 mice were followed in a longitudinal study from a very early phase at post-natal day four (p4) until today 57 (p57). Experiments were conducted in accordance with Canadian animal care guidelines on a 7-Tesla small animal MR system. Specimens were examined under inhalation anesthesia using single-voxel MRS. A cubic volume with edge lengths of 1.9 mm was placed in the thalamus region of animals and point-resolved spectroscopy (PRESS) spectra were acquired with the following parameters (TR/TE/NEX=2500 ms/20 ms/600; Bandwidth=4000 Hz). Absolute metabolite quantification using LCModel was obtained by assigning water signal intensity measured by MRS to water concentrations determined by histobiochemical analysis and interpolation. Optimized anesthesia, immobilization, and careful monitoring led to a survival rate of 100% throughout the study. The brain water content was 84.8, 78.8, and 77.6% at p12, p31, and p66. Variation of metabolites revealed similar patterns for the total of creatine and phosphocreatine (tCr), glutamate and glutamine (Glx), and the total of N-acetyl aspartic compounds (tNAA), with steady increases from p4 to reaching a plateau after p21. The total of Cholinecontaining compounds (tCho) and myo-inositol (Ins) had high concentrations at early exam points, decreased to minima between p14 and p19, and increased to adult levels thereafter. Taurine (Tau) had highest levels at p4, decreased persistently but fast in the early development and slow in the later stages of brain development. Our results indicate that biological variance must be considered if results from studies on mouse models of pathologies are compared with results from healthy controls during development. This aspect seems to be especially important between p10 and p21. Due to the high temporal resolution used at early time points in our study and the inclusion of multiple groups of animals at time points, our data contribute important aspects to the existing literature about mouse brain development.
Keywords
MR-spectroscopy; C57BL/6; Brain metabolites; Absolute quantification
Introduction
Rodent models of human diseases are of high importance for biomedical research as they enable studying diseases under laboratory conditions to provide information on pathophysiology of the disease and to develop and evaluate treatment options. This is of special importance for orphan diseases such as the guanidinoacetate methyltransferase (GAMT) deficiency, where it has not been possible, due to the low number of patients, to evaluate and modify treatment strategies in a clinical setting. Further, in order to understand disease induced changes occurring during brain development, it is essential to have an accurate understanding of normal brain maturation and its biological variability [1].
Proton magnetic resonance spectroscopy (1H MRS) is a wellestablished tool for non-invasive detection and quantification of brain metabolites such as taurine, creatine, choline, glutamate, glutamine, myo-inositol, and N-acetylaspartate [2]. 1H MRS applied to the brain of mice and rats at different magnetic field strengths have revealed metabolic patterns that are comparable to those observed in human brain [2-5]. Studies of cerebral metabolite concentrations in different mouse strains, however, have also demonstrated small (<13%) inter-strain variations, which are limited to specific metabolites [4,6,7]. Metabolite changes occurring during post-natal mouse brain development have been studied in vivo with single-voxel spectroscopy [8-10] or a multi-voxel approach in C57BL/6 mice, and in vitro with whole brain samples from C57BL/6 mice [11,12]. The four in vivo studies used different approaches for absolute metabolite quantification, namely advanced method for accurate, robust and efficient spectral fitting (AMARES), and linear combination of model spectra (LCModel). Additionally, the particular time points, the intervals between them, and the brain regions examined varied between the studies. While the results of studies seem fairly homogeneous, distinct differences in reported development patterns were found in a study by Larvaron et al. [8-11]. Differences between the study results may be attributed to the respective employed quantification strategies, e.g., the choice of using an external quantification or measured brain water content as quantification reference, and corresponding limiting factors, such as the scope of prior knowledge for spectral fitting [13,14].
The aim of the present study was to quantitatively evaluate developmental changes and differences in brain metabolite concentrations of C57BL/6 mice starting in the early phase at p4 until p57 with a focus on high temporal resolution at early time points. The data obtained shall provide additional detail knowledge and strengthen the basis of existing data about developmental changes in mouse brain.
Experimental
Animals
Animal care guidelines were approved by the University of Toronto in accordance with the Canadian Council on Animal Care (CCAC). The studied mouse population consisted of three sets of wild-type C57BL/6 mice obtained commercially (Charles River Laboratories International Inc., Wilmington, MA, USA), which were followed by MRS in a longitudinal study from post-natal day four (p4) today 57 (p57). An overview about the number of animals per group and the measured time points is given in Table 1. After birth, littermates were kept with their mothers until p21, after which they were separated based on sex.
In vivo MR protocol
Mice were positioned prone on a murine slider bed with respiratory monitoring (SA Instruments, Stony Brook, NY, USA). Anesthesia was introduced in a closed chamber with 3% isoflurane in the gas atmosphere. It was maintained via a mixture of oxygen with a flow of 1 L/min and isoflurane. The isoflurane fraction was adapted depending on animal age to values between 0.9% and 2% so that mice were breathing spontaneously and had physiological heart rates during the measurement according to [15]. The body temperature of animals was maintained at 38°C by water heating of the animal bed.
| Animal Type | C57BL/6 wild type (n=5) | C57BL/6 wild type (n=4) | C57BL/6 wild type (n=5) | |
|---|---|---|---|---|
| Age at Exam (Post-natal Day)Â Â Â | Group # | 1 | 2 | 3 |
| 4 | ||||
| 6 | ||||
| 10 | ||||
| 11 | ||||
| 12 | ||||
| 14 | ||||
| 15 | ||||
| 18 | ||||
| 19 | ||||
| 21 | ||||
| 22 | ||||
| 24 | ||||
| 25 | ||||
| 26 | ||||
| 29 | ||||
| 31 | ||||
| 57 | ||||
n= Number of animals per group; red bars indicate ages when animals from a particular group were scanned.
Table 1: Overview about genotypes of mice, number of animals per group and time points of examinations.
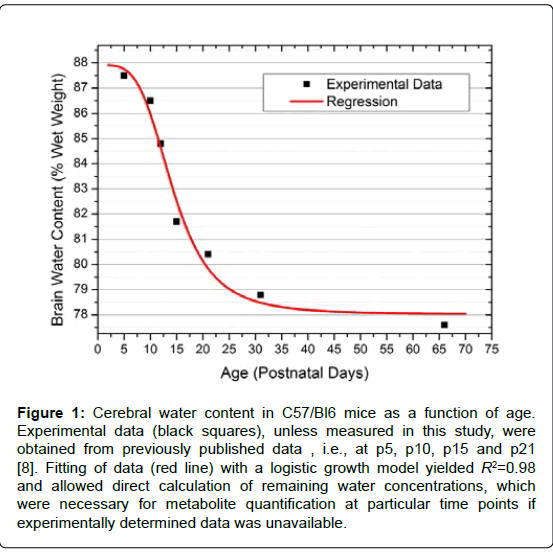
Figure 1: Cerebral water content in C57/Bl6 mice as a function of age. Experimental data (black squares), unless measured in this study, were obtained from previously published data , i.e., at p5, p10, p15 and p21 [8]. Fitting of data (red line) with a logistic growth model yielded R2=0.98 and allowed direct calculation of remaining water concentrations, which were necessary for metabolite quantification at particular time points if experimentally determined data was unavailable.
[1H]-MRI and MRS
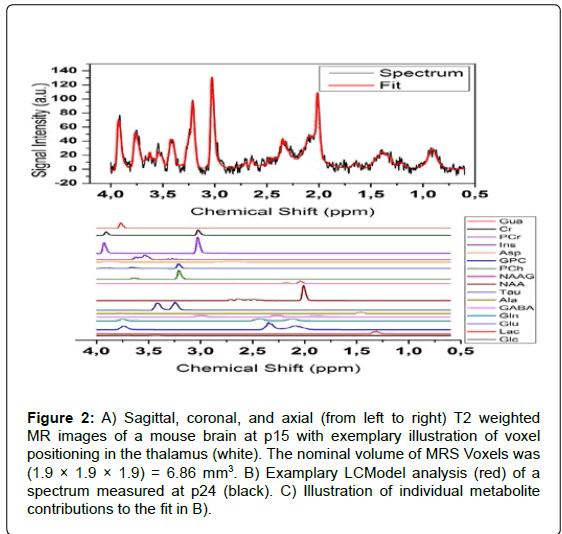
All experiments were performed on a 7 Tesla Bruker BioSpec 70/30 USR MR system (Bruker Biospin, Ettlingen, Germany). A linear, 72 mm inner-diameter volume resonator was used for RF excitation, while signal was acquired with a mouse brain surface coil (12 mm diameter, Bruker Biospin). Transversal T2 weighted rapid acquisition with relaxation enhancement (RARE) images were acquired prior to voxel positioning for MRS. The spectroscopy voxels had a nominal volume of 1.9×1.9×1.9 mm3; and were placed in the thalamus region of brains carefully avoiding contributions from tissue-tissue borders or ventricular spaces (Figure 2A). The line width of the water resonance was typically in the order of 6 to 14 Hz after localized shimming using the FASTMAP technique in a cube with an edge length of 3.4 mm [16]. A standard point-resolved spectroscopy (PRESS) sequence (TR/ TE/NEX=2500 ms/20 ms/600; acquisition bandwidth=4000 Hz) with variable power RF pulses with optimized relaxation delays (VAPOR) water signal suppression was used for acquisition of spectral data. The bandwidths of the excitation and refocusing hermite-type RF pulses were 5.4 kHz, which results in a chemical shift displacement error at the lipid resonance (δ=1.6 ppm) of 0.3 mm.
MRS data analysis
Each spectrum was analyzed with the LCModel software using the unsuppressed water signal as a reference for metabolite quantification [17]. A simulated basis-set, which included estimations for macromolecular and lipid signals, was used for spectral fitting. The Cramér-Rao Lower Bounds (CRLB), which is provided by LCModel as a measure of the reliability of the apparent metabolite concentration quantification, was taken into account for each specimen. Therefore, all metabolite concentration estimates with CRLB values above 25% were excluded from further analysis. Additionally, all spectra with signal-to-noise ratios (SNR) <8 were removed from data analysis. Absolute metabolite quantification was obtained by assigning the water signal intensity measured by MRS to water concentrations, which were determined by histobiochemical analysis as described at the beginning of this section. The concentrations of taurine (Tau), total creatine (tCr=[creatine] + [phosphocreatine]), cholinecontaining compounds (tCho=[choline]+[phosphocholine]), glutamic compounds (Glx=[glutamate]+[glutamine]), myo-inositole (Ins) and total N-acetylaspartic compounds (tNAA=[N-acetylaspartate] + [N-acetylaspartylglutamic acid]) were calculated for each animal and examination (Figures 2B and 2C). Levels of other metabolites such as glucose and gamma-amino butyric acid were removed from the analysis because quantification reliabilities were insufficient (CRLB>25%), especially at early time points. Calculated metabolite concentrations were averaged over all sets of animals measured during a particular time period and expressed as mean values together with corresponding standard errors of the mean (SEM).
Figure 2: A) Sagittal, coronal, and axial (from left to right) T2 weighted MR images of a mouse brain at p15 with exemplary illustration of voxel positioning in the thalamus (white). The nominal volume of MRS Voxels was (1.9 ÃÂ? 1.9 ÃÂ? 1.9) = 6.86 mm3. B) Examplary LCModel analysis (red) of a spectrum measured at p24 (black). C) Illustration of individual metabolite contributions to the fit in B).
Statistical analysis
All statistical analyses were performed with Origin 8.5 (OriginLab, Northampton, MA, USA). Differences between time points within groups were evaluated using one-way ANOVA and the Tukey test for repeated measures. P-values<0.05 were considered statistically significant. Differences between groups at equal time points were evaluated with one-way ANOVA. As a measure for comparing the individual variations of metabolites between animals of groups, the coefficients of variation (CV) were determined at each time point and averaged for each metabolite over all time points and groups examined. The mean coefficients of variation were then compared between metabolites using one-way ANOVA with Bonferroni correction for multiple testing.
Results
Animal survival
Between p4 and p10 unstable physiological parameters required permanent re-adjustment of anesthesia compared to older animals. In the younger animals oxygen flow and isoflurane fraction were varied during experiments, so a constant breathing frequency slightly below a physiologic frequency was maintained [18]. With these measures, a survival rate of 100% was obtained and none of the animals exhibited apparent neurological or behavioral abnormalities during the course of the study.
Weight
Animal weight was measured in one set of wild-type C57BL/6 mice of group #3 from p4 to p31. The mean weight increased from (2.42 ± 0.12, mean ± SD) g at p4 to (15.78 ± 0.61) g at p31. The individual weight differences, as indicated by the SEM at time points, were 4.9 % at p4, 10.9 % at p10 and finally decreased to 3.9 % at p31.
Cerebral water content
The measured relative brain water content was (84.8 ± 0.4)% at p12 (mean ± SEM), (78.8 ± 0.6)% at p31 and (77.6 ± 0.3)% at p66. Data fitting with a logistic growth model resulted in R2 = 0.98. Hence, missing water concentrations required for absolute metabolite quantification were derived directly from the fit function:

with the coefficients A1=(87.9 ± 0.9)% (mean ± SEM), A2=(78.0 ± 0.7)%, x0=(14.4 ± 1.2) days, p=(3.9 ± 1.1).
Brain metabolites
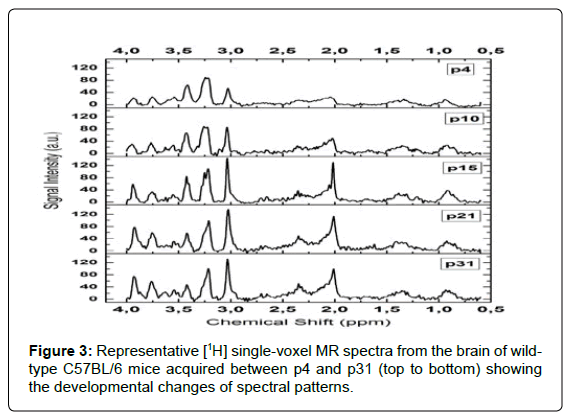
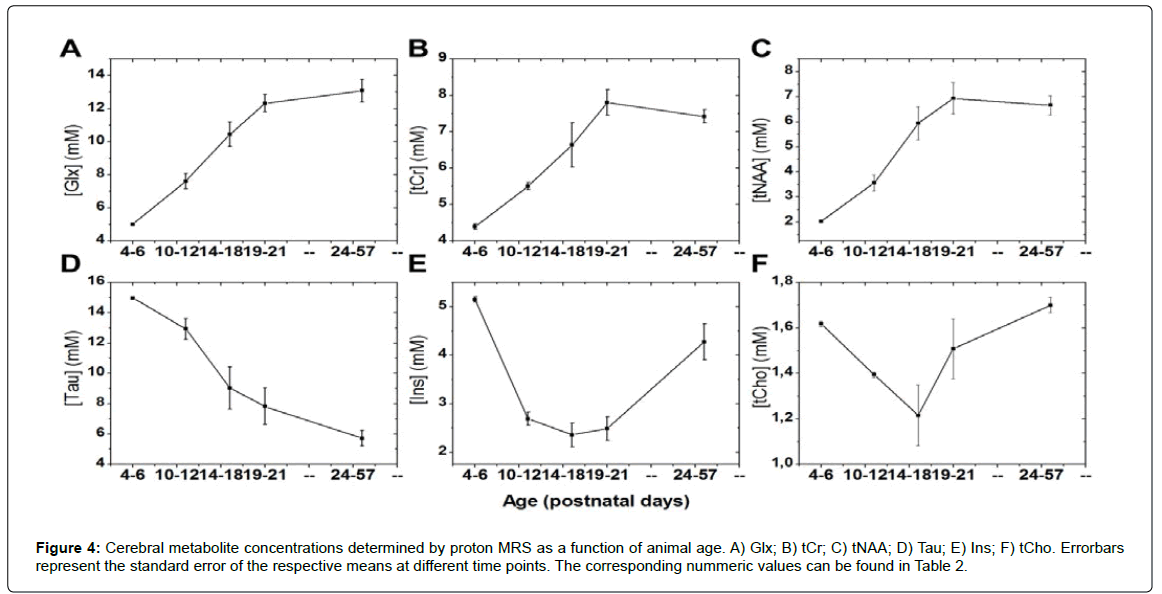
All examined MR spectra consistently exhibited average signal-tonoise ratios (SNR) of (12.3 ± 2.0) (mean ± SD); range: 8-15; (Figure 3). This enabled quantification of the metabolites of interest with CRLB values of (5 ± 2)% (mean ± SD) for the measured concentrations. The development patterns of all investigated brain metabolites could thus be determined with low quantification uncertainties in individual animals. Time courses of metabolite concentrations are displayed in Figure 4 and detailed analysis of differences between time points can be found in (Table 2). tNAA, tCr and Glx levels increased continuously from early time points until p21 (Figure 4A-C). Tau concentrations showed an opposite pattern with highest values at the beginning and a decrease towards a steady state, which was reached at a similar age (Figure 4D). Ins values had high levels at early exam points, then decreased to minimum values between p14 and p19, and finally increased by age (Figure 4E). This was comparable to the time course of tCho concentrations (Figure 4F). When coefficients of variation from different exam points were compared between metabolites, elevated values were found for Tau (26.7% at p10-12; 26.3% at p14-18), Ins (17.6% at p14-18, 18.8% at p19-21) and tCho (18.5% at p14-18). For all other metabolites and time points, CV values were below 16%.
| Animal Age [post-natal days] | Number of animal per group | tNAA [mM] | Glx [mM] | tCr [mM] | tCho [mM] | Tau [mM] | Ins [mM] |
|---|---|---|---|---|---|---|---|
| 4-6 | 10 | 2.04 Â ± 0.03 | 5.02 ± 0.02 | 4.40 Â ± 0.08 | 1.62 ± 0.01 | 14.97 ± 0.09 | 5.15 ± 0.06 |
| 10-12 | 15 | 3.57 ± 0.32*** | 7.62 ± 0.46 *** | 5.51 ± 0.10 *** | 1.39 ± 0.01*** | 12.92 ± 0.68** | 2.69 ± 0.13*** |
| 14-18 | 18 | 5.97 ± 0.66*** | 10.45 ± 0.74*** | 6.55 ± 0.61*** | 1.22 ± 0.13 | 9.03 ± 1.39*** | 2.37 ± 0.25 |
| 19-21 | 14 | 6.95 ± 0.62** | 12.33 ± 0.52** | 7.80 ± 0.35*** | 1.51 ± 0.13 | 7.83 ± 1.197 | 2.49 ± 0.24 |
| 24-57 | 24 | 6.89 ± 0.38 | 13.09 ± 0.68 | 7.45 ± 0.18 | 1.70 ± 0.03 | 5.72 ± 0.51 | 4.28 ± 0.37*** |
Significant differences between subsequent time-points in groups of mice are marked with asterisks (*: p<0.05; **: p<0.01; ***: p<0.001).
Table 2: Concentrations of cerebral metabolites measured with proton MR spectroscopy in mice. Data given here represent mean values (± SEM) averaged over all mice that were measured within certain age ranges. Calculation of SEM values for age ranges was performed taking into account the uncertainties of each individual measurement.
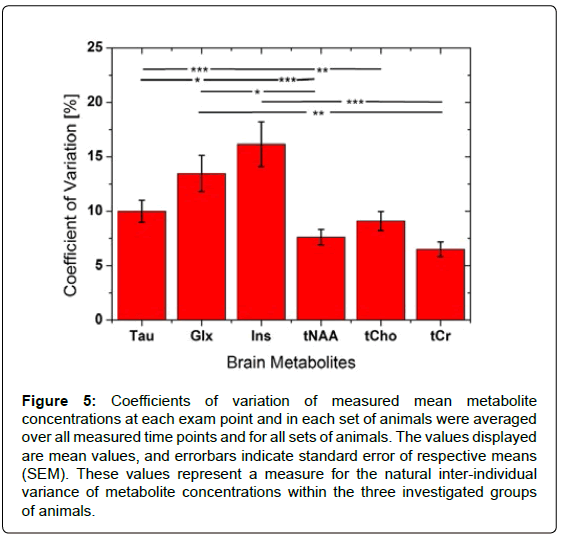
Coefficients of variation were calculated for each exam point, i.e., the CV of the average concentration of a certain metabolite within all animals from the same litter at a certain age. As a measure of interindividual variability of metabolite levels within groups, the CV values obtained for different metabolites were averaged for all time points. The largest average CV was found for Ins (CV=16.2 ± 2.1%, mean±SEM), which was significantly higher than the CV values of all other metabolites except for Glx (Figure 5). A high mean CV=13.5 ± 1.7% was also found for Glx, while all other metabolites exhibited average CV values of less than 10%.
Discussion
In this study, we have followed the early development of metabolite levels in the brain of mice by 1H-MRS, which is a highly useful and sensitive tool for non-invasive monitoring of cerebral metabolite concentrations [13,19]. The metabolite levels were derived by referencing MR signal intensities of metabolite protons to the unsuppressed signal of water protons, which reflects the concentration of bulk water in the examined region. While it is valid to assume stable water content in mature brain because of tightly regulated water homeostasis, this assumption will fail for the developing brain. Thus, in order to avoid significant errors in the calculation of absolute metabolite concentrations, we took the age-dependent variation of brain water content into account for calculation of absolute metabolite concentrations. The water concentrations measured in our study, combined with additional reference values obtained from the literature, could be fitted with very good confidence using a logical growth model, which also shows the good integration of the literature data into our data. This development corresponds well with the course of changing water content in human brain [8,20,21].
To our knowledge, there are three comparable in vivo studies, which also followed the development of metabolites during mouse brain maturation with MRS, and the earliest time point to be investigated was p5 in a previous study compared to p4 in our study. MRS results from the study of Larvaron et al. show considerable differences to results from similar previous studies and to our results, and, hence, are not taken into account for comparison with our data [8-11]. The results reported in the study by Larvaron et al., i.e. a higher variance of metabolite concentrations and occurrence of the most distinct concentration changes rather late between p15 and p21, can be explained by the long echo time of 130 ms used for MRS data acquisition in that study combined with a non model-based quantification approach [8]. The developmental changes of the major metabolites observed in our study, such as the persistent rise (Glx, tCr, tNAA) between p4 and p21 or decrease (Tau) during the whole period of brain maturation starting from the early post-natal period are in good agreement with the results from Weiss et al. [11]. Further, some of the developmental metabolite patterns established in the present study resemble those observed in post-natal development of the human brain, own unpublished data). When comparing our quantitative results to those obtained by Weiss et al. at p5 and p21, we found consistently lower values for Tau, tCr, tCho, and tNAA as well as higher values for Glx [21,22]. The differences in absolute values between the studies are attributed to the different quantification techniques, i.e. the employment of an external reference for quantification compared with the water signal as reference (this study). In terms of absolute quantification, a correction for the individual relaxation times of the metabolites was not performed in our study, which may potentially affect the accuracy of our quantification results. However, errors are considered to be negligible for T2 relaxation times given the echo time of 20 ms used in our study [11]. They are also negligible for T1 relaxation times in all metabolites except for Tau. A T1 of Tau on the order of 2 s, which was reported for Tau in the human brain at 7 Tesla, in combination with the TR of 2500 ms used in our study could have led to a constant underestimation of the true concentrations [23]. However, an explicit correction of signal intensity by applying a correction term that takes into account uncertainties of signal due to SNR, and uncertainties of T1 measurements would have led to increased uncertainty of the resulting values due to error propagation. This increase in turn may have impaired assessment of physiological differences between animals, which is an important aspect of this study. Thus, we decided to exclude an explicit correction of relaxation effects although resulting quantitative values for Tau may be lower than the true values in tissue.
Figure 5: Coefficients of variation of measured mean metabolite concentrations at each exam point and in each set of animals were averaged over all measured time points and for all sets of animals. The values displayed are mean values, and errorbars indicate standard error of respective means (SEM). These values represent a measure for the natural inter-individual variance of metabolite concentrations within the three investigated groups of animals.
In principle, our quantification approach allowed for accurate quantification of spectra via the determination of absolute brain water content, and is similar to other studies in this respect. For studies that were performed at 14.1 T, it may be assumed that quantification results are more accurate compared with our study due to higher spectral resolution and increased SNR. However, these studies only assessed the development at a lower temporal resolution (p10, p20, p30…) compared with our study [9,10]. While there is a good agreement between metabolite time courses at the level of their coarse temporal resolution, it is impossible to compare their data with our data at the time points p6 or p14 to 18. Our data, when expressed as ratios to tCr, are in very good agreement with data measured at 7 T in the hippocampus of wildtype C57BL/6J mice [24]. This agreement may indicate similarity in the neurochemical profile between the two brain areas, while, at least in adult mice, difference have been observed between the hippocampus and the striatum, which is adjacent to the thalamus [4].
Comparing our data with those from Weiss et al., who also used high temporal resolution, our data correlated better with concentrations measured ex vivo by high-performance liquid chromatography during brain maturation in the thalamus and with concentrations in humans, (own unpublished data) [11,21,25,26]. For instance, in our study, the concentrations of tCr, tNAA, and Glx show a steady increase in the developing brain and reach a plateau when the brain maturation is finished. This is in good accordance with observations in the thalamus region of children where the plateau was reached around the age of 3-4 years. Additionally, there is a good agreement between our results and those from in vivo MRS studies on brain metabolites in adult wild-type C57BL/6 mice [4,27].
The significant decrease of tCho and Ins after p4 reaching lowest levels at p14-18 may be interpreted as a physiologic process although such an observation has not been reported before and no data exist to correlate our observation with the development in humans. However, the assumption is supported by the fact that spectral quality and consequently quantification of the underlying spectra at these data points was not different compared with other time points. Instead, the high SEM values for tCho and Ins at p14-18 arise from large differences between animals. This, in turn, indicates a large natural variance of these metabolites at young age with a concentration that, on average, tends to be lower than the concentration at very early time points, i.e. p0 to p6. It will be interesting to see whether similar patterns occur in the first months of life when children get weaned off breast milk and start on solid food. For tCho, it has been speculated that accelerated myelination during a certain period of brain development incorporates a proportion of these MRS-visible choline residues into an ‘invisible’ macromolecule associated with myelin, for example phosphatidylcholine [21]. Ins is known to be an important osmolyte in the brain [28]. The early decline correlates very well with decrease in brain water content. The observed rise after p14-18 is more difficult to explain, but most likely the Ins concentration was much higher on p0 (e.g. ~12 mM in humans, [21]) and the increase could reflect the onset of compensatory mechanisms to keep Ins constant.
The grouping of MRS results from animals with minor age differences became necessary due to organizational issues concerning the planning of scan time according to the (variable) date of birth of litters. The corresponding shifts led to a suboptimal assignment of scan time to animal age. Merging of data according to age ranges was then introduced to obtain sufficiently high numbers of animals per data point in order to perform meaningful statistical analysis. Although data were carefully checked for age-dependent effects within the combined groups and did not exhibit any obvious tendencies, it is generally not possible to exclude an impact of age-dependent effects on our analysis. However, according to previous literature, we certainly do not expect a considerable influence of data merging on data for the p25-57 group because metabolite concentrations showed only minor developmental changes in this age range [9,10].
In conclusion, our study provides additional data that, on one hand, back up and solidify the existing knowledge about metabolite development in the brain of C57BL/6 mice. On the other hand, the high temporal resolution and the inclusion of data from multiple different groups at time points showed that large natural variances between p4 and p14 should be expected for tCho and Ins levels. This is especially important when results from studies that focus on pathological changes at these early time points are to be referenced to literature data about physiologic conditions in healthy controls.
Acknowledgements
We thank Dr. Warren Foltz for his support in method implementation, animal scanning and handling. This study was in part funded by the Heinz/Mead Johnson/ Weston endowment fund.
References
- Schulze A (2003) Creatine deficiency syndromes. Mol Cell Biochem 244: 143-150.
- Pouwels PJ, Frahm J (1998) Regional metabolite concentrations in human brain as determined by quantitative localized proton mrs. Magn Reson Med 39: 53-60.
- Kassem MN, Bartha R (2003) Quantitative proton short-echo-time laser spectroscopy of normal human white matter and hippocampus at 4 tesla incorporating macromolecule subtraction. Magn Reson Med 49: 918-927.
- Tkac I, Henry PG, Andersen P, Keene CD, Low WC, et al. (2004) Highly resolved in vivo 1h nmr spectroscopy of the mouse brain at 9.4 t. Magn Reson Med 52: 478-484.
- Tkac I, Rao R, Georgieff MK, Gruetter R (2003) Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1h nmr spectroscopy. Magn Reson Med 50: 24-32.
- Penet MF, Laigle C, Fur YL, Confort-Gouny S, Heurteaux C, et al. (2006) In vivo characterization of brain morphometric and metabolic endophenotypes in three inbred strains of mice using magnetic resonance techniques. Behav Genet 36: 732-744.
- Schwarcz A, Natt O, Watanabe T, Boretius S, Frahm J, et al. (2003) Localized proton mrs of cerebral metabolite profiles in different mouse strains. Magn Reson Med 49: 822-827.
- Larvaron P, Bielicki G, Boespflug-Tanguy O, Renou J-P (2006) Proton mrs of early post-natal mouse brain modifications in vivo. NMR Biomed 19: 180-187.
- Kulak A, Duarte JM, Do KQ, Gruetter R (2010) Neurochemical profile of the developing mouse cortex determined by in vivo 1h nmr spectroscopy at 14.1 t and the effect of recurrent anaesthesia. J Neurochem 115: 1466-1477.
- das Neves Duarte JM, Kulak A, Gholam-Razaee MM, Cuenod M, Gruetter R, et al. (2012) N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry 71: 1006-1014.
- Weiss K, Melkus G, Jakob PM, Faber C (2009) Quantitative in vivo 1h spectroscopic imaging of metabolites in the early postnatal mouse brain at 17.6 t. Magma (New York, N Y ) 22: 53-62.
- Yao FS, Caserta MT, Wyrwicz AM (1999) In vitro proton and phosphorus nmr spectroscopic analysis of murine (c57bl/6j) brain development. NMR Biomed 12: 463-470.
- Mandal PK (2012) In vivo proton magnetic resonance spectroscopic signal processing for the absolute quantitation of brain metabolites. Eur J Radiol 81: 653-664.
- Kanowski M, Kaufmann J, Braun J, Bernarding J, Tempelmann C (2004) Quantitation of simulated short echo time 1h human brain spectra by lcmodel and amares. Magn Reson Med 51: 904-912.
- Szczesny G, Veihelmann A, Massberg S, Nolte D, Messmer K (2004) Long-term anaesthesia using inhalatory isoflurane in different strains of mice-the haemodynamic effects. Lab Anim 38: 64-69.
- Gruetter R (1993) Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med 29: 804-811.
- Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton nmr spectra. Magn Reson Med 30: 672-679.
- Bissonnette JM, Knopp SJ (2001) Developmental changes in the hypoxic ventilatory response in c57bl/6 mice. Respir Physiol 128: 179-186.
- Duarte JM, Lei H, Mlynarik V, Gruetter R (2012) The neurochemical profile quantified by in vivo 1h nmr spectroscopy. Neuroimage 61: 342-362.
- Kimelberg HK (2004) Water homeostasis in the brain: Basic concepts. Neuroscience 129: 851-860.
- Kreis R, Ernst T, Ross BD (1993) Development of the human brain: In vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med 30: 424-437.
- Pouwels PJ, Brockmann K, Kruse B, Wilken B, Wick M, et al. (1999) Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton mrs. Pediatr Res 46: 474-485.
- Xin L, Schaller B, Mlynarik V, Lu H, Gruetter R (2012) Proton T1 relaxation times of metabolites in human occipital white and gray matter at 7 T. Magn Reson Med 69: 931-936.
- Shi D, Xu S, Waddell J, Scafidi S, Roys S, et al. (2012) Longitudinal in vivo developmental changes of metabolites in the hippocampus of fmr1 knockout mice. J Neurochem 123: 971-981.
- Miranda-Contreras L, Benitez-Diaz PR, Mendoza-Briceno RV, Delgado-Saez MC, Palacios-Pru EL (1999) Levels of amino acid neurotransmitters during mouse cerebellar neurogenesis and in histotypic cerebellar cultures. Dev Neurosci 21: 147-158.
- Miranda-Contreras L, Mendoza-Briceno RV, Palacios-Pru EL (1998) Levels of monoamine and amino acid neurotransmitters in the developing male mouse hypothalamus and in histotypic hypothalamic cultures. Int J Dev Neurosci 16: 403-412.
- Renema WK, Schmidt A, van Asten JJ, Oerlemans F, Ullrich K, et al. (2003) Mr spectroscopy of muscle and brain in guanidinoacetate methyltransferase (GAMT)-deficient mice: Validation of an animal model to study creatine deficiency. Magn Reson Med 50: 936-943.
- Lien YH, Shapiro JI, Chan L (1990) Effects of hypernatremia on organic brain osmoles. J Clin Invest 85: 1427-1435.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15237
- [From(publication date):
December-2013 - Nov 17, 2025] - Breakdown by view type
- HTML page views : 10458
- PDF downloads : 4779