Research Article Open Access
Primitive Stem Cells are Present in the Blood of Adult Equines and Increase with Moderate Exercise or Ingestion of the Cyanobacter, Aphanizomenon Flos-Aquae
| George W McCommon1 , Frank Lochner2 , Asa C Black Jr3 and Henry E Young4 * | ||
| 1Department of Veterinary Sciences, Fort Valley State University, Fort Valley, GA, 31030, USA | ||
| 2Cougar Creek Farms, Fort Valley, GA, 31030, USA | ||
| 3Department of Basic Medical Sciences, Memorial General Hospital - University of South Carolina Medical School, Greenville, SC 29605, USA | ||
| 4Regeneration Technologies, Macon, GA 31201, USA | ||
| Corresponding Author : | Henry E Young, PhD Regeneration Technologies Corporation 778-B Mulberry Street, Macon, GA 31201, USA Tel: 478-319-1983 E-mail: young.hey1@yahoo.com |
|
| Received February 16, 2013; Accepted July 17, 2013; Published July 19, 2013 | ||
| Citation: Common GWM, Lochner F, Black Jr AC, Youn HE (2013) Primitive Stem Cells are Present in the Blood of Adult Equines and Increase with Moderate Exercise or Ingestion of the Cyanobacter, Aphanizomenon Flos-Aquae. Autacoids 2:103. doi: 10.4172/2161-0479.1000103 | ||
| Copyright: © 2013 Common GWM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Autacoids and Hormones
Abstract
Primitive stem cells have been discovered within the blood of adult mammals such as rodents, porcines, and humans. The current study addressed the issue of primitive stem cells in the blood of adult equines. Blood withdrawal by venipuncture from adult equines was performed following the guidelines of Fort Valley State University IACUC. Ten horses were used in this study: one Danish Oldenburg, three Standard breds and six Quarter Horses with age ranges of 5 – 20 years. The blood was processed for stem cell isolation and counting. All horses examined were noted to have circulating levels of primitive stem cells in their blood. Standard breds showed an increase of stem cell number with increasing age of the animals. In contrast, Quarter Horses showed an increase in stem cell number that paralleled an increase in the level of stress to the animal, regardless of age. All horses showed an increase in stem cell number in their blood after moderate exercise (10 minutes of cantering) and at time periods after ingestion of Aphanizomenon flos-aquae. These studies demonstrate the existence of primitive stem cells within adult equine blood. Larger sample sizes are needed to determine the significance of the effects of age, stress, trauma and ingested compounds on the number of circulating primitive adult stem cells in the blood of adult horses. Further studies are also needed to assess the applicability of using circulating primitive stem cells for the restoration and/or repair of tissues in the adult equine damaged by trauma or disease.
| Keywords | |
| Equine; Autologous; Blood; Adult stem cells; Wound healing; Aphanizomenon flos-aquae (AFA) | |
| Introduction | |
| Autologous adult mesenchymal stem cells are being used commercially to treat a variety of musculoskeletal disorders in equines [1]. These mesenchymal stem cells are processed from various body tissues, predominantly from bone marrow or adipose tissue. The harvested stem cells are isolated by enzymatic digestion and are either expanded in vitro, to increase the number of stem cells prior to use, and/ or transfused intravenously and the cells allowed to migrate to the site of tissue damage to repair the damage isolated adult mesenchymal stem cells are then injected into the site of injury or pathology [1]. The harvesting of stem cells from bone marrow or fat is cumbersome and extremely painful to the animal. Moreover, such procedures render the animal prone to infection and leave a site of injury that may be slow to heal. | |
| Young et al. [2,3] described the presence of primitive stem cells within the connective tissue matrices of amphibians, avians, mammals and humans. Studies by these authors described the specific characteristics of these primitive stem cells (Table 1). | |
| Stout et al. [4] examined the effects of trauma on the circulating levels of these primitive stem cells in the blood of adult pigs after 90 minutes of anesthetized trauma. Trauma was defined in this study as splenectomy followed by pancreatectomy. This study documented the presence of a mass migration of primitive stem cells from the connective tissues of skeletal muscle into the blood stream following trauma. Primitive stem cell levels in the blood increased 23.5 times following trauma compared to the baseline levels established before trauma. | |
| Preliminary unpublished data from Young’s colleagues’ laboratory noted that ingestion of a cyanobacter increased the levels of circulating primitive stem cells to 168% within human blood 90 minutes post ingestion. The cyanobacter used was Aphanizomenon flos-aquae, a freshwater species of cyanobacter. It is known to contain a source of nutrients such as vitamins, minerals, essential fatty acids (including omega 3 fatty acids), beta-carotene, chlorophyll, phycocyanin, active enzymes, amino acids, proteins, complex sugars, phytonutrients and other bioactive components [5]. The composition of Aphanizomenon flos-aquae is described in Table 2. | |
| Four hypotheses were tested in this study. 1) Primitive stem cells are present in the blood of adult equines. 2) Moderate exercise will increase levels of primitive stem cells in adult equine blood. 3) Ingestion of Aphanizomenon flos-aquae will increase levels of primitive stem cells in adult equine blood. 4) primitive stem cells can be isolated from adult equine peripheral blood with less injury to the animal than that caused by their isolation from bone marrow or adipose tissue. | |
| Materials and Methods | |
| Animal use | |
| Experiments followed the guidelines of Fort Valley State University’s Institutional Animal Care and Use Committee (IACUC). These guidelines reflect the criteria for humane animal care of the National Research Council as outlined in “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (National Academy Press, 1996). Ten horses were used in this study: one Danish Oldenburg, three Standard breds, and six Quarter Horses, with age ranges of 5 - 20 years. | |
| Moderate exercise | |
| Moderate exercise in this study was defined as 10 minutes of cantering. Adult horses had their blood withdrawn immediately prior to and 1 hour after exercising. The horses had 8-ml of blood withdrawn at each time point by jugular vein venipuncture and placed in standard hemovac tubes containing EDTA (Becton Dickson). The tubes were inverted several times to ensure appropriate mixing of blood with the EDTA, and then stored on wet ice at 4°C until returned to the laboratory. | |
| Ingestion of Aphanizomenon flos-aquae | |
| One dose of Aphanizomenon flos-aquae (AFA) in this study was defined as 1 gram (2 capsules) of Stem Trition (Sea Change Therapeutics, Denver, CO). The AFA was removed from the capsules and placed into one cup of sweet feed. One dose of AFA was fed to each horse. Adult horses had their blood withdrawn immediately prior to ingestion and at 1 hour and six hours after ingestion of AFA. The horses had 8-ml of blood withdrawn at each time point by jugular vein venipuncture and placed in standard hemovac tubes containing EDTA (Becton Dickson). The tubes were inverted several times to ensure appropriate mixing of blood with the EDTA, and then stored on wet ice at 4°C until returned to the laboratory. | |
| Primitive stem cell isolation | |
| Eight ml of equine blood were divided into two equal fractions of 4 ml each. Primitive stem cells were isolated from equine blood using two separate isolation procedures. In the first procedure, each 0.5 ml of whole blood was added to 49.5 ml of hemolysis solution (Moraga Biotechnology Corporation, Los Angeles, CA) in a 50 ml polypropylene tube (Falcon, VWR). Each tube was inverted twice to mix its contents. The tubes were balanced and centrifuged at 1,800 rcf for 10 minutes. The supernatant was removed by aspiration. The cell pellets were resuspended by agitation performed by stroking the tube across an Eppendorf tube holder. The cell suspension was reconstituted with 2 mls of reconstitution solution (Moraga). To each ml of reconstituted cells, 49 mls of clarification solution (Moraga) were added. The tubes were inverted twice to mix their contents. The tubes were balanced and then centrifuged at 1,800 relative centrifugation force (rcf) for 10 minutes. The supernatant was removed by aspiration and the pellets resuspended by agitation. The cell suspension was reconstituted with 2 ml Serum- Free Defined BLSC Adherent Propagation Medium (Moraga) and cell counts performed. | |
| In the second isolation procedure, each tube containing 4 ml of blood was stored vertically at 4°C for 48 hours. At the end of 48 hours the plasma, having separated from the blood cells, was removed and placed into 15 ml polypropylene conical tubes (Falcon, VWR). Sterile Dulbecco’s Phosphate Buffered Saline (DPBS, In Vitrogen) was added to each tube to the 14-ml mark. The tubes were then spun at 4,000 rcf for 5 min. The supernatants were discarded and each cell pellet reconstituted in 1 ml of DPBS. | |
| Cell counts | |
| The final volume of each cell suspension was determined and recorded. Each cell suspension was diluted 1:1000, by serial dilution. Fifteen microliters of each final cell suspension were removed and placed into separate 2.0 ml polypropylene tubes (VWR). Fifteen microliters of sterile 0.4% w/v aqueous Trypan blue, pH 7.4 (Sigma) were added to each tube. The contents were mixed by trituration 5-6 times and 15 microliters of a cell /Trypan blue suspension were placed on a hemocytometer for cell counting. All Trypan blue-positive and negative cells within the nine large boxes of a hemacytometer were counted and then averaged for the number of cells per each large box. The formula to determine final cell number per ml was [(average number)/50] = cell number x 106 cells per ml. Final cell number calculations were based on total number of cells for 4 ml of whole blood times a 1:1000 dilution. For separate individuals, the counts were averaged and standardized per 8 ml of blood. To make comparisons between individuals with variable cell counts, the total number of primitive stem cells in control blood (before any action was undertaken) was divided into the final numbers of primitive stem cells after exercise or at time points after ingestion of AFA and then multiplied by 100 to obtain a percent increase in the number of primitive stem cells generated. | |
| Adult stem cell immune cytochemical staining | |
| Presumptive primitive equine stem cells were initially identified by their size and characteristic staining patterns with Trypan blue. The identity of the cells was later verified using immunostaining with CEA-CAM-1 and SSEA following established procedures [6]. In brief, aliquots of 1 million cells isolated from blood of adult horses were fixed with a 2.5% Glutaraldehyde/4% Formaldehyde/1% Glucose fixative in Dulbecco’s phosphate buffered saline, pH 7.4 (DPBS, Cellgro, Mediatech, Inc, Herndon, VA). The cells were blocked with 0.3% sodium azide (Sigma, St. Louis, MO) and then with the blocking solution from the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) to inhibit endogenous peroxidases on the surfaces of the cells. Individual aliquots of equine cells were incubated with CEA-CAM-1 and SSEA antibodies, respectively. Excess antibody was removed by washing (rinsing / centrifugation) with DPBS. The equine cells were incubated with a secondary antibody bound to biotin (Vector) and the excess antibody removed by washing with DPBS. The equine cells were incubated with the Vectastain ABC reagent (Vector) and the excess removed by washing with DPBS. Finally, the equine cells were incubated with an insoluble horseradish peroxidase substrate (3,3’-diaminobenzidine, DAB, Vector) that precipitates at antibody binding sites. The equine cells were then photographed using a Nikon CoolPix Digital camera coupled to a Nikon TMS Inverted Phase microscope. Photographs were cropped using Photoshop 7.0. | |
| Results | |
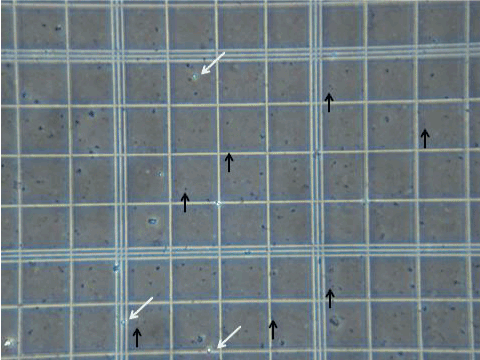
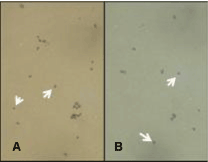
| All horses examined were noted to have both Trypan blue positive spherical entities and Trypan blue negative spherical entities within their blood before exercising and before ingestion of AFA (Figure 1). After exercise and after ingestion of AFA all horses demonstrated an increase in both populations of spherical entities. At six hours after ingestion of AFA both negative Trypan blue-stained spherical entities and positive Trypan blue-stained spherical entities were shown to visibly increase in number (Figure 2). The positively-Trypan blue stained spherical entities were shown to bind to the CEA-CAM-1 antibody, denoting primitive blastomere-like stem cells (Figure 3A, white arrows). The negatively- Trypan blue stained spherical entities were shown to bind to the SSEA antibody, denoting primitive epiblast-like stem cells (Figure 3B, white arrows). Both populations of primitive stem cells were circulating in adult equine blood before any intervention occurred at average baseline levels (Figure 4 and 5) and showed increases in number with either 10 minutes of moderate exercise (Figure 4) of after ingestion of one gram (two capsules) of AFA (Figure 5). In addition, the Standard bred horses in this study showed an increase of stem cell number with increasing age of the animals. In contrast, the Quarter Horses showed an increase in stem cell number that paralleled an increase in stress to the animal, regardless of age (Table 3). Stress in this study was noted as an increase in nervousness (increased respirations and increased heart rate) during visual inspection of the animal and the difficulty encountered while performing venipuncture. Upon ingestion of AFA, all horses examined demonstrated an increasing number of both populations of primitive stem cells through the six hour time point when the study was concluded (Figure 5). | |
| Discussion | |
| Drapeau and colleagues [7] demonstrated that two ligands isolated from Aphanizomenon flos-aquae (StemEnhance, Stem Tech Health, www.Stemtechhealth.com) would increase the quantity of CD34+/ CD133+ erythroblasts in the bloodstream, thereby mimicking the effects of granulocyte macrophage colony stimulating factor (GMCSF) to increase the number of erythrocytes in circulation as a method to stimulate the repair of damaged tissues. Stout and colleagues [4] demonstrated that massive trauma, i.e., anesthetized splenectomy followed by pancreatectomy, would increase the level of circulating epiblast-like stem cells and blastomere-like stem cells in the blood of adult swine. These two primitive stem cell populations were shown to become incorporated in tissues undergoing repair after induced myocardial infarction, Parkinson disease, and diabetes (8). The current study investigated whether moderate exercise or ingestion of intact AFA (StemTrition, Sea Change Therapeutics, www.SeaChangeForLife.com) would have similar effects on circulating primitive stem cell populations in adult equines. | |
| Four hypotheses were tested in this study. 1) Primitive stem cells are present in the blood of adult equines. 2) Moderate exercise will increase levels of primitive stem cells in adult equine blood. 3) Ingestion of Aphanizomenon flos-aquae will increase levels of primitive stem cells in adult equine blood. And 4) primitive stem cells can be isolated from adult equine peripheral blood with less injury to the animal than that caused by their isolation from bone marrow or adipose tissue. | |
| Primitive stem cells | |
| Primitive adult stem cells can be divided into at least two populations based on size, Trypan blue staining, cell surface markers and differentiation potential (Table 1). Blastomere-like stem cells are very small (<2 microns) spherical entities that are Trypan blue positive, their cell surface marker is carcinoembryonic antigen cell adhesion molecule-1 (CEA-CAM-1 and also in humans CD34-/CD66e+) and they will differentiate into at least 66 of the 66 possible cell types based on available phenotypic-specific expression markers [3]. In contrast, epiblast-like stem cells are larger (6-8 microns) spherical entities that are Trypan blue negative, their cell surface marker is stage-specific embryonic antigen (SSEA and also in humans CD34-/CD10+) and they will differentiate into at least 63 of the 66 possible cell types based on available phenotypic-specific expression markers [8]. | |
| Primitive equine stem cells | |
| Primitive adult equine stem cells, isolated from blood, were initially identified by their size and characteristic staining patterns with Trypan blue. The identity of the cells was verified using two antibodies shown to specifically attach to primitive stem cells. Carcinoembryonic antigen - cell adhesion molecule - 1 (CEA-CAM-1) was used to verify the existence of blastomere-like stem cells [3] and stage-specific embryonic antigen (SSEA) was used to verify the existence of epiblast-like stem cells [8] in adult equine blood. Therefore, the isolated cells analyzed in this study demonstrated representative characteristics of primitive adult stem cells, i.e., epiblast-like stem cells and blastomere-like stem cells, previously identified and characterized by Young and colleagues [3,8] (Table 1), and shown to be located within adult skeletal muscle, bone marrow, adipose tissue and blood [4,9,10]. As shown in Figures 1, 3 and 4, base line levels of these two populations of primitive stem cells were found in the blood stream of adult equines before any of the experiments occurred, i.e., moderate exercise or ingestion of AFA. These results suggest that there is a normal contingent of circulating primitive epiblast-like stem cells and blastomere-like stem cells in the blood of adult equines. | |
| Mobilizing primitive stem cells using moderate exercise | |
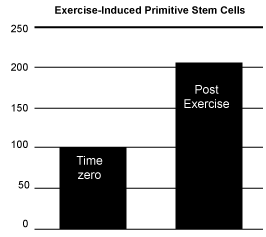
| The second hypothesis tested was to determine if moderate exercise would increase the circulating levels of primitive stem cells in adult equine blood. As a test of this hypothesis ten horses were cantered around the paddock for ten minutes, followed by 50 minutes rest and then subjected to a jugular venous puncture to remove 8 ml of blood for subsequent isolation and analysis of circulating primitive stem cells. As shown in Figure 4, 10 minutes of cantering induced an approximate 98% increase in circulating primitive stem cell numbers compared to base line controls. | |
| Mobilizing primitive stem cells using nutraceuticals | |
| The third hypothesis tested in this study was to determine if ingestion of intact Aphanizomenon flos-aquae would increase the circulating levels of primitive stem cells in adult equine blood. A number of compounds have been found to either mobilize various types of adult precursor cells or support their function. Among these are fucoidans, blueberry powder, red wine, lithium, L-carnosine, vitamin-D3 and Aphanizomenon flos-aquae. | |
| Fucoidans, sulfated polysaccharides, which are found in abundance in brown seaweeds, are thought to influence the mobilization of endothelial progenitor cells. Endothelial progenitor cells are dispatched by the body to help deal with ischemic (low oxygen) tissues [11]. There is also evidence that fucoidans enhance the levels and activity of stromal-derived factor 1 (SDF-1), which plays a crucial role in progenitor and stem cell mobilization. When researchers tested other stem cell mobilizing agents to see if they would have the same effect as fucoidans on endothelial progenitor cells, they found they did not [12]. | |
| A 2006 study [13] found a dose-related effect of blueberries, L-carnosine and vitamin D3 on the proliferation of human bone marrow related stem cells (as compared with an FDA approved drug, recombinant human granulocyte-macrophage colony stimulating factor, GM-CSF). Compounds such as L-Carnosine and vitamin D3 have been shown to support stem cell proliferation [13]. Vitamin D3 has also been shown to be essential for intestinal absorption of calcium and for the regulation of calcium in bones, muscles, and nerves [14]. | |
| A group of Taiwanese researchers [15] explored whether red wine’s established cardioprotective effects might be due to its impact on circulating endothelial progenitor cells. As part of the study, eighty healthy, young subjects were randomized and assigned to groups that consumed water (100 mL= milliliters, 3.4 fluid ounces), red wine (100 mL), beer (250 mL), or vodka (30 mL) daily for 3 weeks. When the study ended the scientists found that the intake of red wine had significantly enhanced the numbers of circulating endothelial progenitor cells and improved their functioning (by virtue of influencing nitric oxide bioavailability). | |
| It is well established that the use of lithium at therapeutic dose levels (typically 600 or more milligrams lithium carbonate daily) liberates various precursor stem cells from bone marrow [16]. However, some researchers and theorists believe that very low dose lithium can help modulate mood in normal people and, by so doing, increase circulating levels of the neurotransmitter serotonin. Serotonin has been shown to play a role in the shuttling of various precursor stem cells from bone marrow [17]. | |
| Aphanizomenon flos-aquae (AFA) is a freshwater species of cyanobacteria. Cyanobacteria (blue-green algae) are an ancient class of bacterial microphytes, part of the cyanobacteria phylum. AFA as a species has both nontoxic and toxic forms [7,18,19]. AFA grows throughout the world and is harvested in Upper Klamath Lake, Oregon in the Pacific Northwest. Most sources worldwide are toxic, containing both hepatic and neuroendotoxins. AFA from Klamath Lake is a non-toxic type of algae of the cyanobacteria phylum [7]. Like other cyanobacter and plants, AFA uses photosynthesis to produce glycogen that is stored and utilized by the cell. While plants’ cell walls are mainly cellulose, AFA’s cell walls are composed of peptides and carbohydrates, the typical cell wall material of bacteria. The cellular structure of AFA is that of a simple prokaryote. Most cyanobacter are highly efficient photosynthesizers, even more so than plants. Algae utilize light energy from the sun, carbon dioxide from the air, and water to synthesize proteins, carbohydrates, and lipids. AFA is also able to directly use molecular nitrogen from the air to produce proteins and other nitrogen-containing biomolecules. This ability is widespread in prokaryotes, but unknown in eukaryotes [7,18-23]. | |
| Aphanizomenon flos-aquae is often used as a health supplement for certain nutritional deficiencies or to increase alertness and focus. Klamath Lake’s diversity of microorganisms (microflora and microfauna) enables AFA to utilize and/or sequester minerals and compounds often chemically unavailable. For example, some microorganisms process inorganic sulfur into organic sulfur. Once those cells die the organic form of sulfur is available for AFA to make into amino acids like cysteine and Methionine. Other microorganisms convert vast amounts of magnesium, a vital component of chlorophyll, into is organic form. Although AFA can utilize magnesium on its own, the excess magnesium in the ecosystem helps AFA attain one of the highest chlorophyll concentrations of any food, five times more than spinach and ten times more than broccoli. This is a key component for the AFA industry. A recent report funded by the United States Department of Agriculture (USDA) affirms almost 70% of Americans fail to meet their recommended daily intake of magnesium. Additionally, one of the most common magnesium supplements, magnesium oxide, is the least bio-available. If you take 100% of your recommended daily intake in magnesium oxide form, you will only absorb 4%, leaving you 96% deficient. The AFA and other natural supplement industries cite that poor bio-availability “is common with supplements that are not derived from balanced ecosystems” and are instead mined from the earth or refined from laboratories. Furthermore, extensive studies on modern diet and nutrition show deficiencies in many of the important factors to maintain health and wellbeing. It is imperative to have the bio-available minerals and nutrients that foods from balanced ecosystems offer. Magnesium, calcium, iron, and potassium are just a few of the ions with increased bio-availability in Klamath Lake’s ecosystem [24-27]. | |
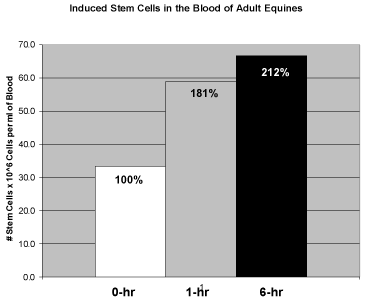
| In the experiment testing the third hypothesis, 2 capsules of intact AFA (StemTrition) were opened and their contents added to sweet feed prior to ingestion by these same horses. At time periods of 1 hour and 6 hours after ingestion, the horses were subjected to jugular venous puncture to remove 8 ml of blood for subsequent isolation and analysis of circulating primitive stem cells. As shown in Figure 5, 1 hour after ingestion the circulating primitive stem cells increased to 181% of baseline, whereas at 6 hours after ingestion the circulating levels of primitive stem cells increased to 212% of baseline. This could also be seen visually, comparing Figure 1 (baseline) to Figure 2 (6 hours after ingestion of AFA). The results from this experiment confirm that ingestion of AFA will lead to an increase in primitive stem cells in the blood of adult equines. | |
| Ease of isolating stem cells from blood | |
| The fourth hypothesis examined whether primitive stem cells could be isolated from adult equine blood with less injury to the animal and expense than that caused by isolation of stem cells from bone marrow or adipose tissue. Bone marrow aspiration to remove stem cells is a painful procedure to any living organism. The procedure uses a large bore needle placed into a marrow space to remove cells by aspiration. The removed cells may be characterized, propagated if present in insufficient numbers, released from their two-dimensional surfaces by enzymes, extensively washed, and then infused into an autologous host [1]. Isolation of stem cells from adipose tissue also involves risk of infection and wounds that are slow to heal. In equines an incisional biopsy is taken from the hip of the animal, about 100 g of adipose tissue is removed, enzymatically disrupted to separate the stem cells from the adipocytes, fibroblasts, endothelial cells and other differentiated cell types, expanded in vitro if their cell numbers are insufficient, released from their two-dimensional culture surfaces by enzymes, extensively washed, and then infused into an autologous host [1]. A jugular venous puncture to derived circulating blood can be accomplished with the animal fully awake and not needing local or general anesthetic. The current study used two different methods to isolate the primitive stem cells from the blood. In the first method, the blood cells were lysed followed by washing to remove cellular debris from the primitive stem cells. The second method utilized the inherent negative charge zeta potential of the cells in solution to allow the cells to self-separate into blood elements in the “cell pellet” and the primitive stem cells in the “plasma” fraction. The primitive stem cells were then collected in the plasma and washed to remove serum proteins. Due to the increased numbers of primitive stem cells in the blood, sufficient quantities of primitive stem cells could be isolated without having to expand cell numbers in vitro. | |
| Precursor cells and their subcategories | |
| Stem cells are a subcategory of cells designated as “precursor cells”, which provide the material to maintain the tissues and organs of the body throughout the lifespan of the individual and potentially for use in tissue replacement and repair [2,3,8]. Four major categories of precursor cells have been identified within postnatal mammals, e.g., tissue-committed progenitor cells [15,28-30], multipotent germ layer lineage stem cells (i.e., ectodermal stem cells, mesodermal stem cells and endodermal stem cells) [31-35], pluripotent stem cells [2,35,36], and totipotent stem cells [3,37]. | |
| Tissue-committed progenitor cells have been found adjacent to their respective differentiated cell types [38-45], while the more plastic multipotent, pluripotent and totipotent stem cells have been found in bone marrow [34,35,46-48], adipose tissue [35,49-53], and various connective tissue compartments within various organ stroma [2,4,8,35,54-57]. | |
| Studies are ongoing in various laboratories to ascertain whether endogenous precursor cells, i.e., progenitor cells and/or stem cells would make sufficient building blocks for the restoration and repair of diseased or damaged tissues [2,8,37,58-65]. | |
| Vitamins and Minerals Comprising AFA and Recommend Daily Allowances | |
| The majority of the vitamins and minerals that compose one gram of Aphanizomenon flos-aquae are either at or below the Recommended Dietary Allowances / Adequate Intake and Tolerable Upper Intake Levels as set forth by the Institute of Medicine (IOM) of the U.S. National Academy of Sciences (Table 4) [66]. However, some of the vitamins and minerals present in the Aphanizomenon flos-aquae might be construed as deleterious if more than a single gram of AFA were ingested (Table 4). | |
| Provitamin-A β-carotene | |
| The most common side effect of excessive provitamin-A β-carotene consumption is carotenodermia, a benign condition that results from deposition of carotenoid in the outer layer of the epidermis of the skin, i.e., the stratum corneum. However, chronic high pharmacologic doses of β-carotene supplements (>30 mg RAE) have been associated with statistically higher rates of lung cancer in those that have smoked more than two packs of cigarettes per day. There are 2000 IU of provitamin-A β-carotene in 1 gram of AFA (Table 2). With a conversion rate of 1 IU β-carotene from synthetic dietary supplements equivalent 0.15 μg RAE, this may equate to 30 mg of RAE. However, if AFA is more equivalent to a food source than as a synthetic source of β-carotene, the conversion rate is 0.05 μg RAE. This then equates to 10 mg of RAE per gram of AFA, or greater than 30 mg if 6 capsules of AFA were ingested. Ingestion of more than 1-3 grams of AFA puts a smoker at risk for lung cancer [67-70]. | |
| Boron | |
| The exact physiological role of boron in the animal kingdom is poorly understood. Boron occurs in all foods produced from plants. The US Department of agriculture conducted an experiment in which postmenopausal women took 3 mg of boron a day. The results showed that supplemental boron reduced excretion of calcium by 44%, and activated estrogen and vitamin D, suggesting a possible role in the suppression of osteoporosis. However, whether these effects were conventionally nutritional, or medicinal, could not be determined. The U.S. National Institutes of Health states that “Total daily boron intake in normal human diets ranges from 2.1-4.3 mg boron/day”. The minimum lethal dose for humans has not been established. An intake of 4 g/day of boric acid was reported without incidents, but more than this is considered toxic for more than a few doses. Intakes of more than 0.5 grams per day for 50 days cause minor digestive and other problems suggestive of toxicity [71-74]. | |
| Cobalt | |
| Cobalt is an essential element for human life in minute amounts. The LD50 value for soluble cobalt salts has been estimated to be between 150 and 500 mg/kg. Thus, for a 100 kg person the LD50 would be about 20 grams. It is a key constituent of cobalamin, also known as vitamin B12, which is the primary biological reservoir of cobalt as an “ultratrace” element. Bacteria in the guts of ruminant animals convert cobalt salts into vitamin B12, a compound which can only be produced by bacteria or archaea. The cobalamin-based proteins use corrin to hold the cobalt. Coenzyme B12 features a reactive C-Co bond, which participates in its reactions. In humans, B12 exists with two types of alkyl ligand: methyl and adenosyl. MeB12 promotes methyl (-CH3) group transfers. The adenosyl version of B12 catalyzes rearrangements in which a hydrogen atom is directly transferred between two adjacent atoms with concomitant exchange of the second substituent, X, which may be a carbon atom with substituents, an oxygen atom of an alcohol, or an amine. Methylmalonyl coenzyme-A mutase (MUT) converts MMl-CoA to Su-CoA, an important step in the extraction of energy from proteins and fats. Although far less common than other metalloproteins (e.g. those of zinc and iron), cobaltoproteins are known aside from B12. These proteins include methionine aminopeptidase-2, an enzyme that occurs in humans and other mammals, which does not use the corrin ring of B12, but binds cobalt directly. Another non-corrin cobalt enzyme is nitrile hydratase, an enzyme in bacteria that are able to metabolize nitriles. After nickel and chromium, cobalt is a major cause of contact dermatitis. In 1966, the addition of cobalt compounds to stabilize beer foam in Canada led to cardiomyopathy, which came to be known as beer drinker’s cardiomyopathy [75-81]. | |
| Germanium | |
| Germanium is not thought to be an essential nutrient to animals. It usually occurs only as a trace element in ores and carbon-based molecules. It has been used as a purported immune system booster, but its efficiency has been dubious. Its role in cancer treatments has been debated, with the American Cancer Society contending that no anticancer effects have been demonstrated. U.S. Food and Drug Administration research has concluded that germanium, when used as a nutritional supplement, “presents potential human health hazard” [83-85,42,73,35]. | |
| Nickel | |
| Minute amounts of nickel are considered non-toxic to the human body. Nickel enters the human body through three routes, absorption through the gastrointestinal system, and inhalation through the lungs. For most individuals the most common route of exposure is the consumption of nuts, which contain 3-10 mg of nickel per kg. According to the World Health Organization, the safe dietary intake is 4.2 μg of nickel per kg per day. For an average 100 kg individual, that is equivalent to 420 μg of nickel per day [86-88]. | |
| Silicon | |
| Although silicon was proposed to be an ultra-trace nutrient, its exact function in the biology of animals is still under discussion. Silicon is known to be needed for synthesis of elastin and collagen; the aorta contains the highest quantity of elastin and silicon [89]. | |
| Tin | |
| Tin has no known natural biological role in living organisms. It is not easily absorbed by animals and humans. The low toxicity is relevant to the widespread use of tin in dinnerware and canned food. Nausea, vomiting and diarrhea have been reported after ingesting canned food containing 200 mg/kg of tin [89-91]. | |
| Titanium | |
| Titanium is non-toxic to the human body even in large doses. It is estimated that approximately 0.8 mg of titanium is ingested by humans each day, but most of it passes through the gastrointestinal system without being absorbed. Titanium is not known to play any natural role inside the human body. However, it does have a tendency to bioaccumulate in tissues that contain silica and therefore has a possible connection with yellow nail syndrome. Since titanium is biocompatible it has been used in medical applications for long term joint replacements (i.e., knee and hip) and for use in dental implants [92,93]. | |
| Vanadium | |
| The National Institute for Occupational Safety and Health (NIOSH) has recommended that 35 mg/m3 of vanadium be considered immediately dangerous to life and health. This is the exposure level of a chemical that is likely to cause permanent health problems or death. However, the amount of vanadium in 1 gram of AFA (Tables 2, 4) is 2.7 μg which is several orders of magnitude less than required for toxic dose. In contrast, vanadium at the lower dose may actually be beneficial. Vanadium is a relatively controversial dietary supplement, used primarily for increasing insulin sensitivity and body-building. Whether it works for the latter purpose has not been proven; some evidence suggests that athletes who take it are merely experiencing a placebo effect. Vanadyl sulfate may improve glucose control in people with type 2 diabetes. Decavanadate and oxovanadates appear to play a role in a variety of biochemical processes, such as those relating to oxidative stress [94-101]. | |
| Benefits versus risks | |
| The ability of AFA to sequester often chemically unavailable minerals and compounds besides being beneficial can also be detrimental. Sequestration of heavy metals, such as arsenic, mercury and lead can lead to deleterious conditions for the ingesting individual. Heavy metals tend to accumulate in the body, a phenomenon called bioaccumulation. Bioaccumulation of heavy metals in soft tissues interferes with normal physiological functions. Generally, these heavy metals exert their toxic effects by forming complexes with organic compounds. When heavy metals bind to nitrogen-, oxygen- or sulfur-containing groups on enzymes, for example, they disrupt proper protein folding and thus can inactivate enzymes that function in key metabolic processes. Increased exposure to heavy metals is associated with impaired mitochondrial function, oxidative stress, DNA damage, deregulated cell growth and cell death [102-106]. | |
| Further studies are necessary to ascertain whether heavy metals, such as arsenic, mercury, lead and/or other chemical compounds were present in AFA, and what affects short term and/or long term exposure of these chemical compounds would have on the human body. | |
| Conclusion | |
| The current project was performed to determine if primitive stem cells were present in adult equine peripheral blood; if primitive stem cells were present in adult equine peripheral blood, whether moderate exercise would increase their levels in the blood; if primitive stem cells were present in adult equine peripheral blood, whether ingestion of AFA would mimic the effects of moderate exercise to increase the numbers of primitive stem cells within the blood; and if primitive stem cells were present in adult equine peripheral blood, whether these primitive stem cells could be obtained with less morbidity from adult equine peripheral blood than from adipose tissue or bone marrow. The experiments reported herein have demonstrated that 1) all adult horses examined were noted to have circulating baseline levels of primitive totipotent stem cells and primitive pluripotent stem cells in their blood; 2) that the baseline levels of primitive stem cells in adult equine blood were shown to increase in the presence of moderate exercise; 3) that the baseline levels of primitive stem cells in adult equine blood were shown to increase after the ingestion of the cyanobacter, AFA; and 4) that isolation of stem cells from the blood by venipuncture is easier and less traumatic than the isolation of stem cells via biopsy from either adipose tissue or bone marrow. Larger sample sizes from each group and all breeds are needed to further explore the significance of age, exercise and ingested AFA on the number of circulating primitive stem cells in the blood of adult horses. Further studies are needed to assess the feasibility of utilizing circulating primitive adult equine stem cells for the restoration and/or repair of tissues that have been damaged by trauma or disease. Further studies are also necessary, if increased quantities of AFA are used to increase stem cell quantity in peripheral blood, to ascertain whether heavy metals, such as arsenic, mercury and/ or lead were present in AFA, and what affects short term and/or long term exposure of these chemical agents would have on the human body. | |
| Acknowledgements | |
| We would like to thank Julie A Collins, Gypsy Long Black and Marie Carreiro for their technical assistance. The CEA-CAM-1 antibody was a gift from D Hixson. The SSEA antibodies, e.g., MC480, MC631 and MC813-70 developed by D Solter, were obtained from the Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Funding for this study was provided from LM & HO Young Estate Trust (HEY) and Rubye Ryle Smith Charitable Trust (HEY). | |
| References | |
|
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
 |
 |
 |
 |
 |
||||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15105
- [From(publication date):
May-2013 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10499
- PDF downloads : 4606
