Research Article Open Access
Primary Prevention of Thyroid Associated Ophthalmopathy by Pentoxifylline
Balázs Cs1* and Korányi K21Teaching Hospital of St. John of God in Budapest, Hungary
2National Institute of Neurosurgery, Budapest, Hungary
- *Corresponding Author:
- Balázs Cs MD. PhD. DSc.
Department of Medicine & Endocrinology
Teaching Hospital of St. John of God in Budapest
Frankel l. Str. H-1027 Budapest, Hungary
E-mail: drbalazs@irgalmas.hu
Received November 03, 2011; Accepted December 09, 2011; Published December 23, 2011
Citation: Balázs Cs, Korányi K (2011) Primary Prevention of Thyroid Associated Ophthalmopathy by Pentoxifylline. J Addict Res Ther 2:118. doi:10.4172/2155-6105.1000118
Copyright: © 2011 Balázs Cs, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Prospective controlled study was designed for comparing the influence of methimazole (MMI) alone (control group) and MMI+ pentoxifylline (PTX) with respect to grades of thyroid associated orbitopathy (TAO). Control group of patients consisted of 112 patients with hyperthyroidism (mean aged 44.0+/-12.4 yr, 83 female and 29 male). PTX treated group of 112 (mean aged 47.7 +/-10.2 yr, 83 female and 29 male) hyperthyroid patients were treated with MMI+ PTX. At the onset of study there was no remarkable differences between control and PTX treated groups. After six and twelve month observation period the manifestation of TAO with moderate and severe forms were significantly lower in PTX treated patients. Various risk factors were analyzed in both groups. Smoking by itself without genetic factors greatly increased the risk of TAO (OR: 7.1: CI 95% 9.3-5.4). If the smoking habit was associated genetic background, the manifestation of TAO significantly increased (OR: 9.2 CI 95%, 12.1 to 6.9, p< 0.0001). PTX therapy had a beneficial preventive effect on manifestation of eye symptoms and decreased the number of patients both in smokers with and without genetic susceptibility (OR: 2.62 CI 95%, 1.5-3.7, p
Keywords
Thyroid associated ophthalmopathy; Graves’disease; Prevention; pentoxifylline; Prospective controlled study
Introduction
Thyroid associated ophthalmopathy (TAO) is a troublesome manifestation of Graves’ disease is not infrequently difficult to manage [1]. Even the symptoms of moderate and severe forms have a negative impact on quality of life of the patients [2]. TAO is considered to be genetically determined autoimmune disorder by infiltration of lymphocytes and enlargement of extra-ocular muscle, accumulation of glycosaminoglycan (GAG) resulting a clinical manifestation of edema, proptosis, diplopia and optical nerve compression [3-5]. The activated lymphocytes have been shown to secrete several cytokines including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL- 1) and interferon gamma (IFN-γ) which are able to express HLADR antigens and stimulate fibroblasts to proliferate, produce GAG and free oxygen radicals. The cytokines can result in induction and perpetuation of autoimmune processes in the retrobulbar tissue [6-8]. The aim of therapy to inhibit the production of inflammatory cytokines by various methods including mostly used corticosteroid treatment and/or retrobulbar irradiation. Recently, it has been clear that the patients with active stage are likely to respond to medical treatment, whereas such therapy is unlikely to be of benefit in patients with inactive stage, therefore, the follow up studies underlined the importance of prevention of manifestation of eye symptoms in the early stage. Some factors are known to actually increase the risk of incidence and severity of TAO. These factors can be divided into two main categories. The unpreventable category consists of sex, age and genetic background. The preventable risk factors encompasses the radioiodine therapy, cigarette smoking [9-11]. Unfortunately, the majority of patients can not able to stop smoking, therefore, its precipitating effect remained during thyrostatic treatment. We have previously demonstrated that pentoxifylline (PTX) therapy resulted in the improvement of mild and moderately severe forms of TAO [12]. PTX exerts its effects by inhibiting the elaboration of cytokines by orbit-infiltrating T lymphocytes. PTX thus prevents the proliferation of orbital fibroblasts, the production of GAG and their evolution to adipocytes [12,13]. We have undertaken a prospective, randomized study to assess the potential preventive effect of PTX used at time of diagnosis in hyperthyroid patients with Graves ’disease. We have tested the clinical symptoms and laboratory data of patients to answer whether PTX can prevent or inhibit progression of TAO and this beneficial effect extends to those patients who continue to smoke, since smoking is the most obvious environmental risk factor for TAO.
Patients and Methods
Diagnosis of Graves’ disease was made by conventional criteria with additional other examinations including radio-isotopic thyroidal diffuse pattern of uptake. The level of TSH, FT4, FT3 was measured by Elecsys 2010 (Roche) (normal range TSH.0.27-4.2 IU/l, FT4 0.71- 1.85 pmol/l, FT3 1.45-3.48 pmol/l, respectively). The diagnosis was confirmed by testing the presence of anti-TSH-R antibodies in patients’ sera measured by competitive radio-receptor assay kit (TRAK) (Brahms Berlin Chemie) (normal range: 0-2 IU).
Study design
We recruited the newly diagnosed patients with Graves’ disease during a period ot January 2005 to December 2010 from the outpatient clinics for endocrinology. PTX with respect to grades of TAO and remission rates of hyperthyroidism at onset midpoint and the end of 12 months of treatment were investigated. Since for prospective analysis is useful in planning stages of a study to determine how large sample size should be in order to obtain a desired power in test of hypothesis (Wright, Hoenig). The sample size of study was determined by statistical power analysis used JMP (Statistics and Graphics Guide, Version 5, SAS Institute Inc. Cary, NC, USA, 2002 by DEO menu) (α=0.05). One arm was the MMI+ placebo (control group) and the other MMI+ PTX. After review of eligibility and consent participants, the research nurse kept the randomization list until the end of the study by computer-generated program and was in charge of dispensing the study medication or placebo to the patients. The placebo medication was identical in appearance and taste to the active medication, the bottle indicated which medication to take each day. Through the study neither patients nor investigators were aware of treatment assignment. Double blind placebo randomized study has designed for comparing the influence of MMI+ placebo and MMI+ Ptx with respect to grades of TAO. The patients of the control group consisted of 112 patients with hyperthyroidism (mean aged 44.0 +/- 12.4 yr) (83 female and 29 male). Of these patients the hyperthyroidism relapsed in 21 cases. The PTX treated group of 112 (mean aged 47.7+/-10.2 yr, 29 male and 83 female) hyperthyroid patients (with 9 relapsed hyperthyroidism) was treated with MMI+ PTX. None of patients was treated previously radioiodine. In control group 56 patients were smokers, in 39 cases were found cumulation of autoimmune thyroid diseases including Graves’ and Hashimoto’s thyroiditis and/or type 1 diabetes mellitus. The PTX treated group contained 58 smokers and in 35 patients were positive family history for autoimmune thyroid diseases.
Follow-up frequency
The control examinations were two monthly or more if clinically indicated. At these times thyroid function and immunological tests, eye examination and goiter palpation, ultra- sonography were carried out. At the time of recruitment, midpoint at the end of study the following investigations were made: determination of TSH and thyroid hormones, anti-thyroid antibodies, blood glucose was made routinely. Grading of objective and subjective eye findings by “blinded” ophthalmologists were made in each patient. If was necessary, other examinations including MRI or orbits were made.
Side-effects and study modification
All adverse effects, both expected or unexpected were reported to a central independent monitor within one week. If TAO has progressed and appeared the severe symptoms of TAO the PTX therapy, it was obligatory to report to the monitor who consulted with an Investigators’ Resource Group (IRG) and recommended to drop patient(s) from the study and managed by the conventional therapy.
End points
Change in TAO were detected during one year observation compared to onset, at mid-point and at the cessation of therapy.
Definition of smoking status
Smoking was defined as having smoked more than 20 cigarettes per day. Further quantification of smoking habit was not made due to uncertainties. Those who had never smoked or who had stopped for more than five years before the time of study were considered to be nonsmokers.
Exclusion criteria
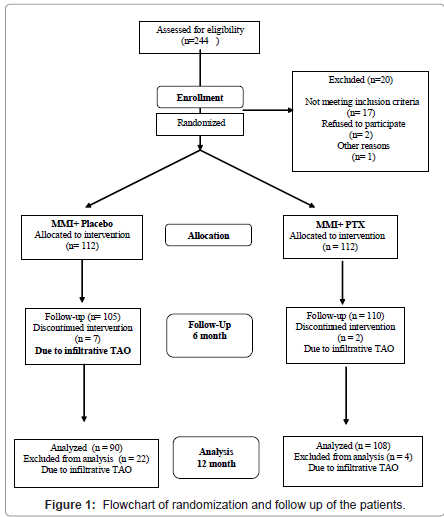
Patients with severe TAO at the time of inclusion and female of reproductive age taking contraceptives were excluded. The study was permitted with local ethical committee and it was in accordance of Helsinki Declaration. Follow-up examinations were made two monthly or more frequently if clinically were indicated. At these times thyroid function was measured, goiter palpated and eye examined as indicated. If it was necessary other investigator(s) were involved. The TAO was estimated of ATA criteria, the mild TAO was categorized by only sign or 2a. Moderate form was diagnosed by 2b3a4o5o6o [14,15]. At the time of recruitment and on each follow up visit examinations for grading of objective and subjective eye findings by independent (“blinding”) ophthalmologists were carried out. Severe forms of TAO proved to be 2c3b or worse were excluded from the study and high dose methylprednisolone pulse therapy was made (1000 mg/day for three consecutive days). In two patients the corticosteroid pulse treatment was completed with retrobulbar irradiation (2.0 Gray/day, total dose 20 Gray).All patients were treated with MMI (mean 30 mg/ day). Patients were treated with PTX of 1200 mg/day until the end of study. If subclinical symptoms of hypothyroidism appeared, the thyroxine substitution (Euthyrox, Merck, Darmstadt, Germany), was made (mean dose in group A 75 +/- 12 μg/day, in B group 60 +/- 9.5 μg/day, respectively). Side effects, all adverse effects of expected and unexpected were regulatory monitored and reported to a central independent monitor within one week. If TAO progressed in severity, or unexpected symptoms appeared in 19 patients of both groups the study was discontinued (Figure 1).
Laboratory methods
If the clinical symptoms indicated, MRI and ultrasonography were made. In all visits the following hormones (including TSH, FT4 and FT3) and immunological parameters were determined: anti-TSH-receptor autoantibodies by TRAK (Brahms Berlin Chemie, Germany) (normal range: 0-2 IU) anti-thyroid peroxidase and antithyroglobulin autoantibodies by Elecsys 2010 (Roche) (normal range were 0.00-63 IU/l and 0.00-60 IU/l, respectively).
Ultrasonography
The thyroid volume was determined, by real-time ultrasonography in patients lying supine with the neck hyper-extended, at the beginning, mid-point and at the end of study. Measurements were carried out by the same experienced investigator using a Hitachi EUB-405 scanner with a 7.5 MHz, 6.25 cm linear transducer (Hitachi Medical Co, Tokyo, Japan). The length (L), width (W) and depth (D) of each thyroid lobe expressed in centimeters was estimated by a modified formula of the ellipsoid (vol= L x W x D x 0.4799) [16]. The within-day mean coefficient of variation was 5.5% and the between day mean coefficient of variation was 6.7%.
Statistics
Statistical analysis was performed using Stat View statistical software package (Version 4.5, SAS Institute Corp., North Carolina, USA). Descriptive statistics, Pearson χ2 and Fischer exact tests, ANOVA and odds ratios (OD) were used as appropriate. P values< 0.05 were considered significant. OR test based 95% confidence interval (CI). The sample size of study was determined by statistical power analysis used JMP (Statistics and Graphics Guide, Version 5, SAS Institute Inc. Cary, NC, USA, 2002 by DEO menu) (α= 0.05) [17,18].
Role of the study sponsor
Neither funding source from any pharmaceutical factories had a role in the collection, analysis, interpretation of the data or in the decision to submit this paper for publication.
Results
| Control group | PTX group | |||
|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |
| TRAK(U/l) | 7.1±0.41 | 3.6±0.23 | 8.9±0.4 | 2.3±0.14* |
| FT4 (pmol/l) | 4.1±0.16 | 1.13±0.03 | 4.2±0.16 | 1.19±0.03 |
| FT3 (pmol/l) | 6.5±0.23 | 2.23±0.07 | 6.3±0.48 | 2.29±0.14 |
| TSH (mU/l) | 0.13±0.01 | 2.9±0.04 | 0.12±0.01 | 3.2±0.08 |
Table 1: Results of laboratory parameters at study initiation and completion * p<0.05.
| CONTROL GROUP | No signs | Only signs | Moderate | Severe | |
|---|---|---|---|---|---|
| Smokers (n=54) | 3 | 18 | 20 | 13 | |
| Non-smokers (n= 58) |
23 | 19 | 14 | 12 | |
| Statistical analysis |
n.s. | p= 0.0002 | p= 0.0001 | ||
| PTX GROUP | No signs | Only signs | Moderate | Severe | |
| Smokers (n=54) | 24 | 16 | 10 | 4 | |
| Non-smokers (n= 58) |
9 | 8 | 0 | ||
| Statistical analysis |
p<0.07 | p<0.49 | p< 0.06 |
Table 2: Effect of pentoxifylline on manifestation of TAO after 12 month therapy.
| ATTRIBUTE CONTROL | OR (95%) | p |
|---|---|---|
| Smoking (n= 56) | 7.1 (9.3-5.4) | < 0.003 |
| Smokinkg+ FH of AITD (n=39) | 9.2 (6.9-12.1) | <0.001 |
| ATTRIBUTE PTX TREATMENT | OR (95%) | p |
| Smoking (n=58) | 2.12 (1.5-3.1) | 0.037 |
| Smoking+ FH of AITD (n=35) | 2.62 (1.5-3.7) | 0.022 |
FH= familiar history of autoimmune diseases.
AITD= autoimmune thyroid disorders.
Table 3: Effects of categorial attribution on the odds for GO.
Both groups were treated by MMI and at the end of one year all patients were in euthyroid stage. There was not found remarkable difference in the TSH. FT4, FT3 between control and PTX treated groups (Table 1). Titers of anti-thyroglobulin and anti-thyroid peroxidase autoantibodies were not significantly elevated at the beginning of MMI therapy and did not change during therapy. In contrast, the level of anti-TSH-receptor autoantibodies decreased in both groups after one year therapy but remained significantly higher in control group in comparison to PTX treated patients (3.6±0.23 and 2.30.13, respectively, p<0.05). At the onset of study there was no remarkable differences in clinical symptoms and laboratory data between control and PTX treated groups. After 12 month observation period the manifestation of TAO with moderate and severe forms were significantly lower in PTX treated patients (Table 2). Various risk factors including smoking habit, genetic background (one or more patients with autoimmune thyroid disorders in the family) and diabetes mellitus (type 1) were analyzed in both groups (Table 3). Smoking by itself without genetic factors greatly increased the risk of TAO (7.1 CI 95% 9.3-5.4, p<0.001). If the smoking habit was associated genetic background the manifestation of TAO significantly increased (OR 9.2 CI 95%, 12.1-6.9, p<0.0001). PTX therapy had a beneficial preventive effect on manifestation of eye symptoms and decreased the number of patients with severe TAO. In smokers with and without genetic susceptibility the OR decreased (OR: 2.62 CI 95%, 1.5-3.7 and 2.12 95% CI 1.5 to 3.1, respectively. The number of moderate and severe forms of TAO was significantly higher in the control group in comparison to the PTX treated group. In addition, diabetes mellitus (1 or 2 type) was observed in 10 cases of the control group and 4 patients of PTX untreated group. The volume of thyroid glands in smokers were significantly larger than non-smokers (24.3 ± 3.2 ml vs. 19.2 ± 2.4 ml, p<0.05). The volume of the goiter at the end of study was smaller in PTX treated group than in non-treated group, however, this difference proved not to be significant. Side effects of PTX with transient nausea was observed in ten cases (it has not indicated to stop the treatment).
The cost effectiveness
One of the most common arguments against the clinical use of a drug has been that the new therapy is not cost-effective. Significant difference in the number of severe TAO was observed between the control group and PTX treated group. Irrespective of quality of life, the average cost for 1 patient with severe TAO including diagnostic procedures and pulse corticosteroid therapy Є 200-250 per year in Hungary. The mean cost of PTX treatment Є 45-50 per year. Krassas provided evidence that the cost for TAO in European countries are very different (from Є 23- Є 10200) depending on the severity and the protocol of treatment [40]. There is an apparent advantage of PTX preventive therapy in Graves’ patients, especially in those patients who are not able to discontinue smoking habit [19,20].
Discussion
The effectiveness of treatment depends on various factors including activity of inflammatory processes and severity of eye symptoms. The severity and activity are not synonymous [27,28]. It has been clear that the patients with active stage are likely to respond to medical treatment, whereas such therapy is unlikely to be of benefit in patients with inactive stage [21]. If TAO is severe, the activity of eye disease is established it can be treated either medically (by high dose corticosteroids or/and orbital radiotherapy) or surgically [21- 23]. In spite of new possibilities, the management of TAO represents a difficult task that does not constantly provide favorable results and causes disfigure as well as an impairment of quality of life [20,23]. Anti-cytokine antibodies, IL-1 receptor antagonist were published to inhibit the GAG synthesis of retrobulbar fibroblasts (REF) [14,24]. Furthermore, recently it was observed that superoxide radicals generated in culture media were able to stimulate REF. MMI as a free radical scavenger drug inhibited superoxide-induced proliferation in a dose-dependent manner [24]. Since the different methods and drugs used for treatment of TAO are not able to cure completely the inflammatory symptoms of TAO and have potential serious side effects or proved to be very expensive, therefore, an effort was made to find new cytokine antagonists interfering with cytokine synthesis, receptor binding or signal transduction [25]. Previously, we published the beneficial effect of PTX in patients with TAO [12]. PTX (1-/5-oxohexyl)-3,7-dimethylxantine) has been widely used for the treatment of chronic occlusive arterial disease because of its rheological action. The effectivity of this drug has been attributed to its influence on erythrocyte deformability, platelet reactivity and plasma viscosity, prostacycline release [1]. Like other methylxanthines, PTX inhibits phospho-diesterase, resulting in a significant increase of intracellular cyclic adenosine mono-phosphate (cAMP), which is known to modulate a number of cellular immune functions [2,3]. It was published that PTX is able to inhibit inflammatory processes including phagocytosis and superoxide anion and nitric oxide (NO) production by polymorphonuclear granulocytes and monocytes [4,5]. This drug has been reported to be an effective drug on T lymphocytes by modulating production of various cytokines involved into immune and autoimmune reactions [6-9]. Recently, the immune modulating effects of PTX was investigated in a randomized double-blind study comparing PTX with placebo in 140 patients receiving cadaveric kidney grafts under cyclosporine and prednisolone treatment [38]. It was found that PTX weakened the consequences of rejection on graft survival and this phenomenon was mediated by reduction of TNFα in sera of transplanted patients [1,36-38]. Furthermore, PTX influenced the cytokine-induced fibroblast proliferation. It was found that PTX exerted a robust inhibitory effects on fibroblast proliferation, extracellular matrix synthesis and myofibroblast differentiation [13]. In addition, Finamor et al. in their prospective randomized trial studied the effect of PTX on quality of life of patients with TAO [41]. They found significant improvement both in proptosis and the quality of life after 6 month of PTX therapy. Although the favourable effects of PTX the randomized monotherapy for patients with TAO might have been effective, however, the administration due to ethical considerations was questionable. It is known that the corticosteroid and retrobulbar irradiation proved to be successful in the active phase (“net eye”) of TAO. If this “honey moon” period is over the consequences of eye symptoms might be potential irreversible by the other recommended forms of therapy. It is important to note that systemic corticosteroid therapy was insufficient approximately 40% in whom TAO had already been present for more than one year [28,29]. Similarly, other studies have shown that little effect was seen on TAO by systemic corticosteroid or radiotherapy if the disease persisted for more than one year [27-29]. This critical period between manifestation of TAO and initiation of therapy can also lead to an unfavourable response to therapy. Prummel et al. showed that orbital irradiation was equally effective as corticosteroid therapy and the efficacy in both treatment groups was 50% [27]. Furthermore, it was observed that although soft tissue signs and eye muscle motility improved with corticosteroid and irradiation therapies, however, the exophthalmos did not. Therefore, it was concluded the primary, secondary and tertier preventions are recommended for all Graves’ patients [10]. Our study provided evidence that PTX resulted in significant decrease in manifestation of severe and modest forms of TAO in patients with high risk including smoking habit and genetic background. The precise mechanism of this observation is not clarified. The role of smoking in the induction of immune-and autoimmune reactions is substantially studied [29-33]. The inhibitory effect of PTX on proinflammatory cytokines might be the responsible for preventive effect on manifestation of eye symptoms. The significant decrease of anti-TSH-R autoantibodies also can be explained by this immune modulating effect of PTX [34-38]. In our pilot study we found “nonresponders” to PTX and in 4 patients the PTX therapy was not able to prevent the development of severe form of TAO. The cause of this failure is not known, the following possibilities are arisen: firstly, lack of compliance from these patients, secondly, the turnover of PTX in some individual is higher than in the control population possibly due to genetic reasons, thirdly, the potential catalytic antibody against the PTX might neutralize the immune modulating effect of this drug [37,39]. It is concluded, that PTX of therapy in prevention of TAO is found a relatively cheap drug without serious side effects. The question is whether PTX therapy is recommended for all patients with Graves’ disease. We think that actually not to do it, because the manifestation of TAO not more than 50% and in a smaller number of patients (10- 20%) precedes the symptoms of hyperthyroidism. However, PTX therapy is considered for all patients who refuse to give up smoking habit and have positive immune genetic background to avoid the irreversible consequences of TAO.
References
- Bartalena L, Marcocci C, Tanda ML, Piantanida E, Lai A, et al. (2005) An update on medical management of Graves' ophthalmopathy. J Endocrinol Invest 28: 469-478.
- Prummel MF, Bakker A, Wiersinga WM, Baldeschi L, Mourits MP, et al. (2003) Multi-center study on the characteristics and treatment strategies of patients with Graves’orbitopathy: the first European Group on Graves’ orbitopathy experience. Eur J Endocrinol 148: 491-495.
- Ludgate M, Baker G (2002) Unlocking the immunological mechanism of orbital inflammation in thyroid eye disease. Clin Exp Immunol 127: 193-198.
- Mack WP, Stasior GO, Cao HJ (1999) Ophthalmic Plastic and Reconstructive Surgery. 15: 260-271.
- Weetman AP, Wiersinga WM (1998) Current management of thyroid associated ophthalmopathy in Europe. Results of an international survey. Clin Endocrinol 49: 21-28.
- Weetman AP (2000) Graves’disease. N Engl J Med 343: 1236-1248.
- Krassas GE, Heufelder AE (2001) Immunosuppressive therapy in patients with thyroid eye disease: an overview of current concept. Eur J Endocrinol 144: 311-318.
- Bartalena L, Marcocci C, Pinchera A (2002) Graves’ophthalmopathy: a preventable disease? Eu J Endocrinol 146: 457-461.
- Bonnema SJ, Bartalena L,Toft AD, Hegedüs L (2002) Controversies in radioiodine therapy: relation to ophthalmopathy, the possible radioprotective effect of antithyroid drugs, and use in large goitres. Eur J Endocrinol 147: 1-11.
- Wiersinga WM, Bartalena L (2002) Epidemiology and prevention of Graves’ophthalmopathy. Thyroid 12: 855-860.
- Bartalena L, Marcocci C., Pinchera A (2002) Graves’ ophthalmopathy: a preventable disease? Eur J Endocrinol 146: 457-461.
- Balázs C, Kiss E, Vámos A, Molnar I Farid NR (1997) Beneficial effect of pentoxifylline on thyroid associated ophthalmopathy (TAO): a pilot study. J Clin Endocrinol Metab 82: 1999-2002.
- Balázs Cs, Kiss E, Farid NR (1998) Inhibitory effect of Pentoxifylline on HLADR expression by retrobulbar fibroblasts. Horm Met Res 30: 496-499.
- Tan GH, Dutton CM, Bahn RS (1996) Interleukin-1 (IL-1) receptor antagonists and soluble IL-1 receptor inhibit IL-1 induced glycosaminoglycan production in cultured human orbital fibroblasts from patients with Graves’ophthalmopathy. J Clin Endocrinol Metab 81: 446-452.
- Wiersinga WM, Prummel MF, Mourits MPh, Koornneef L, Buller HR (1991) Classification of the eye changes of Graves’disease. Thyroid l: 357-360.
- Brunn J, Block U, Ruf G (1981) Volumetrie der Schilddrüssenlappen Mittels Real-time Sonography. Dtsch Med Wochenschr 106: 1338-1340.
- Wright SP, O’Brien RG (1988) “Power Analysis in an enhanced GLM procedure. What is might look like” Proceedings of the Thirteenth Annual Conference, Cary NC: SAS Institute Inc. 1097-1102.
- Hoenig JM, Dinnis MH (2001) The abuse of power: The pervasive fallacy of power calculation for data analysis. American Statistician 55: 19-24
- Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB (1996) Recommendation of the panel on cost-effectiveness in health and medicine. JAMA 276: 1253-1258.
- Terwee CB, Dekker FW, Mourits MP, Gerding MN, Baldeschi L, et al. (2001) Interpretation and validity of changes in scores on the Graves’ophthalmopathy quality of life questinaire (GO-QOL) after different treatments. Clin Endocrinol 54: 391-398.
- Wiersinga WM, Bartalena L (2002) Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid 12: 855-860.
- Wiersinga WM, Prume MF (2001) Pathogenesis of Graves’ ophthalmopathy current under-standing. (editorial). J Clin Endocrinol Metab 86: 501-503.
- Gerding MN, Terwee CB, Dekker FW, Koornneef L, Prummel MF, et al. (1997) Quality of life in patients with Graves’ophthalmopathy is markedly decreased: Measurement by the Medical Outcomes Study Instrument. Thyroid 7: 885- 889.
- Balázs Cs, Kiss E, Leövey A, Farid NR (1986) The immunosuppressive effect of Methimazole on cell-mediated immunity is mediated by its capacity to inhibit peroxidase and to scavenge free oxygen radicals. Clin. Endocrinol (Oxf) 25: 7-16
- Balázs Cs, Kiss E (1994) Immunological aspects of the effects of pentoxphylline (Trental) Acta Microbiol. Immunol. Hung 41: 121-128.
- Hatton MP, Rubin PAD (2002) The pathophysiology of thyroid associated ophthalmopathy. Opthalmol Clin N Am 15: 113-119.
- Prummel MF, Mourits MP, Blank L, Berghout A, Koornneef L, et al. (1993) Randomized double-blind trial of prednisone versus radiotherapy in Graves’ ophthalmopathy. Lancet 342: 949-954.
- Prummel MF, Mourits MP, Berghout A, Krenning EP, van der Gaag R, et al. (1989) Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopath. N Engl J Med 321: 1353-1359.
- Noth D, Gebauer M, Müller B, Bürgi U, Diem P (2001) Graves’ophthalmopathy: natural history and treatment outcomes. Swiss Med Wkly 131: 603-609.
- Balázs Cs, Stenszky V, Farid NR (1990) Association between Graves’ ophthalmopathy and smoking. Lancet 336: 754-755.
- Vestergaard P (2002) Smoking and thyroid disorders- a meta-analysis. Eur J Endocrinol 146: 153-161.
- Hegedüs L, Karsrup S, Veiergang D, Jacobsen B, Skovsted LIS (1985) High frequency of goitre in cigarette smokers. Clin Endocrinol 22: 287-292.
- Bartalena L, Marcocci C, Tanda ML, Manetti L, Dell’Unto E (1998) Cigarettesmoking and treatment outcomes in Graves’ ophthalmopathy. Annals Intern Med 129: 632-635.
- Balázs Cs (2002) Pentoxiphylline in the menagement of Graves’ orbital disease. In: Thyroid Eye Disease, Ed. Dutton MD. Marcel Dekker, Inc. New York 443-449.
- Marcinkiewicz J, Grabowska A, Lauterbach R, Bobek M (2000) Differencial effects of pentoxifylline, a non-specific phosphodiesterase inhibitor, on the production of IL-10, IL-12 p40 and p35 subunits of murine perinoneal macrophages. Immunopharmacology 49: 335-343.
- Raetsch C, Jia JD, Boigk G, Bauer M, Hahn EG, et al. (2002) Pentoxifylline downregulates profibrogenetic cytokines and procollagen I expression in rat secondary biliary fibrosis. Gut 50: 241-247.
- Briggs WA, Eustace J, Mathew S, Gimenez LF, Choi M, et al. (1998) Pentoxifylline potentiates in vitro lymphocyte suppression by glucocorticoids and immunosuppressive drugs. J Clin Pharmacol 38: 561-566.
- Hewitson TD, Martic M, Kelynack KJ, Pedagogos E, Becker GJ (2000) Pentoxifylline reduces in vitro renal myofibroblast proliferation and collagen secretion. Am J Nephol 20: 82-88.
- Nishi Y (2002) Evolution of catalytic antibody repertoire in autoimmune mice. J Immunol Methods 268: 213-233.
- Krassas GE (2004) The cost of immunosuppressive therapies currently used in patients with thyroid eye disease. J Endocrinol Invest 27: 919-923.
- Finamor FE, Martins JRM, Nakanami D, Paiva ER, Manso PG, et al. (2004) Pentoxifylline (PTX)-An alternative treatment in Graves’ ophthalmopathy (inactive phase): Assessment by a disease specific quality of life questionnaire and by exophthalomomemtry in a prospective randomized trial. Eur J Ophthalmology 14: 277-283.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14299
- [From(publication date):
December-2011 - Jun 30, 2024] - Breakdown by view type
- HTML page views : 9944
- PDF downloads : 4355