Research Article Open Access
Proteome Analysis of B. subtilis in Response to Calcium
Delfina C. Domníguez1*, Rosana Lopes1, I. Barry Holland2 and Anthony K. Campbell3
1500 W University Ave. Rm 420, College of Health Sciences, El Paso, Texas, The University of Texas at El Paso
2Institut de Génétique et Microbiologie, UMR 8621 Orsay CNRS, Université de Paris-Sud, Orsay
3Welsh School of Pharmacy, Cardiff University, Cardiff, Wales, UK
- *Corresponding Author:
- Delfina C. Domínguez
500 W University Ave. Rm 420
College of Health Sciences, El Paso
Texas, The University of Texas at El Paso
Tel: (915) 747-7238
Fax: (915) 747-7207
Received date: July 01, 2011; Accepted date: August 08, 2011; Published date: Augutst 31, 2011
Citation: Domníguez DC, Lopes R, Holland IB, Campbell AK (2011) Proteome Analysis of B. subtilis in Response to Calcium. J Anal Bioanal Techniques S6:001. doi:10.4172/2155-9872.S6-001
Copyright: © 2011 Domníguez D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
While the role of calcium binding proteins (CaBPs) in cell signaling pathways and homeostasis is well established in eukaryotic cells, the physiological function of CaBPs in prokaryotes is unknown. Although several CaBPs have been identified and sequences predicted in a variety of prokaryotic genomes, biochemical and functional characterization is lacking. We hypothesize that CaBPs play an important role in Ca2+ homeostasis and that Ca2+ ions regulate several processes in bacterial cells. The purpose of this work was to study the effects of Ca2+ in the B. subtilis proteome, to identify CaBPs altered (increased or decreased) by the addition of Ca2+ -chelators (EGTA, BAPTA) or CaCl2, and to examine Ca2+ homeostasis in B. subtilis cells utilizing various analytical techniques. 45Ca-autoradiography and antibody-crossreactivity were used to detect CaBPs. These proteins were identified by LC-MS/MS. Intracellular calcium levels [Ca2+]i were measured using the photoprotein aequorin. Our results show that remarkable global changes in protein abundance occurred in the B. subtilis proteome as a result of CaCl2 or chelator 58 treatments compared to control cells. Six proteins appeared to be modulated by high levels of extracellular Ca2+. These proteins were increased after Ca2+ -chelator treatments and reduced upon Ca2+ addition. Moreover, these proteins bound radioactive 45Ca2+, and showed a shift in molecular weight in the presence of Ca2+ /EGTA. B. subtilis cells thightly regulate cytosolic Ca2+ levels. Taken together, these results suggest an important role of Ca2+ ions in B. subtilis.
Introduction
The importance of calcium (Ca2+) as a cell regulator is well established in eukaryotes [1-3]. Cells respond to various stimuli by transient changes in intracellular free Ca2+ concentration. Nevertheless, prolonged high intracellular Ca2+ levels may be toxic and/or cause cell damage [3]. Calcium homeostasis is maintained by efflux and influx transport systems, calcium binding proteins (CaBPs) that act as reservoirs or buffers, and more specialized CaBPs such as calmodulin, which act as signal transducers activating Ca2+ phosphorylation cascades leading to gene expression, and control of Ca2+ channel activity. These proteins regulate a wide variety of biological processes including cell differentiation, movement, the cell cycle, transport mechanisms, and gene expression [5-8].
While the role of Ca2+ in eukaryotes has been extensively studied, the role of Ca2+ in prokaryotes still remains elusive. Indirect evidence suggests that Ca2+ may play a role in various bacterial physiological processes such as spore formation, chemotaxis, heterocyst differentiation, transport and virulence [9-12]. Several reports have shown that bacteria are capable of maintaining intracellular Ca2+ homeostasis, and Ca2+ transients are produced in response to adaptation to nitrogen starvation, environmental stress [13-16], and metabolites of carbohydrate metabolism [17,18]. These findings suggest a regulatory role for Ca2+ in bacteria. Moreover, a recent transcriptase analyses in E. coli and (Domínguez et al., unpublished results) showed that the expression of >100 genes are regulated by changes in the [Ca2+]i.
Gangola & Rosen proposed that the level of bound intracellular Ca2+ in E. coli was 100 to 1000-fold higher than that of free Ca2+ and more recent studies showed that the level of free cytosolic Ca2+ in various bacteria is very low (100-300 nM), similar to that found in eukaryotic cells [14,15]. Therefore, such a system requires tight control of the intracellular Ca2+ which suggests the involvement of not only transporters but perhaps CaBPs or other proteins participating in the adaptive response.
The existence of bacterial CaBPs has been documented in several genera of bacteria [22,24] and sequence analyses of various prokaryotic genomes indicate the presence of EF-hand motifs proteins as well as other Ca2+ -binding motifs. [10,25-29]. However, none of the proteins or corresponding genes has been fully characterized. While the genes of some CaBP’s have been cloned and partial characterization has been done, the functional activity remains to be investigated [30-32].
The aim of the present work was to identify proteins responding to elevated or reduced amounts of extracellular Ca2+ and to examine Ca2+ homestasis in B. subtilis cells. A variety of techniques were employed including: 45Ca autoradiography, Western blotting, antibody crossreactivity, measurements of intracellular calcium, two-dimensional electrophoresis and LC-MS/MS. We anticipated that proteins reduced in the presence of high concentrations of extracellular Ca2+ could be functioning as scavengers or participating in import mechanisms. Conversely, proteins reduced by the action of EGTA/BAPTA (lower extracellular Ca2+) could be acting as a buffer or as a sink or may be involved in Ca2+ export mechanisms.
We show for the first time in B. subtilis that major global changes in protein abundance occurred as a result of Ca2+ or chelator treated cells compared to control cells. Levels of cytosolic Ca2+ appear to correlate with the changes in protein abundance according to treatment. We identified six CaBPs whose synthesis appears to be modulated by Ca2+ levels. These proteins identified by LC-MS/MS, bound radioactive 45Ca2+ and showed a shift in molecular weight in the presence of Ca2+ or EGTA. All these results are consistent with an important role of Ca2+ in B. subtilis.
Materials and Methods
Bacterial strain, growth conditions and treatments
Bacillus subtilis 168 ATCC 23857 was used in all experiments and was grown in LB medium at 35oC (250 rpm). When cells reached mid-logarithmic growth (OD600 0.4-0.5) different concentrations of CaCl2, 116 EGTA or BAPTA were added to culture media. Final concentrations for Ca2+ chelators were as follows: BAPTA (0.25 mM and 0.5 mM) and EGTA (0.5 mM and 1mM). Calcium chloride was added to culture media at a final concentration of 5 mM and 10 mM. Bacterial cells were grown for additional 10 min.
After CaCl2 or chelator treatment, bacterial cells were harvested by centrifugation at 3000 xg for 10 min. Cell pellets were stored at -70°C or used immediately for lysis procedures.
Protein extraction preparation
Bacterial cell suspensions were treated with lysozyme (5 mg/ml) and incubated at 4°C for 30 min before cell lysis. Bacterial cell-pellets were resuspended in lysis buffer containing 7 M urea, 20 mM DTT, 1mM 127 CaCl2 and protein inhibitor cocktail (Sigma-Aldrich, St. Louis MO.). Cell suspensions were lysed by sonication, according to the methods of Herbert et al. [31]. After sonication the cell lysate was centrifuged at 20,000 xg for 10 min at 4°C. The protein in supernatant was precipitated by the addition of 4 volumes of acetone. The precipitated proteins were recovered by centrifugation at 5,000 xg for 10 min at 4°C. The proteins were resuspended in lysis buffer containing 7 M urea, 20 mM dithiothreitol (DTT), 20 mM Tris-HCl pH 7.5, and protease inhibitor cocktail. The Bicinchoninic acid protein assay kit (Sigma-Aldrich) was used to measure the protein concentration.
2D-PAGE separation of proteins
The Bio-Rad Criterion™ system was utilized for two dimensional separations of proteins. The linear immobilized pH gradient (IPG) strips in a range of 3-10 were used for protein separation by isoelectric focusing in the first dimension. First-dimension gels were loaded onto 10-20% 11cm polyacrylamide gel electrophoresis (PAGE) for second-dimension separation after equilibration. The sample was diluted to a total volume of 200 μl in rehydration buffer (5 M urea, 2 M thiourea, 2 mM TBP, 2% CHAPS, 2% SB 3- 10, 40 mM Tris, 0.0002% bromophenol blue and 0.2% ampholytes 3/10). Each sample was run in quadruplicate, and 300g of protein sample was added to each gel, along with 2-D gel electrophoresis standards. All gels were stained with Silver Stain Plus (Bio-Rad, Hercules, and CA). The gels were scanned in the FX imager and the resulting differential protein maps analyzed using the 2-D gel analysis software PDQuest, version 7.2 (Bio-Rad). A standard master gel image was selected and all other images were compared to this in order to construct a match set. Relative molecular mass (M) and isoelectric point (pI) values were automatically calculated according to pI of protein standards for the remaining protein spots. The PDQuest software removes the background and assigns signal intensity value to each protein spot detected without altering the original data. Normalization of data was done according to the total density of spots. Replicate groups containing four gels each were formed for each treatment and controls. Altered protein spots showing a two-fold or greater increase or decrease in intensity between treatments and controls, were recorded. Statistical analysis of protein maps was performed utilizing Mann-Whitney Signed Rank test with 90% confidence interval comparing the synthesis of proteins in the controls and treated cultures. Calcium autoradiography and antibody crossreactivity by Western blotting were performed to detect CaBPs.
45CaCl2 autoradiography
Calcium autoradiography (45CaCl2) was done following the methods of [32]. Briefly, after gel electrophoresis, proteins were transferred to a nitrocellulose membrane (350 mA for 1 h at 4°C) and washed several times in 60 mM KCl, 5 mM MgCl2 and 10 mM imidazole pH 6.8. The low pH in the buffer and the presence of Mg2+ ions reduces non-specific binding. The membrane then was soaked in 20 ml of the same buffer containing 50 μCi 45CaCl2 for 10 min. The membrane was washed and blotted to dry. The membrane was exposed to Kodak Xar-5 X-ray film for 20 h.
Western blot assays
For immunoblotting, proteins were transferred to PVDF membranes at 350 mA for 1 h at 4°C. Membranes were blocked with 5% non-fat milk in Tris-HCl-Saline-Buffer (TBS), 500 mM NaCl, 20 mM Tris-HCL pH 7.4. Two primary antibodies were used to probe the membranes, monoclonal anti-calmodulin antibody (C7055, Sigma) 1:500 TBS-milk, and Polyclonal anti-EFD2 1:1000 TBS-milk (ab24368, Abcam, Inc.). Anti-IgG (L+H) antibodies HRP-conjugated were used as secondary antibodies (1:3000 TBS-milk) to develop the color reaction. The Opti-4CN™ substrate kit (Bio-Rad) was used to develop the reaction following manufacturer’s instructions.
Protein spot identification
Protein spots were excised from silver stained gels and analyzed by LC-MS/MS. The LC-MS analysis was performed at the Protein Sciences Facility, School of Chemical Sciences, at the University of Illinois at Urbana using standard procedures.
Intracellular Ca2+ measurement
Construction and Expression of the Aequorin System in Bacillius subtilis 168: The expression vector pDGAEQ containing the apoqequorin coding sequence was used to measure free cytosolic calcium. The apoaequorin coding sequence was verified by PCR and DNA sequencing. The oligonuclotides AQ440LICS 5’-AAGGAGGAAGCAGGTATGGTCAAGCTTACATCAGACTTCGAC- 3’ and AQ440LICAS: 5’-GACACGCACGAGGTTTAGGGGACAGCTCCACCGTAG- 3’ was used for DNA amplification [16]. The PCR reaction was performed under the following conditions: initial denaturing at 95°C for 4 min, 30 cycles of denaturization at 95°C for 30 sec and anneal/extend at 46°C for 30 sec, and final extension at 72°C for 7 min. B. subtilis 168 was then transformed with pDGAEQ using the Groningen method [35]. The pDGAEQ plasmid confers kanamycin resistance to B. subtilis cells. The aequorin gene was induced with 1 mM (final concentration) isopropyl-β-D-thiogalactoside (IPTG) incubating for 2 h at 35°C.
Aequorin reconstitution: The transformed cells were grown in trypto-casein soy broth (TS medium) containing 20 μg/ml kanamycin at 35ºC with strong shaking. Bacteria were harvested by centrifugation (3000 xg, 5 min) and the cell pellets were resuspended in TS medium at 1/10 of the initial culture volume containing 20 μg/ml kanamycin and 2.5 μM coelenterazine (Biotium, Hayward, CA). The sample was incubated for 1h at room temperature in the dark with gentle rocking. Cells were washed with buffer A (10 mM MOPS, 100 200 mM, KCl pH 7.2), containing 0.5 mM EGTA and centrifuged at 3000 xg for 5 min to remove excess coelenterazine. The cell pellet was resuspended in 1 ml of buffer A. At this stage cells were ready to be used for chemiluminescence measurements.
B. subtilis treatments: It should be noted that the terms aequorin and apoaequorin refer to photoprotein with and without its’ prosthetic group, coelenterazine respectively. The ability of B. subtilis to maintain internal Ca Ca2+ levels was studied by adding increasing concentrations of CaCl2 (0.5 mM, 1.0 mM, 5 mM and 15 mM), EGTA (0.5 mM and 1 mM) or LaCl3 (200 μM) to the cultures and incubated at 35°C for 15 min (CaCl2,EGTA) and 30 min (LaCl3). Bacterial cells without any treatment, cells lacking the apoaequorin plasmid, or cells expressing apoaequorin were used as controls. To study protein abundance in relation to cytosolic Ca2+ levels B. subtilis cells were treated with selected Ca2+ and EGTA concentrations for the proteomic analysis (see Materials and Methods in the Bacterial strain, growth conditions and treatments section).
Detection and measurement of chemiluminescence: Chemiluminescence was measured using a luminometer VICTOR3™ (Perkin Elmer) equipped with two dispenser modules and allowing reading from 96-well Microfluor microtiter plates (Dynatec). Briefly, measurements were made in duplicate on 100 μl aequorin-loaded cells resuspended in buffer A. Reagents (1 mM CaCl2 and 4% NP40, 15 mM CaCl2 ) were injected and the kinetics of luminescence change vs. time was recorded. The total reconstituted aequorin was estimated by injecting 50 μl of 4% Nonidet P40 (NP40) and 100 mM CaCl2 at the end of each experiment. In vitro calibration of apoqequorin was done by exposing B. subtilis lysates of apoaequorin-expressing cells to solutions with known concentrations of calcium (Kit #1 and Kit #2, Molecular Probes™, Invitrogen, Carlsbad, CA). To ensure that the luminescence measured was due to intracellular Ca2+ and not to discharged aequorin into the medium from cell lysis, we measured luminescence before and after the addition of Ca2+. Free [Ca2+]i was converted and calculated from relative luminescence unit (RLU) by using the calcium measurement template designed and formatted according to the equation: pCa = 0.612 (-log k) + 3.745 where k denotes the rate constant for decay of chemiluminescence (s-1).
Results
Ca2+ homeostasis in B. subtilis - Cytosolic Ca2+ measurements
Calcium mediated regulation of various cellular processes such as cell division, cell differentiation, and gene expression require a fine tuned control of free cytosolic Ca2+ levels. Therefore, cells possess mechanisms (efflux, influx and CaBPs) to restore and maintain low cytosolic free Ca2+ levels. Based on this concept, our aim was to study cytosolic proteins as a function to cytosolic Ca2+ levels. We used the photoprotein aequorin to monitor intracellular Ca2+ in B. subtilis 168 cells. CaCl2 and the Ca2+ chelators BAPTA and EGTA were used to manipulate Ca2+ levels (see Materials and Methods).
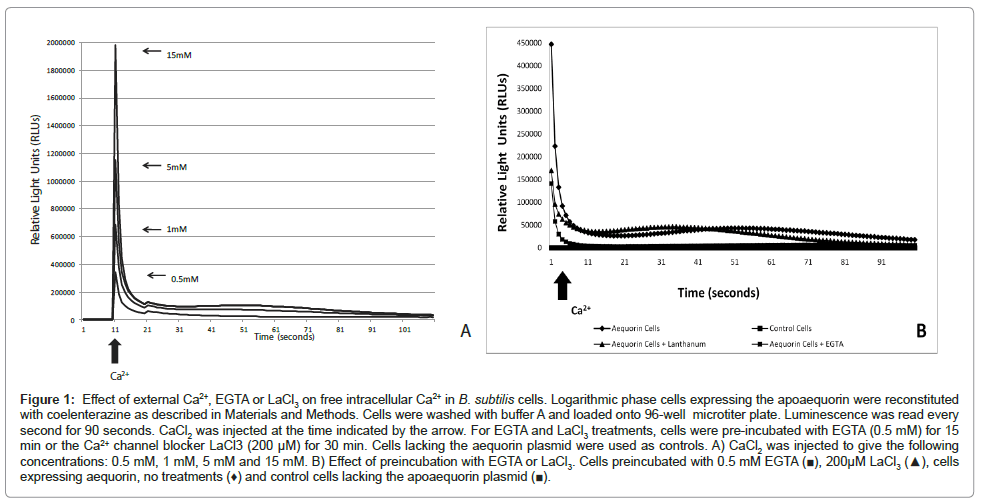
In agreement with previous work reported by [16] our results showed that B. subtilis cells maintain Ca2+ homeostasis. Bacterial cells, loaded with aequorin, resuspended in buffer A showed a luminescence of about 100 relative light units (RLU). When Ca2+ was added to bacterial cells (0.5 mM, 1 mM, 5 mM and 15 mM CaCl2) a transient increase in aequorin luminescence took place followed by a quick decline within seconds. As shown in (Figure 1A), the largest spike was observed when the highest Ca2+ concentration was injected. To confirm that the Ca2+ transients observed were indeed a response to the challenge of external Ca2+ we pre-incubated the bacterial cells with the Ca2+ -chelator EGTA (0.5 mM) and/or the Ca2+ channel blocker LaCl3 (200 μM) (see Materials and Methods). We anticipated that the Ca2+ -chelator EGTA would remove Ca2+ from the extracelluar fluid and therefore will decrease the influx of Ca2+ ions to the cytosol. In B. subtilis, primary and secondary Ca2+ transporters including Ca2+ uptake systems sensitive to channel blockers have been described [21,36,37]. We used LaCl3 to inhibit Ca2+ influx through Ca2+ channels. Treatment with EGTA or LaCl3 resulted in a decrease in luminescence (3-fold and 2.5-fold respectively) compared to cells not treated with the Ca2+ -channel inhibitor. No luminescence was detected in control cells (cells lacking the aequorin plasmid) (Figure 1B).
Figure 1: Effect of external Ca2+, EGTA or LaCl3 on free intracellular Ca2+ in B. subtilis cells. Logarithmic phase cells expressing the apoaequorin were reconstituted with coelenterazine as described in Materials and Methods. Cells were washed with buffer A and loaded onto 96-well microtiter plate. Luminescence was read every second for 90 seconds. CaCl2 was injected at the time indicated by the arrow. For EGTA and LaCl3 treatments, cells were pre-incubated with EGTA (0.5 mM) for 15 min or the Ca2+ channel blocker LaCl3 (200 μM) for 30 min. Cells lacking the aequorin plasmid were used as controls. A) CaCl2 was injected to give the following concentrations: 0.5 mM, 1 mM, 5 mM and 15 mM. B) Effect of preincubation with EGTA or LaCl3. Cells preincubated with 0.5 mM EGTA (â�?ª), 200μM LaCl3 (â�?²), cells expressing aequorin, no treatments (♦) and control cells lacking the apoaequorin plasmid (â�?ª).
These results confirmed that the cellular concentration of free Ca2+ is homeostatically regulated to sub micromolar levels in B. subtilis as previously shown in E. coli. In fact, the results demonstrate that following addition of Ca2+ to the external medium the resulting transient increase in intracellular Ca2+ decays very rapidly to the basal level in B. subtilis suggesting a very tight control.
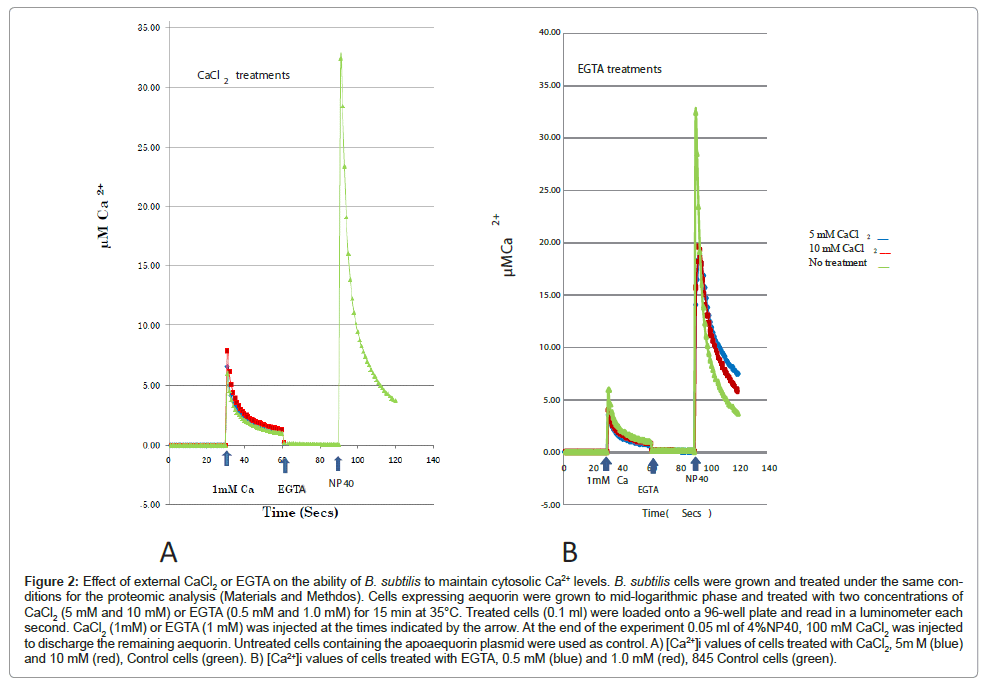
In addition to measuring cytosolic Ca2+ levels we sought to correlate protein synthesis and [Ca2+]i. We grew and treated B. subtilis cells under the same conditions we previously described for our proteomic analysis. CaCl2 treatment (5 mM and 10 mM CaCl2) resulted in an increase in cytosolic Ca2+ levels whereas treatment with EGTA (0.5 mM and 1 mM) decreased [Ca2+]i compared to control cells (no treatment) (Figure 2). These results concur with previous findings, which showed that Ca2+ ions regulate protein synthesis, in E. coli, according to the intracellular Ca2+ levels [19].
Figure 2: Effect of external CaCl2 or EGTA on the ability of B. subtilis to maintain cytosolic Ca2+ levels. B. subtilis cells were grown and treated under the same conditions for the proteomic analysis (Materials and Methdos). Cells expressing aequorin were grown to mid-logarithmic phase and treated with two concentrations of CaCl2 (5 mM and 10 mM) or EGTA (0.5 mM and 1.0 mM) for 15 min at 35°C. Treated cells (0.1 ml) were loaded onto a 96-well plate and read in a luminometer each second. CaCl2 (1mM) or EGTA (1 mM) was injected at the times indicated by the arrow. At the end of the experiment 0.05 ml of 4%NP40, 100 mM CaCl2 was injected to discharge the remaining aequorin. Untreated cells containing the apoaequorin plasmid were used as control. A) [Ca2+]i values of cells treated with CaCl2, 5m M (blue) and 10 mM (red), Control cells (green). B) [Ca2+]i values of cells treated with EGTA, 0.5 mM (blue) and 1.0 mM (red), 845 Control cells (green).
Effect of EGTA and BAPTA treatments on protein abundance
In order to identify potential CaBPs or other proteins involved in a possible adaptive response to changes in Ca2+ levels to maintain Ca2+ homeostasis in B. subtilis, a proteomic analysis was performed. We anticipated that the Ca2+ chelators and addition of high concentration of CaCl2 to the culture media will perturb cytosolic Ca2+ levels with cells responding by expressing genes encoding CaBPs, or other proteins, to restore free Ca2+ levels. Therefore, protein synthesis was analyzed after bacterial cells were grown in the presence of 5 mM and 10 mM CaCl2, or in the presence of Ca2+ -chelators (1mM and 5 mM EGTA; 0.25 mM, and 0.5 mM BAPTA). Identified differentially synthesized proteins, were categorized arbitrarily into 4 classes: Class I proteins whose synthesis was induced by EGTA/BAPTA and reduced by CaCl2; Class II proteins, induced by EGTA/BAPTA; Class III proteins reduced by EGTA/BAPTA, and Class IV proteins, synthesis reduced by CaCl2.
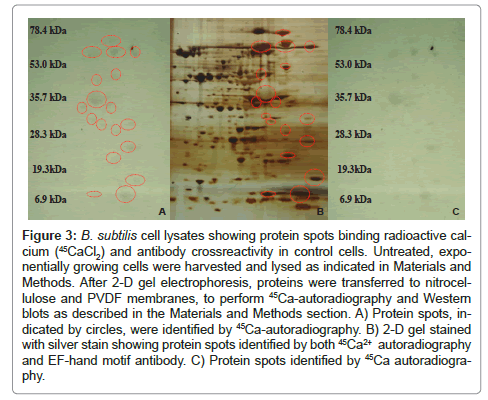
Bacterial growth, EGTA/BAPTA treatments and cell lysates were prepared as described in Materials and Methods. Proteins spots demonstrating changes (induced or decreased) after Ca2+ chelator treatment were further screened by 45Ca2+ -autoradiography and antibody crossreactivity. A control gel is shown in Figure 3. To prevent non-specific calcium binding the nitrocellulose membrane was washed in a low pH buffer containing 5mM MgCl2. The higher H+ concentration and the Mg2+ will compete for the binding of calcium thus increasing specificity. Proteins showing a radioactive signal or crossreactivity with antibodies were selected and identified by LC-MS/ MS. Statistical analysis was performed using the Mann-Whitney test.
Figure 3: B. subtilis cell lysates showing protein spots binding radioactive calcium (45CaCl2) and antibody crossreactivity in control cells. Untreated, exponentially growing cells were harvested and lysed as indicated in Materials and Methods. After 2-D gel electrophoresis, proteins were transferred to nitrocellulose and PVDF membranes, to perform 45Ca-autoradiography and Western blots as described in the Materials and Methods section. A) Protein spots, indicated by circles, were identified by 45Ca-autoradiography. B) 2-D gel stained with silver stain showing protein spots identified by both 45Ca2+ autoradiography and EF-hand motif antibody. C) Protein spots identified by 45Ca autoradiography.
Protein analysis revealed that Ca2+ ions have a significant effect in the B. subtilis proteome. A total of (141 and 128) protein spots were induced in B. subtilis cells when treated with two different concentrations of BAPTA (0.25 mM, 0.5 mM) and EGTA (0.5 mM, 1 mM) respectively, as compared to untreated cells. A total of protein spots (171 BAPTA and 221 EGTA) in comparison to controls were decreased when treated with the same two chelator concentrations. A total of 284 protein spots were statistically significant when compared to controls. Of these, proteins showed changes in response to BAPTA whereas 75 proteins changed when cells were treated with EGTA. A total of 33 proteins showed a similar induction response when cells were treated with BAPTA, while 63 protein spots showed a similar response in both EGTA concentrations.
Protein spots demonstrating at least two-fold change (increased or decreased) after Ca2+ chelator treatments were further analyzed by 45Ca autoradiography. Twenty proteins bound radioactive calcium. Three of these protein spots, the heat shock protein GrpE, acyl-carrier protein, and an unidentified protein, crossreacted with polyclonal antibodies to an EF-hand motif, the C-terminal amino acids 230-240 of human EFHD2 (Figure 4). One protein, flagellin, crossreacted with monoclonal anti-calmodulin antibodies. These later antibodies are directed against the fourth EF-hand domain of Dictyostelium calmodulin (MabC- 7055). Seventeen of the twenty proteins binding 45CaCl2 were identified by LC-MS/MS. Of these, 2 were identified as possible membrane proteins (transporters), 2 are extracellular proteins, and 13 are cytosolic proteins including 7 enzymes involved in intermediary metabolism; 2transcription factors, 1 heat-shock protein, 2 proteins involved in translation and a hypothetical protein.
Figure 4: Western blot analysis of differentially expressed proteins after EGTA treatment. B. subtilis cell lysate proteins were separated by 2-DE and blotted to PVDF as described in Materials and Methods. A) Protein spots crossreacting with the EF-hand antibody. B) 2-D gel stained with silver stain. Red circles indicate the proteins recognized by the antibody.
Protein abundance profile after CaCl2 treatments
Considering the toxic effects of prolonged cytosolic Ca2+ levels in cells we examined the possibility that high concentrations of extracellular Ca2+ will upregulate genes encoding efflux transport systems, or CaBPs acting as Ca2+ sinks or for storage of Ca2+.
Calcium chloride was added to 5 mM and 10 mM final concentration. Cultures without CaCl2were used as controls. Protein spots exhibiting changes were further analyzed by antibody crossreactivity and 45Caautoradiography. Protein spots showing a radioactive signal were selected and identified by LC-MS/MS. A total of proteins showed 2-fold changes after Ca2+ treatment as compared to controls. Proteomics analysis revealed that 127 (52 and 75) protein spots were increased when cells treated with 5mM CaCl2 and 10 mM CaCl2 respectively. A total of 160 (81 and 79) protein spots were reduced after treatment with the same CaCl2 concentrations. Twenty seven protein spots showed a similar induction response with both CaCl2 concentrations, whereas 25 were decreased. Excised protein spots showed a molecular weight between 19.9 to 74.75 and an isolectric point (pI) 6.6-8.0. The differential protein profiles after CaCl2 treatments are shown in Table 1.
| PDQUEST QUANTITATIVE ANALYSIS | ||
| CaCl2 concentrations | 5mM CaCl2 | 10mMCaCl2 |
| Total number of protein spots increased* | 52 | 75 |
| Total number of protein spots decreased* | 81 | 79 |
| PDQUEST QUALITATIVE ANALYSIS | ||
| Number of protein spots that showed analogousUp regulation response in both CaCl2 concentrations | 27 | |
| Number of protein spots that showed analogousDown regulation response in both CaCl2 concentrations | 25 | |
*Compared to Control Cells
Table 1: Differential expression of proteins after calcium treatments (5mM CaCl2 and 649 10mM CaCl2).
Twenty proteins were radioactively labeled. Ten of these protein spots were identified by LC-MS/MS and of these 1 was identified as a membrane protein (transporter), 8 were cytosolic proteins including 4 enzymes involved in metabolism, 1 heat shock protein, 1 transcription elongation factor, 1 translation elongation factor, 1 hypothetical protein, and 1 extracellular protein. Table 2 summarizes the CaBPs identified by LC-MS/MS their relevant characteristics and abundance profiles.
| SSP # calcium class Matchset | Fold change | Protein expression changes | Detected by | LC-MS protein identification | Location | Gene name | Protein class |
|---|---|---|---|---|---|---|---|
| 6312 | 2.41 | ↓Cx 10mMCa | 45Ca | Fructose biphos- phate aldolase 99% coverage | Cytosolic | fbaA | Class I |
| 7816 | 2.11 | ↓Cx 10mMCa | 45Ca | Probable Cation Transport ATPase 92%coverage | Transmembrane | copA | Class I |
| 8205 | 8.41 | ↓Cx 5mMCa | 45Ca | Adenylate kinase 99%coverage | Cytosolic | Adk | Class I |
| 6514 | 2.49/ 3.01 | ↓Cx5mMCa ↓Cx10mMCa |
45Ca | Flagellin 99% coverage | Appendage | Hag | Class I |
| 7603 | 4.62 | ↓Cx10mMCa | 45Ca | No ID | - | - | Class IV |
| 7905 | 2.24 | 10mMCa | No ID | - | - | ||
| 8105 | 7.46 | ↓Cx 10mM Ca | 45Ca | Thiol peroxidase 99% coverage | Cytosolic | tpx | Class |
| 8103 | 4.77 | ↓Cx 5mMCa ↓Cx 10mMCa |
45Ca | Transcription elon- gation factor GreA 99% coverage | Cytosolic | greA | Class IV |
| 8202 | 5.61 | ↓Cx 10mMCa | 45Ca EF-Hand | Heat-shock protein 99% coverage | Cytosolic | grpE | Class I |
| 8501 | 2.02 | ↓ Cx 10mMCa | 45Ca | Elongation factor Tu 99% coverage | Cytosolic | tufA | |
| 9102 | 2.50 | ↓Cx 5mMCa | 45Ca | Hypothetical protein BS12280 99% coverage | Cytosolic | rex | Class I |
| 8106 | 2.85 4.34 | ↓Cx 5mMCa ↓Cx 10mMCa |
45Ca EF-Hand | 3-oxoacyl- (acyl- carrier-protein) synthase 97% coverage | Cytosolic | fabF | Class IV |
| 8706 | 2.0 2.10 | ↓Cx 5mMCa ↓Cx 10mMCa |
45Ca EF-Hand | No ID | - | - | - |
Table 2: Identification and characteristics of proteins differentially expressed after 676 CaCl2 treatments.
CaBPs apparently modulated by calcium ions
Six calcium-binding proteins appear to be specifically regulated by Ca2+ ions in B. subtilis. The synthesis of all these proteins was reduced by extracellular Ca2+, but induced by chelator’s treatment (Table 3). In addition to detection by 45Ca2+ overlay technique, some of these proteins crossreacted with anti-CaM or anti-EF-hand antibodies. The Ca2+ -binding proteins identified by LC-MS/MS were identified as follows: a probable cation transport ATPase, flagellin, adenylate kinase, fructosebisphosphate aldolase, heat-shock protein (the adenine exchange GrpE factor of Dnak 70), and the hypothetical protein BSU12280 (uncharacterized protein yjlC). Interestingly, all these proteins showed a molecular weight shift after Ca2+/Ca2+ -chelator treatment. A molecular weight reduction was observed when subjected to Ca2+ and an increase with Ca2+ -chelator treatment (Table 3), a property that is known to be unique of CaBPs.
| SSP # Ca/ Chelator | MW Ca/Chelator *Fold change | Protein expression change Ca | Protein expression change BAPTA/EGTA | Detected by | LC/MS ID | Gene name | Protein class |
|---|---|---|---|---|---|---|---|
| 7816/6807 | 63.6/87.4 * 2.11; 2.41 | ↓10mMCa | ↑Bapta 0.5mM | 45Ca | Probable Cation transport ATPase 92% coverage | copA | Class I |
| 6514/7503 | 42.27/53.51 * 2.49; 4.94 | ↓5mMCa ↓10mMCa | ↑0.25mM Bapta | 45Ca Anti-CaM Ab | Flagellin 99% coverage | hag | Class I |
| 8205/7205 | 28.37/32.01 * 5.69; 8.41 | ↓5mMCa | ↑EGTA 1mM | 45Ca | Adenylate kinase 99% coverage | adk | Class I |
| 6312/6401 | 35.05/47.58 * 2.41; 2.4 | ↓10mMCa | ↑0.25Bapta ↑1mM EGTA | 45Ca | Fructose- biphos- phate aldolase 99% coverage | fbaA | Class I |
| 8202/8501 | 30.19/52.32 *5.69; 5.69 | ↓10mMCa | ↑0.25mM Bapta ↑0.5mM Bapta | 45Ca EF-Hand Ab | Heat-shock protein 99% coverage | grpE | Class I |
| 9102/9103 | 19.99/23.52 *2.5; 2.5 | ↓5mMCa | ↑0.25 Bapta ↑0.5mM Bapta | 45Ca | Hypothetical protein BS12280 99% coverage | rex | Class I |
Table 3: Characteristics of differentially expressed proteins identified by 45Ca- 760 autoradiography, induced by Ca2+- chelators and reduced by high CaCl2 761 concentra-tion.
Discussion
We present here, for the first time, the effect of Ca2+ in the B. subtilis proteome as a function of cytosolic Ca2+. First, we looked at the global changes produced by the Ca2+ chelators BAPTA/EGTA, and 357 high extracellular Ca2+ in B. subtilis protein abundance, and second, we sought to identify among proteins whose level of synthesis appeared to be regulated by intracellular calcium levels.
Our results show that substantial changes in protein abundance occur as a result of Ca2+ / Ca2+ chelator treatments compared to control cells. Comparison of the total numbers of proteins (increased or decreased) at different concentrations of EGTA and BAPTA showed only slight differences between the two chelators. But the total number of statistically significant changes in protein spots analyzed by the Mann-Whitney test was higher in cells treated with BAPTA than with EGTA. This difference could be due to the slower rate of Ca2+ association and dissociation exhibited by EGTA compared to BAPTA or to the greater affinity of BAPTA to bind Ca2+ compared to Mg2+ or perhaps [38,39] to the binding of other ions such as Zn2+ ions by BAPTA as recently reported by Hyrc et al. [40].
Consistent with our data a previous proteomic study on the effects of Ca2+ in Pseudomonas aeruginosa showed that high extracellular Ca2+ (10 mM) elicits major changes in the production of cytosolic proteins and that proteins involved in stress responses such as heat shock proteins, chaperons, and oxidative stress proteins appeared be regulated by Ca2+ [41,42]. However, in this report we used both CaCl2 and Ca2+ -chelators to manipulate external Ca2+ levels, and 45Ca overlay and immunoassays to detect CaBPs. Furthermore, measurement of absolute Ca2+ levels were done to investigate Ca2+ homeostasis and to correlate to protein synthesis.
To identify potential CaBPs or other proteins regulated by [Ca2+]i. Differentially synthesized proteins were further analyzed by 45Ca autoradiography and antibody crossreactivity. Proteins showing a positive signal were selected and identified by LC-MS/MS. Not all proteins selected were identified by LC-MS/MS due to protein quantity. Only those proteins that were grouped arbitrarily into four classes based on their differential synthesis after Ca2+ or Ca2+ chelator treatment. Class I proteins (Table 3) appeared to be specifically regulated by Ca2+. These proteins were induced by both EGTA and BAPTA but reduced by high extracellular Ca2+. Notably, in agreement with our results, the genes encoding adenylate kinase, fructose-bisphosphate aldolase, and the heat-shock protein were also found to be regulated by Ca2+ ions in E. coli [19]. Surprisingly some of these proteins (adenylate kinase and fructose 1,6-biphosphatase aldolase) have been linked to Ca2+ regulation in eukaryotic organisms [43-45]. For example, the action of the membrane-bound adenylate kinase (AK) in rod outer segments of bovine retina that mediates reactions needed for phototransduction is Ca2+ -dependent [44]. AK is a small ubiquitous enzyme involved in energy metabolism and nucleotide synthesis, and it is essential for metabolic maintenance and cell growth. This protein has been found to be involved in channel regulation and as a metabolic sensor [46,48]. Recently, it was shown that ATP regulates Ca2+ efflux and growth in E. coli [19]. It could be possible that this enzyme may be involved in transport and signaling in prokaryotes. The levels of fructose biphosphate aldolase (FBA) were found to be regulated by cytosolic Ca2+ in rice, and to activate a vacuolar H+-ATPase mediating cell elongation [49]. Furthermore, a recent transcriptome analysis showed that the levels of [Ca2+]i regulate the expression of the genes encoding AK and FBA [19].
The GrpE protein is a heat shock protein of the Hsp70 family, which promotes the exchange of ADP for ATP and also augments peptide release from DnaK, and may function as a “thermosensor” [24,50]. It has been reported that DnaK has autophosphorylation activity, which is stimulated by Ca2+ in vitro [24]. It may be significant to point out that DnaK has 60% identity to the 21 residue calmodulin binding site. DnaK is required for chromosome replication in E. coli [24]. Therefore GrpE and DnaK may mediate stress response and growth according to [Ca2+]i levels.
The role of Ca2+ in chemotaxis has been documented in several reports [11,22]. The switch from tumbling to swimming, after the addition of repellants and attractants, is modulated by Ca2+ [11, 50]. However, the molecular events for Ca2+ regulation in bacterial chemotaxis are still unknown. Flagellin has been previously reported as a CaBP [16]. Flagellin is the component of the flagellar filament but since this protein is extracellular it is unlikely to be a target for Ca2+ regulation. Nevertheless, the present study, and other reports indicate that Ca2+ levels affects the production of this protein [42,52].
Finally, the proteins 3-oxoacyl-acylcarrier-protein synthase (ACP), elongation factor-Tu (EF-Tu), and a thiol peroxidase (class IV) were reduced by high extracellular Ca2+. The B. subtilis ACP in addition to binding radioactive 45Ca, crossreacted with anti-EF-hand antibodies. These findings are supported by previous reports, which showed that the E. coli ACP, and more recently Vibrio harvey ACP bind Ca2+ with high affinity [54-56]. However, the role of Ca2+ and ACP has not been analyzed in vivo.
Bacterial EF-Tu has long been suspected of being an actin homologue. It forms filaments in vitro, associates with the membrane and its over-production results in loss of shape in E. coli (V. Norris personal communication). It is interesting to mention that a genomic analysis of EF-hand related sequences in Arabidopsis found several elongation factors as EF-hand containing proteins [57]. In addition, a 2DE proteomic analysis showed that the synthesis of EF-Tu is affected by Ca2+ levels [43].
Seeking to correlate protein synthesis and [Ca2+]i we measured cytosolic Ca2+ levels. While [Ca2+]i levels have been more frequently studied in E. coli and cyanobacteria [13-15,19], limited studies have been done in B. subtilis. Our results indicate that indeed B. subtilis tightly controls cytosolic calcium and we concur with other studies [16] in that intracellular calcium levels regulate the expression of many genes and the synthesis of proteins.
In summary, the data presented provides evidence that Ca2+ ions have a significant effect on the B. subtlis proteome. Thus, changes in the level of cytosolic calcium are accompanied by major changes in gene expression. As evidenced in this report, genes regulated by Ca2+ levels may have an impact in a wide variety of cellular events including growth, stress response, transport, and signal transduction. The present study therefore presents evidence that Ca2+ ions have an important physiological role in bacteria.
Acknowledgements
We are pleased to acknowledge funding from the National Institutes of Health to support these studies (grant #2S066M008012-39). We are grateful to Marie- Claude Kilhoffer for providing the aequorin plasmid. We like to thank Srilaxmi Nerella for performing sequence analysis of proteins (Bioinformatics Program, UTEP) and Nai Guy for helping with the luminescence assays. I.B. Holland is grateful to Université Paris-Sud for support.
References
- Carafoli E (2002) Calcium signaling: A tale for all seasons. Proc Natl acad Sci USA 99: 1115-1122.
- Clapham DE (1995) Calcium signalling. Cell 80: 259-268.
- Campbell AK (1983) Intracellular calcium: Its universal role as regulator. John Wiley and Sons, New York, NY.
- Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signaling: dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol 4: 517-529.
- Ikura M, Osawa M, Ames JB (2002) The role of calcium-binding proteins in the control of transcription: structure to function. BioEssays 24: 625-636.
- Corbett EF, Michalak M (2000) Calcium, a signaling molecule in the endoplasmic reticulum?. Trends Biochem Sci 25: 307-311.
- Sanders D, Brownlee C, Harper JF (1999) Communicating with calcium. The Plant Cell 11: 691-706.
- Poovaiah BW, Reddy ASN (1993) Calcium and signal transduction in plants. Crit Rev Plant Sci 12: 185-211.
- Rosch JW, Sublett JG, Gao Wang YD, Tuomanen EI (2008) Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol Microbiol 70: 435-444.
- Zhao Y, Shi Y, Zhao W, Huang X, Wang D, et al. (2005) CcbP, a calcium-binding protein from Anabaena sp. PCC 7120, provides evidence that 468 calcium ions regulate heterocyst differentiation. Proc Natl Acad Sci 102: 5744-5748.
- Tisa LS, Adler J (1995) Cytoplasmic free Ca2+ level rises with repellents and falls with attractants in Escherichia coli chemotaxis. Proc Natl Acad Sci USA 92: 10777-10781.
- Trombe MC, Rieux V, Baille F (1994) Mutations which alter the kinetics of Calcium transport alter the regulation of Competence in Streptococcus pneumonia. J Bacteriol 475 176: 1992-1996.
- Leganés F, Forchhammer K, Fernández-Pinas F (2009) Role of calcium in acclimation of the cynobacteriumSynechococcuselongatus PCC 7942 to nitrogen starvation. Microbiology 155: 25-34.
- Torrecilla I, Leganès F, Bonilla I, Fernàndez-Piñas F (2000) Use of Recombinant aequorin to study calcium homeostasis and monitor calcium transients in response to heat and cold shock in cyanobacteria. Plant Physiol 123:161-175.
- Jones HE, Holland IB, Baker HL, Campbell AK (1999) Slow changes in cytosolic free Ca2+ in Escherichia coli highlight two putative influx mechanisms in response to changes in extracellular calcium. Cell Calcium 25: 265-274.
- Herbaud ML, Guiseppi A, Denizot F, Haiech J, Kilhoffer MC (1998) Calcium Signalling in Bacillus subtilis. BiochemBiophys Acta 1448: 212-226.
- Campbell AK, Naseem R, Holland IB, Matthews SB, Wann KT (2007) Methylglyoxal and other carbohydrate metabolites induce lanthanum-sensitive Ca2+ 494 transients and inhibit growth in E. coli. Arch BiochemBiophys 468: 107-113.
- Naseem R, Davies SR, Jones H, Wann KT, Holland IB, et al. (2007) Cytosolic Ca2+ regulates protein expression in E. coli through release from inclusion 499 bodies. Biochem. Biophys Res Comm 360: 33-39.
- NasseemR,Wann KT, Holland IB, Campbell AK (2009) ATP regulates calcium efflux and growth in E. coli. J Mol Biol 391: 42-56.
- Gangola P, Rosen BP (1987) Maintenance of intracellular calcium in Escherichia coli. J BiolChem 262: 12570-12574.
- Fujisawa M, Wada Y, Tsuchiya, Ito M (2009) Characterization of Bacillus 549 subtilis YfkE (ChaA): a calcium-specific Ca 2+/H+ antiporter of the CaCA family. Arch Microbiol 191: 649-657.
- Domínguez DC (2004) Calcium signalling in bacteria. Mol Microbiol 54: 291-297.
- Reusch RN, Sadoff HL (1988) Putative structure and functions of a poly-ß-555 hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membrane. Proc Natl Acad Sci USA 85: 4176-4180.
- Norris V, Grant S, Freestone P, Canvin J, Sheikh FN, et al. (1996) Calcium signalling in bacteria. J Bacteriol 178: 3677-3682.
- Aravind P, Mishra A, suman SK, Jobby MK, Sankaranarayanan R, (2009) The ßγ-crystallin superfamily contains a Universal Motif of Binding Calcium. Biochemistry 48: 12180-12190.
- Zhou Y, Yang W, Kirberger M, Lee HW, Ayalasomayajula G, et al. (2006) Prediction of EF-Hand Calcium-Binding Proteins and Analysis of Bacterial EFhand Proteins. Proteins 65: 643-644.
- Rigden, JD, Jedrzejas MJ, Galperin MY (2003) An extracellular calcium-binding do ain in bacteria with a distant relationship to EF-Hands. FEMS Microbiol Lett 221: 103-110.
- Michiels J, Xi C, Verhaert J, Vanderleyden J (2002) The functions of Ca2+ in bacteria: a role for EF-hand proteins? Trends Microbiol 10: 87-93.
- Yang K (2001) Prokaryotic Calmodulins: Recent Developments and Evolutionary Implications. J Mol MicrobiolBiotechnol 3: 457-459.
- Tossavainen H, Permi P, Annila A, Kilpelainen I, Drakenberg T (2003) NMR solution structure of calerythrin, and EF-hand calcium- binding protein from Saccharopolysporaerythraea. J Biochem 270: 2505-2512.
- Bylsma N, Drakenberg T, Andersson I, Leadlay PF, Forsen S (1992) Prokaryotic calcium-binding protein of the calmodulin superfamily calcium binding to a 537 Saccharopolysporaerythraea 20 kDa protein. FEBS Lett 299: 44-47.
- Swan DG, Cortes J, Hale RS, Leadlay PF (1989) Cloning, characterization, and heterologous expression of the Saccharopolysporaerythraea (Streptomyces erythraeus) 541 gene encoding and EF-Hand calcium-binding protein. J Bacteriol 171: 5614-5619.
- Herbert BR, Grinyer J, McCarthy JT, Isaacs M, Harry EJ, et al. (2006) Improved 2-DE of microorganisms after acid extraction. Electrophoresis 27: 1630-1640.
- Maruyama K, Mikawa T, Ebashi S (1984) Detection of calcium binding proteins by Ca autoradiography nitrocellulose membrane after sodium dodecyl sulfate gel electrophoresis. J Biochem 95: 511-519.
- Harwood CR, Cutting SM (1990) Molecular Biological Methods for Bacillus. John Wiley & Sons, New York, NY.
- Matsuchita T, Ueda T, Kusaka I (1986) Purification and characterization of Ca2+/ H+ antiporter from Bacillus subtilis. Eur J Biochem 156: 95-100.
- Matsuchita T, Hirata H, Kusaka I (1989) Calcium channel blockers inhibit chemotaxis. FEBS Lett 236: 437-440.
- Harrison SM, Bers DM (1987) The effect of temperature and ionic strength on the apparent Ca-affinity of EGTA and the analogous Ca-chelators BAPTA and dibromo-563 BAPTA. Biochemica et Biophysica Acta 925: 133-143.
- Tsien R (1980) New Calcium Indicators and Buffers with high selectivity against magnesium and protons: Design, Synthesis and Protrotype Structures. Biochemistry 19: 2396-2404.
- Hyrc KL, Bownik JM, Goldberg MP (2005) Ionic selectivity of low-affinity ratiometric calcium indicators: mag-Fura-2, Fura-2FF and BTC. Cell Calcium 27: 75-86.
- Patrauchan MA, Sarkisova SA, Sauer K, Franklin MJ (2005) Calcium influences cellular and extracellular product formation during biofilm-associated growth of a marine Pseudoalteromonas sp. Microbiology 151: 2885-2897.
- Patrauchan MA, Sarkisova SA, Franklin MJ (2007) Strain-specific proteome responses of Pseudomonas aeruginosa to biofilm-associated growth and to calcium. Microbiology 153: 3838-3851.
- Kim HK, Park WS, Kang SH, Warda M, Kim N, et al. (2007) Mitochondrial alterations in human gastric carcinoma cell line. Am J Physio Cell Physiol 293: 761-771.
- Notari L, Pepe IM, Cugnoli C, Morelli A (2001) Adenylate kinase activity in rod outer segments of bovine retina. BiochemBiophys Acta 1504: 438-443.
- Nakamura H, Satoh W, Hidaka S, Kagaya Y, Ejiri S, et al. (1996) Genomic structure of the rice aldolase isozyme C-1 gene and its regulation through a Ca2+ -mediated protein kinase-phosphatase pathway. J Plant Mol Biol 30: 381- 385.
- Randak CO, Welsh MJ (2005) Adenylate kinase activity in ABC Transporters. J BiolChem 280: 34385-34388.
- Randak CO, Welsh MJ (2007) Role of CFTR’s intrinsic adenylate kinase activity in 595 gating of the Cl –channel. J BioenergBiomembr 39: 473-479.
- Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman, et al. (2001) 600 Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive 601 potassium channels. Proc Natl acad Sci USA 98: 7623-7628.
- Konishi H, Yamane H, Maeshima M, Komatsu S (2004) Characterization of fructose-604 bisphosphate aldolase regulated by gibberellin in roots of rice seedling. Plant Mol Biol 56: 839-848.
- Harrison C (2003) GrpE, a nucleotide exchange factor for DnaK. Cell Stress and 608 Chaperones 8: 218-224.
- Watkins JN, Knight MR, Trewavas J, Campbell AK (1995) Free Calcium transients 611 in chemotactic and non-chemotactic strains of Eschericia coli determined by using 612 recombinant aequorin. Biochem J 306: 865-69.
- Oomes SJ, Jonker MJ, Wittink FR, Hehenkamp JO, Breit TM, et al. (2009) The effect of calcium on the transcriptome of sporulating B. Subtilis cells. Int J 616 Food Microbiol 133: 234-242.
- Theodorou MC, Tiligada E, Kyiakidi AD (2009) Extracellular Ca2+ transients affect poly-(R)-3-hydoxybutyrate regulation by the AtoS-AtoC system in Escheichia coli. 620 Biochem J 417: 667-672.
- Chan DI, Chu BCH, Lau CKY, Hunter HN, Byers DM, et al. (2010) NMR solution structure and biophysical characterization of Vibrio harveyi acyl carrier protein A75H: effects of divalent metal ions. J 623 BiolChem 285: 30558-30566.
- Hovarth LA, Sturtevant JM, Prestegard JH (1994) Kinetics and thermodynamics 676 of thermal denaturation in acyl carrier protein. Protein Science 3: 103-108.
- Tener DM, Mayo KH (1990) Divalent cation binding to reduced octanoylacylcarrier 626 protein. Eur J Biochem 189: 559-565.
- Day IS, Reddy VS, Ali GS, Reddy ASN (2002) Analysis of EF-hand-containing 629 proteins in Arabidopsis. Genome Biology 3: 0056.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14891
- [From(publication date):
specialissue-2014 - Apr 06, 2025] - Breakdown by view type
- HTML page views : 10240
- PDF downloads : 4651