Research Article Open Access
Quantification of Acyclovir in Human Plasma by Ultra-High-Performance Liquid Chromatography - Heated Electrospray Ionization - Tandem Mass Spectrometry for Bioequivalence Evaluation
Changxing Shao1, Thomas C Dowling2, Sam Haidar3, Lawrence X Yu3, James E Polli1, and Maureen A Kane1*
1Department of Pharmaceutical Sciences, University of Maryland, Baltimore, Maryland, USA
2Clinical Pharmacology Unit, Department of Pharmacy Practice and Science, School of Pharmacy, University of Maryland, Baltimore, Maryland, USA
3Food and Drug Administration, Rockville, Maryland, USA
- *Corresponding Author:
- Maureen A Kane
Department of Pharmaceutical Sciences, University of Maryland
20 N. Pine St.; Pharmacy Hall North 723
Baltimore, MD21201,USA
Tel: 11-410-706-5097
Fax: 11-410-706-5017
E-mail: mkane@rx.umaryland.edu
Received date: June 27, 2012; Accepted date: July 31, 2012; Published date: August 06, 2012
Citation: Shao C, Dowling TC, Haidar S, Yu LX, Polli JE, et al. (2012) Quantification of Acyclovir in Human Plasma by Ultra-High-Performance Liquid Chromatography - Heated Electrospray Ionization - Tandem Mass Spectrometry for Bioequivalence Evaluation. J Anal Bioanal Tech 3:139. doi: 10.4172/2155-9872.1000139
Copyright: © 2012 Shao C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Pharmacokinetic studies are essential towards determining bioequivalence and establishing pharmacokinetic profiles for drug moieties requires accurate quantification. We report a rapid, sensitive, and robust method for the determination of acyclovir in human plasma and its validation towards evaluating the bioequivalence of drug formulations. After a simple liquid-liquid extraction from plasma, acyclovir is quantified using ultra-high-performance liquid chromatography - heated electrospray ionization - tandem mass spectrometry (UHPLC-HESI-MS/MS). The assay has a total analysis time is 5 min, a linear range of 1.0 - 2000 ng/mL, a lower limit of detection of 0.5 ng/mL, and a lower limit of quantification of 1.0 ng/mL. Intra- and inter-day precision is no more than 10.3% and intraand inter-day accuracy was within 13% at various concentrations in human plasma. Validation according to FDA guidelines for bioanalysis indicates that the described UHPLC-HESI-MS/MS method provides rigorous quantification of acyclovir in human plasma and representative data demonstrates successful application towards the determination of pharmacokinetic profiles as part of an evaluation of drug formulation bioequivalence.
Keywords
Acyclovir; Ultra high performance liquid chromatography; Tandem mass spectrometry; Quantification; Pharmacokinetics; Plasma
Abbreviations
UHPLC: Ultra-High-Performance Liquid Chromatography; HESI: Heated Electrospray Ionization; MS: Mass Spectrometry; MS/ MS: Tandem Mass Spectrometry; BCS: Biopharmaceutics Classification System; FDA: Food and Drug Administration; PK: Pharmacokinetic; IS: Internal Standard; m/z: mass-to-charge ratio; Cmax: Peak Plasma Concentration of Drug; Tmax: Time required to reach the peak plasma concentration of drug
Introduction
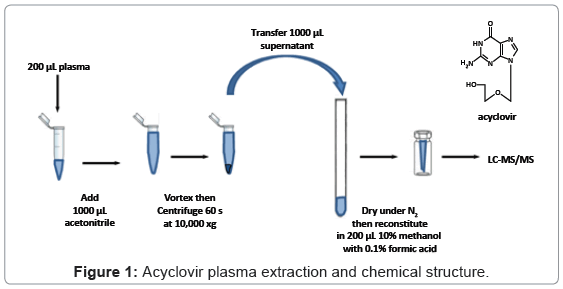
Acyclovir is a nucleoside analogue with antiviral activity for some members of the herpes virus group of DNA viruses (Figure 1) [1-3]. This drug is tolerated by different populations and has a high therapeutic index due to its highly selective biological activity [1]. It has been shown that acyclovir has high solubility and low intestinal permeability, and it is considered as a typical class 3 drug according to the Biopharmaceutics Classification System (BCS) from the Food and Drug Administration (FDA) of the United States [4,5]. Previous studies show acyclovir concentration in plasma peaks between 1.5- 2.5 hours with a concentration range of 460 - 830 ng/mL after a single oral administration of 200 mg [6,7]. The average acyclovir half-life in plasma is 3 hours in adults with normal renal function [8]. Studies also show the average oral bioavailability of acyclovir is ~10-20%,and approximately 80% of an oral dose is never absorbed and is excreted through feces [9]. A recent study demonstrates that acyclovir formulation effects bioequivalency due to variable and incomplete absorption in the gastrointestinal tract [10]. A contributing factor to incomplete absorption may be the effect of excipients on drug permeability [11]. Previously, the impact of common excipients on drug permeability, including acyclovir, was investigated in Caco-2 cell systems [12]. Several formulations with various common excipients were subsequently developed and orally administrated to healthy subjects in order to evaluate in vivo drug pharmacokinetic (PK) profiles (manuscript in preparation).
To determine in vivo acyclovir PK profiles for different formulations, an analytical method that accurately quantifies acyclovir in plasma without interference is essential. In addition, a simple sample preparation and rapid analysis is desirable for data collection of clinical samples in a time efficient manner. Previous analytical methods for acyclovir include immunological techniques [13], high-performance liquid chromatography (HPLC) with ultraviolet (UV) detection [14-18], HPLC with fluorescence detection [19-22], and LC-MS/MS [23-28]. Unfortunately, many UV-, fluorescence-, and LC-MS/MS-based assays lack sensitivity with lower limit of quantification (LLOQ) between 10 ng/mL and 250 ng/mL [14-21,23,25-28]. Recently, MS/MS detection has improved sensitivity 5-fold, lowering reported LLOQs to 2 ng/mL, but the analysis time of 20 min for this method is undesirable for clinical studies with thousands of samples [24].
Ultra-high-performance liquid chromatography (UHPLC) separations using sub-2 μm particle columns have been shown to reduce separation times while maintaining separation efficiency [29-31]. UHPLC separations are compatible with MS/MS detection. The higher resolution provided by UHPLC coupled to MS/MS detection can increase MS/MS sensitivity as much as 10-fold in side-by-side comparisons with traditional HPLC coupled to MS/MS detection [32]. However, UHPLC efficiency is maximized at higher flow rates, which can often overwhelm traditional ESI sources resulting in inefficient ionization [33,34]. Heated ESI (HESI) has been shown to increase ionization efficiency at higher flow rates and assist in eliminating interfering signal, thus, increasing observed sensitivity [35]. In addition to sensitivity, MS/MS detection imparts excellent specificity and is the preferred method for quantification of drug molecules in biological matrices [36]. Here we describe a UHPLC-HESI-MS/MS method for determination of acyclovir in human plasma that combines an appropriately low LLOQ with reduced analysis time and the specificity of MS/MS detection. Furthermore, we demonstrate its successful application in determining PK profiles for bioequivalence evaluation.
Experimental
Chemicals and standards
Acyclovir (acycloguanosine; 9-((2-hydroxyethoxy) methyl) guanine; 2-amino-9-[(2-hydroxyethoxy) methyl]-3,9-dihydro-6Hpurin- 6-one) and ribavirin (1-b-D-Ribofuranosyl-1,2,4-triazole-3- carboxamide) were obtained from Sigma at >99% purity (St. Louis, MO). Ammonium acetate, formic acid, methanol, acetonitrile, ethyl acetate, and water were purchased from Fisher Scientific. All solvents were LC-MS grade except ethyl acetate which was HPLC grade.
Instruments and methods
UHPLC-HESI-MS/MS:The UHPLC system consisted of an Accela degasser and quaternary pump and an HTC PAL thermostatted autosampler (Thermo Scientific; San Jose, CA). Chromatographic separation of acyclovir was effected on a Waters BEH C18 column (50 mm × 2.1 mm, 1.7 μm) (Milford, MA) using a linear gradient of the following mobile phases: A, 2 mM aqueous ammonium acetate with 0.1% formic acid and B, 100% methanol with 0.1% formic acid. The gradient conditions were as follows: 0-0.3 min, 98% A with flow of 400 μL/min; 0.3-1.5 min, 98% to 5% A with flow increasing from 400 μL/min to 500 μL/min; 1.5-2.9 min, 5% A with flow of 500 μL/min; 2.9-3.0 min, 5% to 98% A with flow increasing from 500 μL/min to 650 μL/min; 3.0-5.0 min, 98% A with flow of 650 μL/min. A TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific; San Jose, CA) equipped with a heated electrospray ionization source (HESI-II probe) was operated in positive ionization mode using selected reaction monitoring (SRM). ESI optimization was performed by direct infusion (via a “tee”) of a 0.5 μM acyclovir or ribavirin standard solution in methanol at 10 μL/min into a mobile phase composed of 90% B flowing at 500 mL/min. Acyclovir was monitored with a (precursor → product) mass transition of m/z 226.1 → m/z 152.0. Ribavirin (internal standard, IS) was monitored with a mass transition of m/z 245.1 → m/z 113.0 The mass spectrometer was operated at unit resolution (peak width at halfheight = 0.7 Da) for both the first (Q1) and third (Q3) quadrupoles. The optimum HESI-MS/MS conditions were as follows: Spray voltage, 3500 V; vaporizor temperature, 450°C; sheath gas, 25; aux gas, 10; capillary temperature, 330; collision energy, 12 eV; S-lens, 77.
Sample preparation
Control, drug-free human plasma was obtained from the University of Maryland hospital blood bank (Baltimore, MD) and clinical samples were collected from healthy volunteers enrolled in a 4-way crossover bioequivalence study. During the study, volunteers were orally administered a 200 mg capsule containing acyclovir and combinations of excipients at each of four visits and blood samples were collected at 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 8.0, and 10.0 hours after the dose. The plasma aliquots were stored at -20°C until analysis. Fifty microliters of a 2 μg/mL ribavirin solution (IS) was added prior to sample preparation. Plasma samples were prepared by adding 1000 μL acetonitrile to 200 μL plasma, which were then vortex-mixed briefly. Following centrifugation for 1 min at 10,000 × g, 1000 μL of supernatant was collected and evaporated to dryness under nitrogen (N2) and then reconstituted in 10% methanol with 0.1% formic acid to a final volume of 200 μL (Figure 1). Standard and QC samples were similarly prepared using drug-free plasma spiked with acyclovir stock solution (10.0 mg/mL in methanol).
Data collection and analysis
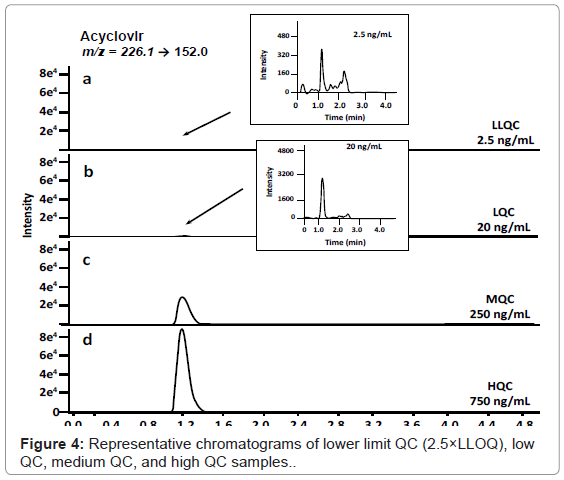
Data were collected and processed using Xcalibur v 2.1 (Thermo Scientific; San Jose, CA). Calibration curves were obtained for each set of data / day of analysis from plots of acyclovir peak area as a function of concentration (10 points ranging from 1.0 ng/ml to 2000 ng/mL) that were analyzed by linear least squares regression with a weighting factor of 1/x2. QC samples were collected for each data set / day of analysis at the following levels: lower limit QC (LLQC; 2.5 x LLOQ), 2.5 ng/mL; low QC (LQC), 20 ng/mL; medium QC (MQC), 250 ng/mL; and high QC (HQC), 750 ng/mL.
Method validation
The current method was validated by evaluating selectivity, accuracy, precision, linearity, sensitivity, and sample stability according to FDA guidelines for Bioanalytical Method Validation [37]. Sensitivity and linearity of the method was established by spiking acyclovir into blank plasma with concentration range from 0.5 ng/mL to 2000 ng/mL. The lowest concentration showing consistent, acceptable accuracy and precision was determined to be the lower limit of quantitation or LLOQ (within 80-120% at LLOQ). Selectivity was evaluated by screening at least six lots of blank plasma and comparing with plasma spiked with acyclovir standard solution. Intra-day precision values were assessed by calculating coefficient of variation (% CV) for seven replicates at four different concentrations spiked into blank plasma. Inter-day precision was assessed by calculating % CV of similarly obtained replicate data obtained on three separate days. Accuracy was determined as the percentage difference of measured concentration compared to intended (spiked) concentration. The stability of acyclovir in plasma was evaluated for 6, 24 and 72 hours at room temperature and at 4°C, and after three freeze-thaw cycles. The stability samples were prepared at the low and high-QC level in triplicate at each concentration level for each condition and time point of the study, and extracted and analyzed following the sample preparation procedures.
Results and Discussion
Our objectives for this work were to (1) develop a robust, rapid, and reproducible analytical assay for acyclovir in human plasma using UHPLC-HESI-MS/MS and (2) to validate the methodology so that it would be appropriate for application in a clinical study evaluating the bioequivalence of various acyclovir formulations. We aimed to employ UHPLC to reduce chromatographic separation time, the HESI probe to increase ionization efficiency, and MS/MS detection to ensure specificity, provide sensitivity, and eliminate interference from components of complex matrices.
Sample preparation and stability
Human plasma samples were stored at -20°C until analysis. A previous study reported acyclovir was stable in human plasma at -20°C for long term storage [9]. Ribavirin was added as an internal standard to monitor analyte recovery during preparation and to monitor MS instrument response. Ribavirin and acyclovir have been previously shown to extract and chromatograph similarly in an LCMS/ MS assay [38]. Sample preparation consisted of a one-step organic extraction followed by nitrogen evaporation and resuspension in a solvent compatible with the initial mobile phase composition (Figure 1). Although it was not employed in this study, this preparation scheme could be automated to increase analysis throughput. Extraction efficiency was assessed at each of the quality control analyte levels: LLQC (2.5 x LLOQ), low (LQC), medium (MQC), and high (HQC) with mean recoveries of 73.9 ± 8.2, 77.0 ± 4.0, 82.9 ± 2.3, and 81.7 ± 1.6, respectively. The average recovery obtained here is similar to that from other work [19].
Short-term stability was evaluated using drug-free plasma spiked with either 2.5 ng/mL or 750 ng/mL subjected to 6 h, 24 h, or 72 h at either 25°C or 4°C as compared to freshly prepared samples (Table 1). Analysis of both 25°C and 4°C stability samples (all time points) recovered acyclovir concentrations that were statistically equivalent to freshly prepared and were less than 8.3 % different from the intended value. Acyclovir quantified after storage at 25°C had a % CV of 7.3 % or less and after storage at 4°C had a % CV of 11.8% or less. Freeze-thaw stability was evaluated by subjecting samples to three freeze-thaw cycles after which recovered acyclovir concentration levels were statistically equivalent to freshly prepared (within 2.8 % of intended value) and had a % CV of 2.3% or less. Stability results were consistent with previous reports [25].
| Plasma storage Condition | Storage Time (hour) | % of intended value ± % CV | |
|---|---|---|---|
| 2.5 (ng/mL) | 750 (ng/mL) | ||
| --- | 0 | 100.0 ± 7.3 | 100.0 ±1.9 |
| 25°C | 6 | 108.3 ± 0.8 | 100.9 ± 3.7 |
| 24 | 102.9 ± 4.9 | 97.7 ± 1.3 | |
| 72 | 102.8 ± 2.3 | 99.2 ± 3.1 | |
| 6 | 104.3 ± 11.8 | 97.4 ± 1.5 | |
| 4°C | 24 | 101.4 ± 2.5 | 98.3 ± 1.6 |
| 72 | 104.2 ± 0.2 | 100.6 ± 0.8 | |
| Freeze and Thaw 3x | --- | 102.8 ± 2.3 | 97.9 ± 1.1 |
|N = 3 for all sample storage conditions, except time = 0 where N = 6
Table 1: Acyclovir Stability in Plasma.
Mass Spectrometry
Tandem mass spectrometry (MS/MS) was used to detect acyclovir in human plasma using SRM. The full scan mass spectrum showed a molecular ion of m/z 226.1 [M+H]+. The product ion (m/z 152.0), a characteristic fragment produced by collision of the precursor ion with Ar gas, was selected for combination of greatest intensity and lowest background. Thus, the m/z 226.1 → m/z 152.0 transition was determined to be optimum in positive ion mode for acyclovir. The m/z 245.1 → m/z 113.0 transition was determined to be optimum for ribavirin. Monitoring a precursor-to-product ion mass transition in MS/MS detection requires that an analyte meet two m/z requirements which can reduce background signal from complex matrices by 100- 1000-fold [39].
Chromatography
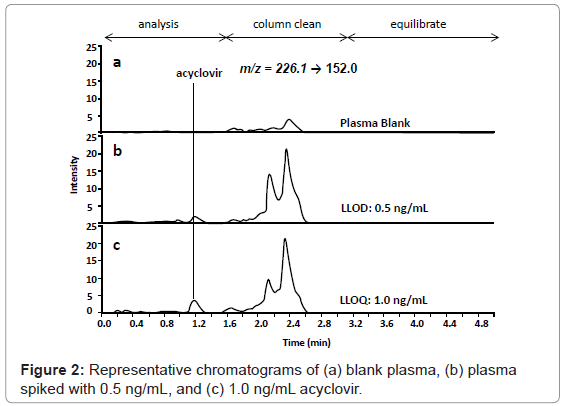
Chromatography conditions were optimized to reduce run time and maximize resolution. The presence of 2 mM ammonium acetate and 0.1 % formic acid in the mobile phase improved peak shape and increased intensity of the observed acyclovir signal. Acyclovir eluted at a retention time of 1.2 min (Figure 2). Ribavirin (IS) eluted at a retention time of 0.6 min (data not shown). The total chromatographic run time is 5 min, including analysis, column cleaning and column equilibration, which is amenable to the high-throughput requirements of clinical study analyses. Previous literature methods that report comparable analysis run times (3 to 8 minutes) cited 10- to 250-fold higher LLOQs (10 ng/mL to 200 ng/mL) [25-28]. Previous LC-MS/MS methodology that reports a comparable LLOQ (2 ng/mL) had a 4-fold longer analysis run time (20 min) [24]. A 4-fold reduction in analysis run time is substantial over the course of an entire study. For example, our study consisted of ~1250 patient plasma samples, which analyzed with replicates, calibration curve standards, and QC samples produces ~3500 sample runs. With a 5 min run time, the entire study could be accomplished in just over 12 days. A 20 min run time would require 48 days of instrument time - over 5 full weeks more than a 5 min analysis run time. Throughput could be further improved by incorporating a multiplexed pump system with column switching between two analytical columns which could bring the analysis run time to < 2.5 min and reduce the total instrument usage time for the entire study to ~6 days [40,41].
Specificity
Isobaric interferences are a well documented source of inaccuracy in LC-MS/MS analyses with chromatographic separation cited as the most effective way to combat non-specific signal [42,43]. No signal was observed in the blank plasma during the time window of acyclovir elution (0.5-1.4 min) (Figure 2). Acyclovir preparations in plasma matrix provided the same response as acyclovir standard solutions indicating that matrix effects did not interfere with acyclovir MS/MS detection (data not shown). No interfering signal and/or matrix effects were observed for the internal standard, ribavirin. Isobaric interferences from the plasma matrix can be observed during the column cleaning phase of the chromatographic run but are well separated from acyclovir during the analysis phase and are absent during the equilibration phase (Figure 2). Additionally, these isobaric interferences are only apparent at very low concentrations (< 20 ng/mL) and do not contribute significantly to overall signal at most of the therapeutic levels of acyclovir measured over the course of the PK profiles.
Sensitivity
The lower limit of detection (LLOD), defined as the concentration of acyclovir in plasma that can be detected with a signal-to-noise ratio of 3, was determined to be 0.5 ng/mL (Figure 2B). The lower limit of quantification (LLOQ), defined as the concentration of acyclovir in plasma that can be detected with a signal-to-noise ratio of 10 and the lowest concentration on the standard curve and that can be measured with acceptable accuracy and precision (within 20%), was determined to be 1.0 ng/mL (Figure 2C). This method is the most sensitive, to our knowledge, with a LLOQ that is between 2-fold and 250-fold better than other assays [14-28]. Typical LLOQs varied for previous acyclovir assays with the most sensitivity reported for MS/MS detection: MS/ MS detection, 2 ng/mL to 200 ng/mL [24-28]; UV detection, 10 ng/ mL to 250 ng/mL [14-18]; and fluorescence detection, 3.25 ng/mL to 30 ng/mL [19-22]. This methods 1 ng/mL LLOQ is important to be able to detect and accurately quantify acyclovir concentrations at all of the study timepoints at the given dose and study conditions: patient plasma at the longest time points frequently contained < 10 ng/mL acyclovir.
Linearity
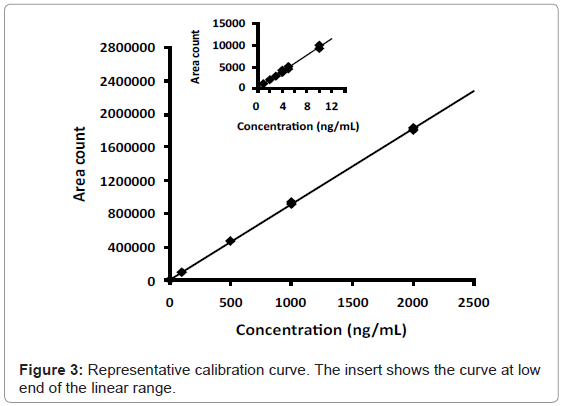
The method was validated using a ten-point calibration curve ranging from 1.0 ng/mL to 2000 ng/mL acyclovir. A representative calibration curve showing linear least squares regression analysis with 1/x2 weighting exhibits linearity over greater than three orders of magnitude (Figure 3). The correlation coefficient of weighted calibration curves was routinely greater than r2 = 0.99. The equation for the best fit to the data in Figure 3 is y = 937x + 244; r2 = 0.9976.
Reproducibility
Precision and accuracy were assessed using quality control samples (Table 2). Intra- and inter-day variation were assessed by assaying seven replicate samples of acyclovir in plasma at four different concentration levels on three separate days. Intra-day precision, expressed as % CV, was between 0.7 % and 10.3 %. Inter-day precision ranged between 2.9 % and 7.9 % CV. Both intra- and inter-day accuracy (for all concentrations) was less than 13% different from the intended value which is within the FDA guidelines of ≤ 15% [37]. Representative chromatograms for quality control samples (acyclovir spiked into drugfree plasma) are shown in Figure 4.
| 2.5 ng/mL (LQC) | 20 ng/mL (LQC) | 250 ng/mL (MQC) | 750 ng/mL (HQC) | |
|---|---|---|---|---|
| 112.7 | 98.1 | 98.6 | 97.7 | |
| 105.8 | 97.4 | 98.3 | 97.6 | |
| Day1 | 91.2 | 95.6 | 98.2 | 98.2 |
| 89.1 | 93.2 | 98.9 | 98.9 | |
| 92.4 | 94.1 | 98.0 | 95.2 | |
| 90.1 | 94.8 | 100.0 | 98.6 | |
| 86.4 | 95.5 | 98.1 | 99.2 | |
| intra-day 1 % intended value ± % CV | 95.4 ± 10.3 | 95.5 ± 1.8 | 98.6 ± 0.7 | 97.9 ± 1.4 |
| 105.4 | 109.6 | 103.5 | 105.6 | |
| 94.6 | 106.3 | 104.4 | 99.8 | |
| Day 2 | 103.2 | 110.9 | 102.4 | 96.4 |
| 97.3 | 104.6 | 106.9 | 99.8 | |
| 94.6 | 107.8 | 103.8 | 104.2 | |
| 99.1 | 109.9 | 104.8 | 104.2 | |
| 97.6 | 110.5 | 100.2 | 96.3 | |
| intra-day 2 % intended value ± % CV | 98.8 ± 4.2 | 108.5 ± 2.2 | 103.7 ± 2.0 | 100.9 ± 3.8 |
| 88.3 | 104.4 | 97.9 | 102.3 | |
| 91.6 | 101.6 | 93.9 | 98.6 | |
| Day3 | 107.5 | 105.3 | 105.2 | 104.5 |
| 97.8 | 106.2 | 104.5 | 99.4 | |
| 104.8 | 107.4 | 101.9 | 101.4 | |
| 102.8 | 106.0 | 105.5 | 102.2 | |
| 110.8 | 107.3 | 108.5 | 101.6 | |
| intra-day 3 % intended value ± % CV | 100.5 ± 8.3 | 105.5 ± 1.9 | 102.5 ± 4.9 | 101.4 ± 1.9 |
| inter-day % intended value ± % CV | 98.2 ±7.9 | 103.2 ± 5.8 | 101.6 ± 3.7 | 100.0 ± 2.9 |
Table 2: Precision and accuracy for determination of acyclovir in plasma samples
Clinical application
The simple sample preparation and rapid analysis time were designed to make this UHPLC-HESI-MS/MS method applicable to clinical studies. A typical clinical data collection consists of calibration standards, QC samples, unknown samples, and another set of QC samples. Acceptance criteria for data collection were established as follows: calibration curve coefficient of correlation (r2) of no less than 0.98; intra-day and inter-day precision and accuracy for sample concentrations above the LLOQ that are no more than 15% variable; accuracy and precision at LLOQ that are no more than 20% variable.
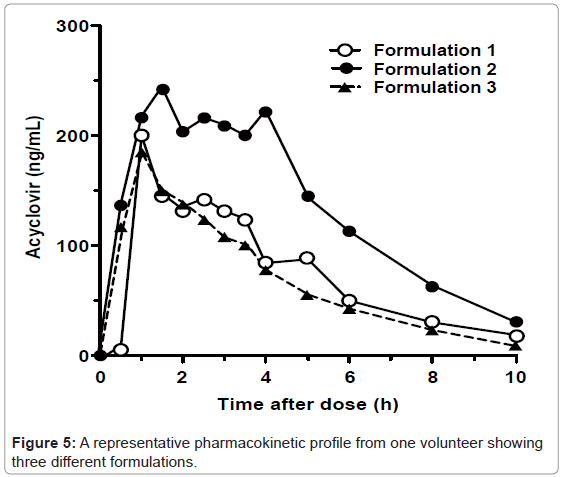
We applied the described method to evaluate the effect of excipients on BCS class 3 drug absorption. Over 1,000 plasma samples obtained from 24 volunteers have been analyzed successfully. A representative acyclovir concentration-time profile obtained from one volunteer for three different formulations is shown (Figure 5). The peak plasma concentration (Cmax) of acyclovir for the three drug formulations are between 185.5 ng/mL and 242.6 ng/mL, and the time to reach the maximum observed concentration of drug (Tmax) is between 1.0 and 1.5 hours. The data obtained using this assay will be used to evaluate the effects of common excipients on the bioequivalence of various formulations (manuscript in preparation).
The combination of reduced analysis run time and increased sensitivity are important features of this assay and key to its clinical performance. In comparison, Yadav et al. reported a shorter method with a 3 min run time, however the LLOQ was 47.6 ng/mL for acyclovir [25]. This almost 50-fold less sensitive LLOQ would result in nonquantifiable data for the last three to four time points (5, 6, 8, and 10 h) for most patients in our study. Other short analysis run time methods that have even higher LLOQs (100 ng/mL to 200 ng/mL) would result in substantial portions of the concentration-time profile in our study being unable to be quantified [26,27]. It is highly desirable to be able to quantify acyclovir concentrations at all the study time points in order to collect complete concentration-time curves for each patient/ formulation for PK analysis. Maes et al. [24] reported a method with sensitivity only 2-fold more than we report that would be able to quantify all points in our clinical study; however, the analysis run time was 20 min. As discussed, a four-fold greater analysis time would substantially increase instrument time and, thus, total analysis costs.
Conclusion
The UHPLC-HESI-MS/MS method described here was validated according to FDA guidelines for drug bioanalysis [37]. The method can detect acyclovir concentrations as low as 0.5 ng/mL, has a working range from 1.0 ng/mL to 750 ng/mL, and demonstrates acceptable accuracy and precision for acyclovir concentrations occurring in human plasma during a bioequivalence study. Using the current method, over 1,000 samples were analyzed efficiently, and the bioequivalence metric Cmax and AUC were determined for each formulation for each volunteer in the bioequivalence study. Incorporation of current advances in analytical methodology, including UHPLC and HESI, in combination with MS/MS detection, provides a validated, sensitive, and rapid method for determining acyclovir in human plasma that is appropriate for clinical applications. The combination of reduced analysis time and lower LLOQ allows for the determination of acyclovir in plasma at all time points up to 10 h after oral administration of 200 mg acyclovir in combination with common excipients. Accurate quantification of drug moieties, as is possible with LC-MS/MS, will aid in clarifying the influence of formulation composition on bioequivalence.
Acknowledgements
This study was supported by contract HHSF223200810041C from the Food and Drug Administration (FDA).
References
- Posner TN (1998) Herpes Simplex. Routledge, New York.
- O'Brien JJ, Campoli-Richards DM (1989) Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 37: 233-309.
- Gonik B (1994) Viral diseases in Pregnancy. Springer-Verlag, New York.
- Arnal J, Gonzalez-Alvarez I, Bermejo M, Amidon GL, Junginger HE, et al. (2008) Biowaiver monographs for immediate release solid oral dosage forms: aciclovir. J Pharm Sci 97: 5061-5073.
- Polli JE, Abrahamsson BS, Yu LX, Amidon GL, Baldoni JM, et al. (2008) Summary workshop report: bioequivalence, biopharmaceutics classification system, and beyond. AAPS J 10: 373-379.
- Fletcher C, Bean B (1985) Evaluation of oral acyclovir therapy. Drug Intell Clin Pharm 19: 518-524.
- McEvoy G (2008) AHFS Drugs information. American Society of Health System Pharmacists, Atlantic Books, Bethesda, MD.
- Wagstaff AJ, Faulds D, Goa KL (1994) Aciclovir. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 47: 153-205.
- Bangaru RA, Bansal YK, Rao AR, Gandhi TP (2000) Rapid, simple and sensitive high-performance liquid chromatographic method for detection and determination of acyclovir in human plasma and its use in bioavailability studies. J Chromatogr B Biomed Sci Appl 739: 231-237.
- Amini H, Javan M, Gazerani P, Ghaffari A, Ahmadiani A (2008) Lack of bioequivalence between two aciclovir tablets in healthy subjects. Clin Drug Investig. 28: 47-53.
- Aungst BJ (2000) Intestinal permeation enhancers. J Pharm Sci 89: 429-442.
- Rege BD, Yu LX, Hussain AS, Polli JE (2001) Effect of common excipients on Caco-2 transport of low permeability drugs. J Pharm Sci 90: 1776-1786.
- Tadepalli SM, Quinn RP, Averett DR (1986) A competitive enzyme-linked immunosorbent assay to quantitate acyclovir and BW B759U in human plasma and urine. Antimicrob Agents Chemother 29: 93-98.
- Brown SD, White CA, Chu CK, Bartlett MG (2002) Determination of acyclovir in maternal plasma, amniotic fluid, fetal and placental tissues by high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 772: 327-334.
- Fernández M, Sepúlveda J, Aránguiz T, von Plessing C (2003) Technique validation by liquid chromatography for the determination of acyclovir in plasma. J Chromatogr B Analyt Technol Biomed Life Sci 791: 357-363.
- Stagni G, Ali ME, Weng D (2004) Pharmacokinetics of acyclovir in rabbit skin after i.v.-bolus, ointment, and iontophoretic administrations. Int J Pharm 274: 201-211.
- Bahrami G, Mirzaeei Sh, Kiani A (2005) Determination of acyclovir in human serum by high-performance liquid chromatography using liquid-liquid extraction and its application in pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci 816: 327-331.
- Hasan S, Chander P, Ali J, Baboota S, Ali M (2011) A new validated ultra performance liquid chromatographic method for determination of acyclovir. Drug Test Anal 3: 187-190.
- Mascher H, Kikuta C, Metz R, Vergin H (1992) New, high-sensitivity high-performance liquid chromatographic method for the determination of acyclovir in human plasma, using fluorometric detection. J Chromatogr 583: 122-127.
- Peh KK, Yuen KH (1997) Simple high-performance liquid chromatographic method for the determination of acyclovir in human plasma using fluorescence detection. J Chromatogr B Biomed Sci Appl 693: 241-244.
- Svensson JO, Barkholt L, Säwe J (1997) Determination of acyclovir and its metabolite 9-carboxymethoxymethylguanine in serum and urine using solid-phase extraction and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 690: 363-366.
- Dao YJ, Jiao Z, Zhong MK (2008) Simultaneous determination of aciclovir, ganciclovir, and penciclovir in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 867: 270-276.
- Brown SD, White CA, Bartlett MG (2002) Hydrophilic interaction liquid chromatography/electrospray mass spectrometry determination of acyclovir in pregnant rat plasma and tissues. Rapid Commun Mass Spectrom 16: 1871-1876.
- Maes A, Garré B, Desmet N, van der Meulen K, Nauwynck H, et al. (2009) Determination of acyclovir in horse plasma and body fluids by high-performance liquid chromatography combined with fluorescence detection and heated electrospray ionization tandem mass spectrometry. Biomed Chromatogr 23: 132-140.
- Yadav M, Upadhyay V, Singhal P, Goswami S, Shrivastav PS (2009) Stability evaluation and sensitive determination of antiviral drug, valacyclovir and its metabolite acyclovir in human plasma by a rapid liquid chromatography-tandem mass spectrometry method. J Chromatogr B Analyt Technol Biomed Life Sci 877: 680-688.
- Sasanya JJ, Abd-Alla AM, Parker AG, Cannavan A (2010) Analysis of the antiviral drugs acyclovir and valacyclovir-hydrochloride in tsetse flies (Glossina pallidipes) using LC-MSMS. J Chromatogr B Analyt Technol Biomed Life Sci 878: 2384-2390.
- Cohen-Wolkowiez M, White NR, Bridges A, Benjamin DK Jr, Kashuba AD (2011) Development of a liquid chromatography-tandem mass spectrometry assay of six antimicrobials in plasma for pharmacokinetic studies in premature infants. J Chromatogr B Analyt Technol Biomed Life Sci 879: 3497-3506.
- Chan D, Tarbin J, Sharman M, Carson M, Smith M, et al. (2011) Screening method for the analysis of antiviral drugs in poultry tissues using zwitterionic hydrophilic interaction liquid chromatography/tandem mass spectrometry. Anal Chim Acta 700: 194-200.
- MacNair JE, Lewis KC, Jorgenson JW (1997) Ultrahigh-pressure reversed-phase liquid chromatography in packed capillary columns. Anal Chem 69: 983-989.
- Chesnut SM, Salisbury JJ (2007) The role of UHPLC in pharmaceutical development. J Sep Sci 30: 1183-1190.
- Guillarme D, Ruta J, Rudaz S, Veuthey JL (2010) New trends in fast and high-resolution liquid chromatography: a critical comparison of existing approaches. Anal Bioanal Chem 397: 1069-1082.
- Churchwell MI, Twaddle NC, Meeker LR, Doerge DR (2005) Improving LC-MS sensitivity through increases in chromatographic performance: comparisons of UPLC-ES/MS/MS to HPLC-ES/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 825: 134-143.
- Goodlett DR, Yi EC, Mottay P (2007) Preparation and Use of an Integrated Microcapillary HPLC Column and ESI Device for Proteomic Analysis. CSH Protoc.
- Kuprowski MC, Konermann L (2007) Signal response of coexisting protein conformers in electrospray mass spectrometry. Anal Chem 79: 2499-2506.
- Boysen G, Collins LB, Liao S, Luke AM, Pachkowski BF, et al. (2010) Analysis of 8-oxo-7,8-dihydro-2'-deoxyguanosine by ultra high pressure liquid chromatography-heat assisted electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 878: 375-380.
- Van Eeckhaut A, Lanckmans K, Sarre S, Smolders I, Michotte Y (2009) Validation of bioanalytical LC-MS/MS assays: evaluation of matrix effects. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2198-2207.
- Center for Drug Evaluation and Research, Center for Veterinary Medicine (2001) Guidance for Industry: Bioanalytical Method Validation. Food and Drug Administration.
- Lin CC, Yeh LT, Lau JY (2002) Specific, sensitive and accurate liquid chromatographic-tandem mass spectrometric method for the measurement of ribavirin in rat and monkey plasma. J Chromatogr B Analyt Technol Biomed Life Sci 779: 241-248.
- Kane MA, Folias AE, Wang C, Napoli JL. (2008) Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem 80: 1702-1708.
- Cass RT, Villa JS, Karr DE, Schmidt DE Jr (2001) Rapid bioanalysis of vancomycin in serum and urine by high-performance liquid chromatography tandem mass spectrometry using on-line sample extraction and parallel analytical columns. Rapid Commun Mass Spectrom 15: 406-412.
- Wang H, Hanash SM (2008) Increased throughput and reduced carryover of mass spectrometry-based proteomics using a high-efficiency nonsplit nanoflow parallel dual-column capillary HPLC system. J Proteome Res 7: 2743-2755.
- Sherman J, McKay MJ, Ashman K, Molloy MP (2009) How specific is my SRM?: The issue of precursor and product ion redundancy. Proteomics 9: 1120-1123.
- Vogeser M, Seger C (2010) Pitfalls associated with the use of liquid chromatography-tandem mass spectrometry in the clinical laboratory. Clin Chem 56: 1234-1244.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 19333
- [From(publication date):
August-2012 - Nov 18, 2025] - Breakdown by view type
- HTML page views : 14351
- PDF downloads : 4982