Research Article Open Access
Properties of Alkaline Protease C45 Produced by Alkaliphilic Bacillus Sp. Isolated from Chitu, Ethiopian Soda Lake
Gizachew Haile1* and Amare Gessesse21Jimma University, College of agriculture and veterinary medicine, Biotechnology program, Jimma, Ethiopia
2Addis Ababa University Biotechnology grogram, Addis Ababa, Ethiopia
- Corresponding Author:
- Gizachew Haile
Addis Ababa University Biotechnology grogram
Addis Ababa, Ethiopia
E-mail: gizachew.gh@gmail.com
Received date: March 15, 2012; Accepted date: April 14, 2012; Published date: April 16, 2012
Citation: Haile G, Gessesse A (2012) Properties of Alkaline Protease C45 Produced by Alkaliphilic Bacillus Sp. Isolated from Chitu, Ethiopian Soda Lake. J Biotechnol Biomater 2:136. doi:10.4172/2155-952X.1000136
Copyright: © 2012 Haile G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
A total of 240 alkaliphilic bacteria were isolated from samples collected from alkaline soda lakes of Ethiopia and were screened for the production of alkaline proteases. Of these, 30% were protease positive indicating the abundance of protease producing microorganisms in these habitats. The Bacillus sp. designated as C45 that grow in solid state medium was selected based on the property of the enzyme for further study. The protease produced by the Bacillus sp. was active in the pH range of 6.5-11.5, with optimum activity at pH 8-9; and stable at alkaline pH. The optimum temperature for activity was 40°C and 50°C in absence and presence of 5 mM of Ca+2, respectively. The enzyme displayed appreciable activity and stability at low temperature. These properties suggest that protease C45 could find potential application for dehairing and detergent at moderate temperature. When protease C45 was added to raw hide it enabled dehairing, suggesting the potential usefulness of the enzyme in the leather industry.
Keywords
Alkaline protease; Bacillus sp. C45; Solid state fermentation
Introduction
Proteases are essential constituents of all forms of life on earth which are classified based on chemical nature of the active site, the reaction they catalyze, and their structure and composition [1]. The major classes are again classified in to sub classes based on pH, catalytic site on polypeptide, occurrence, and so on. Based on the catalytic site on the substrate, proteases are mainly classified in to endoproteases that prefer to act at the inner region of the polypeptide chain and exoproteases preferentially act at the end of the polypeptide chain [1]. Based on their optimal pH proteases are also classified as: acid proteases which are active in the pH ranges of 2-6 [1] and are mainly of fungal in origin [2] , alkaline proteases which are optimally active in the alkaline range (pH 8-13), though they maintain some activity in the neutral pH range as well [3] and are obtained mainly from neutralophilic and alkaliphilic microorganisms such as Bacillus and Streptomyces species, and neutral proteases which are active at neutral, weakly alkaline or weakly acidic pH and are mainly of plant in origin [2]. Neutralophilic and alkaliphilic microbial alkaline proteases that are isolated from alkaline soda lakes possess a considerable industrial potential due to their biochemical diversity and stability at extreme pH environments, respectively [4]. These application areas include: as processing aid like dehairing in leather tanning industries [5], as detergent additive to remove dirt and stains that are difficult to remove [1,6], in protein hydrolysis for production of high quality protein hydrolysates [1], in silver recovery from waste X-ray films [7,8], in pharmaceuticals production, and in chemical synthesis [9]. Ethiopia is endowed with a range of alkaline habitats in Rift valley region. However, only few reports have been found on isolation of alkaline protease producers from these soda lakes [10,11]. Therefore, the aim of this study was to isolate proteolytic alkaliphilic bacteria from Ethiopian soda lakes and characterize its enzyme.
Materials and Methods
Protease assay
Proteolytic activity in the culture supernatant was determined by using casein as a substrate. A mixture of 450 ml of 1% (w/v) of casein in 50 mM Tris-HCl buffer, pH 8.5 and 50 ml crude enzyme extract were incubated in a water bath at 40ºC for 20 minutes. After 20 minutes, the enzyme reaction was terminated by the addition of 500 ml of 10% (w/v) Trichloroacetic acid (TCA) and was kept at room temperature for 15 minutes. Then, the reaction mixture was centrifuged to separate the unreacted casein at 10,000 rpm for 5 minutes. Sample containing 500 ml supernatant was taken, and mixed with 2.5 ml of 0.5 M Na2CO3. After adding 0.5 ml of 1 N Follin Ciocalteus phenol reagent, the mixture incubated in the dark for 30 minutes and absorbance measured at 660 nm against a reagent blank. One U corresponds to the amount of enzyme required to release 1 micromole of amino acid equivalent to tyrosine per minute under the standard assay conditions.
Characterization of alkaline proteases
The protease activity was tested with the following buffers of varying pH: Phosphate (6-8), Tris-HCl (7.5-9), and Glycine-NaOH (8.5-11.5) each at a concentration of 50mM. pH stability was determined by incubating the crude supernatant at buffers of varying pH(6-11.5) for 1 hr at temperature of 25ºC following protease assay. The effect of temperature on protease activity was determined at temperature of 30ºC, 40ºC, 50ºC, 60ºC and 70ºC according to the procedures described in protease assay section. Thermal stability of the protease was determined by pre-incubating the protease with buffer (pH 8.5) at temperature of 30ºC, 40ºC, 50°C, 60ºC and 70ºC in absence and presence of 5mM of Ca+2 following determination of residual activity every 10 minutes. To determine the protease ionic strength, the crude supernatant was pre-incubated with 2 M NaCl for 1hr at temperature of 25ºC following protease assay. Relative protease activity (expressed in %) was defined as the percent protease activity compared with the maximum value.
Test for potential use in enzymatic dehairing
Two set of cow hides were washed and cut into 10 × 15 cm pieces. One set from the pairs (controls) was put in to flask containing buffer solution. The other halves were put in to flasks containing enzyme solution by keeping liquid (ml) to gram hide proportion one to one [12-14] shaker (121 rpm) at room temperature for 24 hrs. To asses dehairing extent, one flak at a time taken for hair removal trail with fingers. The skin pieces after treatments were examined for dehairing time, dehairing extent, and scud.
Results and Discussion
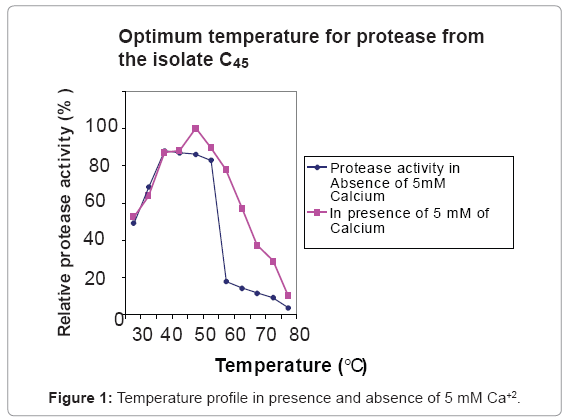
Effect of temperature on activity and stability of the protease
The enzyme was optimally active at 40ºC and 50ºC in the absence and presence of 5 mM of Ca+2, respectively (Figure 1). At 65ºC the enzyme displayed 50% and 10% of the maximum activity in the presence and absence of 5 mM of Ca+2 respectively
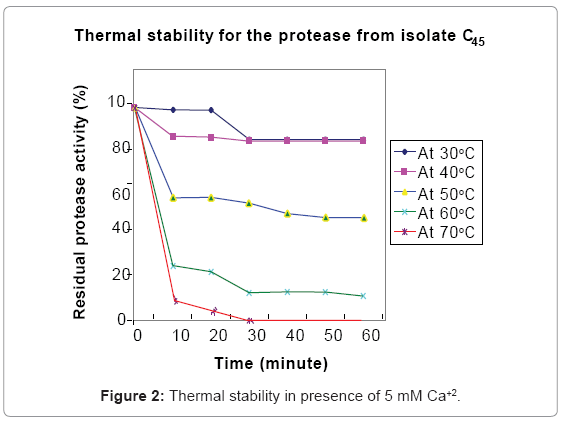
The alkaline protease was stable at moderate temperatures (30ºC to 50ºC (Figure 2)). The enzyme was completely inactivated after 30 minute incubation at 60ºC and 10 minutes incubation with buffer at 70ºC indicating potential applicability to reduce operating energy and cost in protease assisted industrial process such as washing and dehairing [1,15].
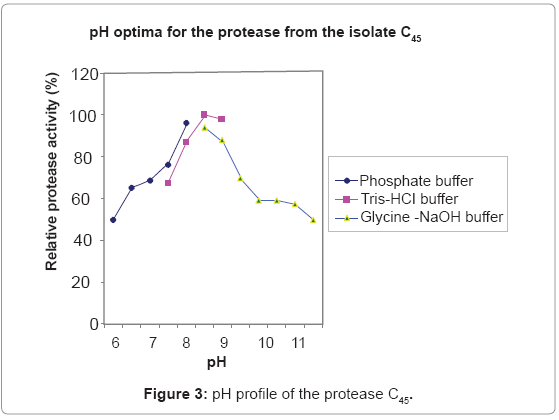
Effect of pH on activity and stability of the protease C45
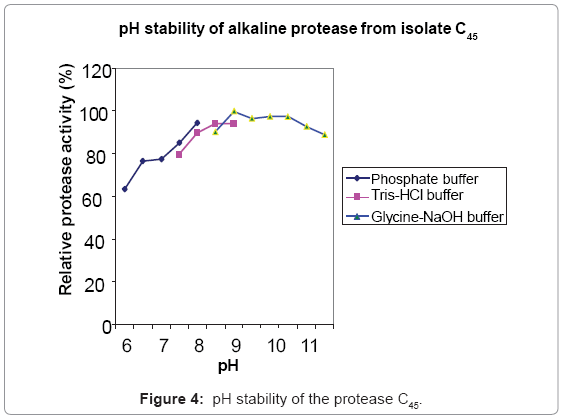
Protease C45 was active in a broad pH range of 6.5-11.5, with optimum activity in the pH range of 8-9 (Figure 3). Protease C45 was stable in a broad pH range (Figure 4). After 1 hr incubation at different pH values at room temperature maintains more than 80% of its original activity in the pH range of 7.5- 11.5.
The enzyme produced by the bacillus isolate C45 under SSF was further characterized to evaluate its potential usefulness for different applications. The protease from this isolate was optimally active between pH 8-9 and stable at alkaline pH. Proteases active in the pH range of 8-12 and stable at alkaline pH are known as potential candidates for detergent application, dehairing of hides, and silver recovery from waste X-ray and photographic films [1,6,9]. This indicates that protease C45 has a good potential for such applications.
Effect of sodium chloride on protease C45 activity
The protease was stable and maintained 100% of its original activity after hour incubation with 2 M NaCl. The observed stability at high ionic strength was due to the presence of high proportion of acidic amino acids such as glutamic acid on its surface [16]. This high salt tolerance of the enzyme implies its importance to use for bioorganic synthesis for protease assisted peptide synthesis [17]. Halophilic protease production has been also reported in alkali tolerant Bacillus patagoniensis [15] and by other halophilic and alkaliphilic bacterial isolates [18,19] at 2 M NaCl.
Therefore, stabilizing ability of active site conformation even in presence of high competing solutes for water and at dry state by halophilic enzymes show their usefulness in proteases assisted synthesis of unique chemicals.
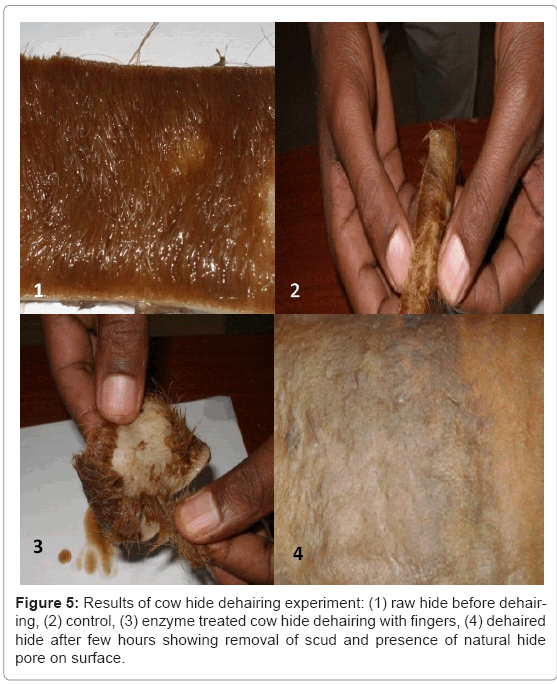
The enzyme produced by the bacillus isolate C45 under SSF was further characterized to evaluate its potential usefulness for dehairing of hides (Figure 5). Complete removal of hair achieved with in 12 hrs with low scud and visible natural hide pore. Proteases active in the pH range of 8-12 and stable at alkaline pH are known as potential candidates for detergent application, dehairing of hides, and silver recovery from waste X-ray and photographic films [1,6,9]. This indicates that protease C45 has a good potential for dehairing applications in leather industries.
Conclusions and Recommendations
Based on the results of this work, the following conclusions can be drawn:
• The alkaline protease produced by this Bacillus sp. was active and stable at high alkaline and salt concentration.
• The protease stability and high enzyme activity at moderate (30ºC-50ºC) and at room temperatures, respectively, was an attractive feature to develop enzyme based industrial processes at room temperatures.
• Hide dehairing experiment at laboratory also confirmed the usefulness of this enzyme for application in leather industries.
Therefore, the following activities for future research recommended:
1. Searching for more potent alkaline protease producers from Ethiopian soda lakes.
2. Testing the alkaline protease from C45 for other suggested potential industrial applications.
3. Test of the enzyme dehairing capability at the tannery experimental level.
Acknowledgements
I would like to thank Dr. Amare Gessesse for his support and guidance throughout the course of this study.
References
- Rao MB, Tanksale AM, Ghatge MS, Deshpande VV (1998) Molecular and Biotechnological aspects of microbial proteases. Microbiol Mol Bio Rev 62: 597-635.
- Aguilar CN, Gutierrez-Sanchez G, Rado-Barragan PA, Rodriguez-Herrera R, Martinez-Hernandez JL et al. (2008) Perspectives of solid state fermentation for production of food enzymes. Am J Biochem Biotechnol 4: 354-366.
- Horikoshi K (1999) Alkaliphiles: some applications of their products for biotechnology. Microbiol Mol Biol Rev 63: 735–750.
- Moon SY, Oh TK and Rho HM (1994) Purification and characterization of an extra cellular alkaline protease from Bacillus subtilis RM 615. Korean Biochem J 27:323-329.
- Sivasubramanian S, Manohar BM, Puvanakrishnan R (2008) Mechanism of enzymatic dehairing of skins using a bacterial alkaline protease. Chemosphere 70: 1025-1034.
- Maurer KH (2004). Détergent protéases. Cur Opin Biotechnol 15: 330–334.
- Fujiwara N, Yamamato K, and Masui A (1991) Utilization of thermostable alkaline protease from an alkaliphilic thermophile for the recovery of silver from used X-ray film. J Fermt Bioeng 72: 306-308.
- Nakiboglu N, Toscali D and Yasa I (2001) Silver recovery from waste photographic films by an enzymatic method. Turk J Chem 25: 349-353.
- Gupta R, Beg QK and Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59: 15-32.
- Gessesse A and Gashe BA (1997) Production of alkaline protease by an alkaliphilic bacteria isolated from an alkaline soda lake. Biotechnol Lett 19: 479-481.
- Gessesse A, Hatti-kaul R, Gashe BA and Mattiasson B (2003) Novel Alkaline protease from alkaliphilic bacteria grown on chicken feather. Enzyme Microb Technol 32: 519-524.
- Macedo AJ, Da Silva WO, Gava R, Driemeier D, Henriques JA, et al. (2005). Novel keratinase from Bacillus subtilis S14 exhibiting remarkable dehairing capabilities. Appl Env Microbiol 71: 594-596.
- Malathi S, Chakraborty R (1991) Production of alkaline proteases by a new Aspergillus flavus isolate under solid substrate fermentation conditions for use as a depilation agent. Appl Environ Microbiol 57: 712-716.
- Najafi MF, Deobagkar D, Deobagkar D (2005) Potential application of protease isolated from Pseudomonas aeruginosa PD100. Electron J Biotechnol 8: 197-203.
- Olivera N, Sequeiros C, Sineriz F, Breccia J (2006) Characterization of alkaline proteases from novel alkali-tolerant bacterium Bacillus patagoniensis. World J Microbiol Biotechnol 22: 737-743.
- Madern D, Ebel C, Zuccai G (2000) Halophilic adaptation of enzymes. Extremophiles 4: 91-98.
- Martinek K, Semenov AN (1981) Enzymatic synthesis in biphasic aqueous-organic systems. II. Shift of ionic equilibria.. Biochem Biophys acta 658: 90-101.
- Dodia MS, Joshi RH, Patel RK, Singh SP (2006) Characterization and stability of extracellular alkaline protease from halophilic and alkaliphilic bacteria isolated from saline habitats of costal Gujarat, India. Braz J Mirobiol 37: 276-282.
- Patel RK, Dodia MS, Joshi RH and Singh SP (2006) Production of extracellular halo-alkaline protease from newly isolated halophilic Bacillus sp.isolated from sea water in Western India. World J Microbiol Biotechnol 22: 375-382.
- Niadu KSB, Devi KL (2005) Optimization of thermostable alkaline protease production from species of Bacillus using rice bran. Afr J Biotechnol 4: 724-726.
- Kumar CG, Takagi H (1999) Microbial alkaline proteases: from bioindustrial viewpoint. Biotechnol Adv 17: 561–594.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16775
- [From(publication date):
July-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11981
- PDF downloads : 4794