Plasticity in the Primary Auditory Cortex, Not What You Think it is: Implications for Basic and Clinical Auditory Neuroscience
Received: 04-Jun-2012 / Accepted Date: 06-Mar-2012 / Published Date: 12-Mar-2012 DOI: 10.4172/2161-119X.S3-002
Abstract
Standard beliefs that the function of the primary auditory cortex (A1) is the analysis of sound have proven to be incorrect. Its involvement in learning, memory and other complex processes in both animals and humans is now wellestablished, although often not appreciated. Auditory coding is strongly modified by associative learning, evident as associative representational plasticity (ARP) in which the representation of an acoustic dimension, like frequency, is re-organized to emphasize a sound that has become behaviorally important. For example, the frequency tuning of a cortical neuron can be shifted to match that of a significant sound and the representational area of sounds that acquire behavioral importance can be increased. ARP depends on the learning strategy used to solve an auditory problem and the increased cortical area confers greater strength of auditory memory. Thus, primary auditory cortex is involved in cognitive processes, transcending its assumed function of auditory stimulus analysis. The implications for basic neuroscience and clinical auditory neuroscience are presented and suggestions for remediation of auditory processing disorders are introduced.
Keywords: Acetylcholine, Cognitive processes, Learning, Learning strategy, Memory code, Receptive field
257151Introduction
A central goal of neuroscience is to discover the functions of brain structures. However, primary sensory cortices (auditory, somatosensory and visual) have been exempt from this quest because their functions presumably have always been known, viz., to analyze their respective sensory stimuli. The analytic function of primary sensory fields is so fundamental to neuroscience that it has remained unquestioned in the face of decades of evidence to the contrary.
During the past twenty years, the discovery and elaboration of associative representational plasticity in the primary auditory cortex (A1) of humans and non-human animals alike has produced a crisis for this standard model. As workers increasingly amass evidence that A1 is deeply involved in “cognitive” functions, such as attention, learning, memory concept formation and problem solving, it has become incontrovertible that the behavioral importance of sounds involves a systematic reorganization of the representation of fundamental acoustic parameters, such as frequency and stimulus level.
This brief review provides a concise, updated account of salient findings about basic learning/memory and the primary auditory cortex. Several prior scholarly reviews with different emphases are available (e.g., [1–6]). The purpose of the current account is to inform the community about some counterintuitive findings regarding auditory cortical plasticity, rather than to provide a comprehensive review. Therefore, citations are kept to a minimum and largely confined to the first report of a particular type of finding. An equally important goal is to discuss implications of the findings for both basic and clinical auditory neuroscience.
Primary Auditory Cortex as an Acoustic Analyzer
The traditional model, which remains dominant, holds that the primary auditory field is purely an acoustic analyzer. That is, its function is essentially to respond selectively to particular physical parameters of sound, e.g., sound frequency, sound level, locus in space, amplitude and frequency modulation, spectral bandwidth, stimulus duration,repetition rate, etc. Relationships between acoustic parameters and cellular responses provide the bedrock of auditory neuroscience, both in the auditory cortex and the subcortical auditory system. Their foundational importance for understanding the neural bases of hearing is unquestionable. However, as will be seen, this is not the entire story.
The clear enunciation of the “pure acoustic analyzer” account may be traced to the early 20th century. It holds that sensory analysis and sensory comprehension must be performed in separate parts of the brain. The source philosophical and psychological considerations that fostered such false certainty need not concern us here. Rather, we can conveniently refer to the highly influential monograph of Campbell [7]. He performed histological analyses of the cerebral cortex of man and other animals and believed that function could be ascertained on the basis of the appearance of cortical layers and related anatomical considerations. Campbell concluded that the function of the primary sensory cortical fields was “sensory-analytic” while the function of “higher” adjacent sensory fields was “sensory-psychic”, i.e., concerned not with analysis but with discerning the comprehension and psychological meaning of sensory stimuli. In so doing, he was instrumental in effectively “removing” learning, memory and other cognitive processes from primary sensory cortices [8]. These “psychic” auditory fields are better known today as “belt” areas [9].
With the advent of electrophysiology in the 1920s–30s, it became possible to seek cortical regions that responded (e.g., evoked potentials) to sound, and eventually to successfully demarcate A1 and its cochleotopic and tonotopic frequency organization. Thus, neighboring cells are tuned best to neighboring acoustic frequencies in A1 (e.g., [10]). Following World War II and continuing to the present, auditory neurophysiology expanded greatly. The coding of acoustic parameters by single neurons and groups of cells in many species has yielded the functional organization of auditory fields adjacent to A1 and refinement of the latter’s tonotopic organization, as well as providing insights into other stimulus domains, e.g., binaural interactions, bandwidth, temporal and spectral modulations. That cortical responses to a given set of acoustic parameters were found to be highly reliable provided empirical support for the “pure analysis” account. However, the classical auditory neurophysiological studies were conducted almost exclusively in anesthetized animals. As experiments were extended to waking subjects, it became obvious that the fixed relationship between a stimulus parameter and a cortical response was not as rigid as had been assumed.
Learning and Memory Processes in the Primary Auditory Cortex
From the mid-1950s to the mid-1980s, there was a little-noted parallel line of inquiry into associative learning and the auditory cortex, which necessarily was conducted in waking subjects. Cortical responses to a sound increased when it became a signal for a reward or punishment. For example, Galambos et al. [11] found significant increases in the amplitude of A1 evoked potentials in the cat, when a click was followed by a mild shock. Since that time, scores of laboratories have replicated this finding and extended it to rewards as well as punishments, various types of tasks, numerous species and methods of recording (single units to brain imaging; reviewed in [12,13]). While this basic finding was first established in non-human animals, studies of the human auditory cortex have been confirmatory (e.g., [14–16]) and continue to provide new insights into associative plasticity in the auditory cortex [17].
Despite the indisputable involvement of A1 in learning and memory, auditory neurophysiologists, who studied the coding of physical parameters of sound, largely ignored the findings. This disregard was perfectly rational because standard learning tasks employ only one or two different sounds whereas studies of auditory coding necessarily involve the presentation of many stimuli (e.g., different tones) to yield receptive fields, the fundamental building blocks of sensory processing. Using the restricted stimulus set used by Galambos et al. and their successors could not provide such information. Of course, while further research on learning revealed that stimulus meaning was an important determinant of cortical response, it was never intended to shed light on the basic problems of acoustic coding. Thus, the two lines of auditory coding and the neural bases of learning and memory continued on parallel paths which, like parallel lines, apparently would not intersect short of infinity.
A New Approach: Synthesis of Auditory Coding and Associative Learning
The Galambos et al. study [11], and virtually all subsequent similar experiments until the mid-1980s, had demonstrated “associative neural plasticity” in the adult primary auditory cortex. That is, they had shown that when a sound is associated with another event (usually reward or punishment), its processing in the auditory cortex is changed. Indeed, essentially all electrophysiological studies of learning and memory are concerned with detecting such plasticity, which must exist when knowledge and behavior are changed due to learning. However, unless studies of learning and memory could directly contribute to fundamental research in auditory coding, continued demonstration of learning-based auditory cortical plasticity would be of little utility.
A synthesis of these fields could be accomplished by combining protocols from auditory coding studies with those from learning and memory in a single experiment. Let us assume that we want to find out if the tone-evoked responses of neurons in the auditory cortex change when that tone becomes a signal for an important event, like the availability of food to a hungry subject. The first step would be to simply run an auditory coding experiment protocol, to obtain baseline tuning information. The second step would then be to perform a learning protocol, such as presenting a tone paired with food. The third step would be to repeat the first step, yielding responses to the signal tone and many other tones after learning had taken place. To determine the effects of learning, one need merely subtract the baseline tuning curve from the tuning curve obtained after learning; the difference would be the effects of learning on responses to tones. Short and longterm retention (memory) of any learning-induced change could be determined by repeating the third phase at desired intervals of minutes to days following training.
This type of “unified” experimental design could reconcile the foundational findings of auditory coding studies with the dynamics of everyday experience, in which we learn, either with studied purpose or merely by exposure to our acoustic worlds, that sounds change their meaning due to experience. Language learning certainly comes readily to mind, but auditory learning can affect the psychological or behavioral meaning of any sounds, be they a car horn or music evocative of a mother’s sung lullaby. It is well to constantly bear in mind, then, that hearing is not simply a matter of detecting and analyzing sounds, but also of comprehending them. And since humans are born with little innate information about the meaning of sounds, the vast majority of sounds accrues their meaning by experience, i.e., learning, and maintains their meaning only by neural storage, i.e., memory.
Associative Representational Plasticity: Auditory Coding is Modified by Learning
The first report that auditory learning resulted in a change in auditory coding appeared in 1984 [18]. Cats underwent tone–shock pairing and exhibited rapid learning that the tone predicted the shock. Tuning curves of neurons in “higher” auditory cortical fields in the cat (second auditory cortex and the ventral ectosylvian field) were modified so that responses to the tone were altered whereas responses to other tones were little affected (see also [19,20]). This first demonstration that learning specifically modified sensory receptive fields was given little notice, perhaps because “plasticity” was expected in higher sensory fields, in accord with the 1905 formulation of Campbell [7].
The Zeitgeist was apparently primed for reconciling auditory coding with learning and memory. In 1986, Gonzalez-Lima and Scheich [21], using metabolic methods of determining increased neural activity, reported that tone–shock pairing produced a specific increase in uptake of a metabolic marker in the area of the primary auditory cortex of the rat that processed the frequency of the tone signal. This was the first demonstration that learning specifically modified auditory coding in the primary auditory cortex where such effects had not been anticipated.
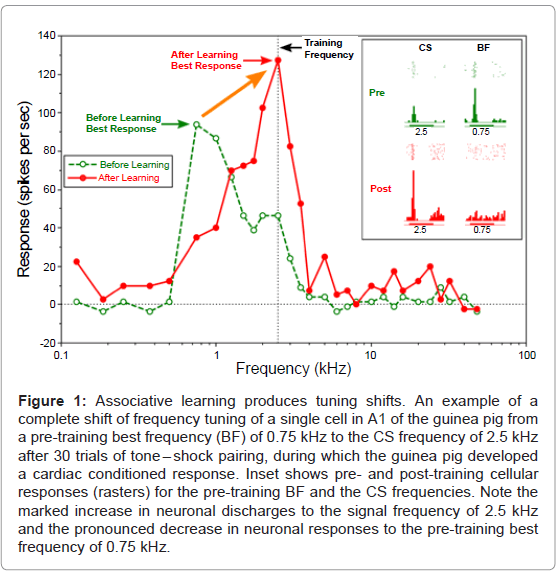
Since that time, electrophysiological experiments on tuning curves (“frequency receptive fields”) have been directed to the primary auditory cortex. The first such study found that when a tone was followed by a shock in the guinea pig, tuning curves were shifted toward, and even to, the frequency of the tone signal [22] (Figure 1).
This “re-tuning” happened in a matter of minutes and required the formation of an actual association between tone and shock, ruling out general excitability as a possible explanation. Moreover, tuning shifts were directed toward the signal frequency not away from it, ruling out random change in tuning.
Studies of the effects of learning on auditory coding initiated a new phenomenon, that of “associative representational plasticity” (ARP). Neural associative plasticity had long been known in the field of learning and memory. Thus, starting with the Galambos et al. [11] experiment, it was clear that when animals learned an auditory-based association (tone → shock), responses to the tone in A1 were increased. The novel aspect of the new line of research was the revelation that learning did not simply change responses to the signal tone, but rather reorganized the processing of a stimulus dimension, in this case, the cortical representation of acoustic frequency. When a particular tone becomes behaviorally relevant by being paired with reinforcement (reward or punishment) a cell’s tuning can shift from its “preferred” frequency to the frequency of the signal tone [5].
Other forms of ARP have also been discovered when animals have to solve different types of auditory problems. For example, tuning shifts (and gain of signal representational area, see next section) typically develop when subjects have merely to detect the signal tone. However, if they have to discriminate the signal tone from among numerous other tones, then responses to the signal can decrease relative to adjacent “side-band” tones, which results in emphasizing the target by “contrast enhancement” [23,24]. Finally, if subjects have to classify numerous physically different sounds into two groups (e.g., rising and falling tones irrespective of their absolute frequency), thus making “categorical” distinctions, then more complex types of neural plasticity are observed [4].
It must be clearly understood that these forms of learning-based specific plasticity in the primary auditory cortex readily develop in the adult. This is of critical importance because the traditional assumption has been that such plasticity can only occur during development, prior to attaining sexual maturity and before the end of a presumed “critical period”. Nonetheless, it is essential to recognize and discard invalid beliefs regardless of how long they have been held and how reasonable they may have seemed. In short, specific learning-based plasticity in the primary auditory cortex is a life-long process.
Follow-up studies of tuning shifts have revealed that the tuning shift form of ARP has the major characteristics of behavioral associative memory: in addition to associativity, it can develop very rapidly (within five trials), is highly specific to the training tone, exhibits consolidation (post-training increased strength over hours and days without further training) and exhibits long-term retention (tracked to eight weeks post-training) [25]. Associative representational plasticity has now been found in a wide variety of tasks (including auditory signaling of reward), in all species tested (e.g., guinea pig, ferret, rat, bat, monkey and human) and for all acoustic stimulus dimensions investigated (e.g., stimulus level, duration tuning, FM envelope, localization in space, repetition rate and tone sequence). Thus, the tuning shift form of ARP may be considered to constitute a basis of auditory memory traces [5]. Other forms of ARP remain to be as thoroughly investigated for their relationships to the characteristics of memory.
Cortical Representational Area as a “Memory Code” for the Importance of a Sound
As noted above, the primary auditory cortex contains a “tonotopic map” in which neurons best tuned to particular acoustic frequencies are adjacent to neurons best tuned to adjacent frequencies, roughly like a keyboard. The number of cells that are best tuned to a given frequency constitutes the area of that frequency representation. It had been assumed that the tonotopic map is fixed, simply reflecting the frequency organization of the cochlea. However, this map is essentially a collection of the tuning of individual neurons. Therefore, as learning modifies frequency receptive fields (tuning curves) in A1 to emphasize behaviorally important sounds, one would expect that learning also modifies tonotopic maps. Specifically, the number of cortical loci that become tuned to a tonal cue during learning should increase, resulting in a gain of representational area. In short, behaviorally important frequencies should become over-represented. This prediction was first supported in a study of frequency discrimination learning in the monkey. The frequencies that had to be discriminated exhibited a greater area of representation in A1 than other frequencies in the same animals or the same frequencies in other (control) animals [26].
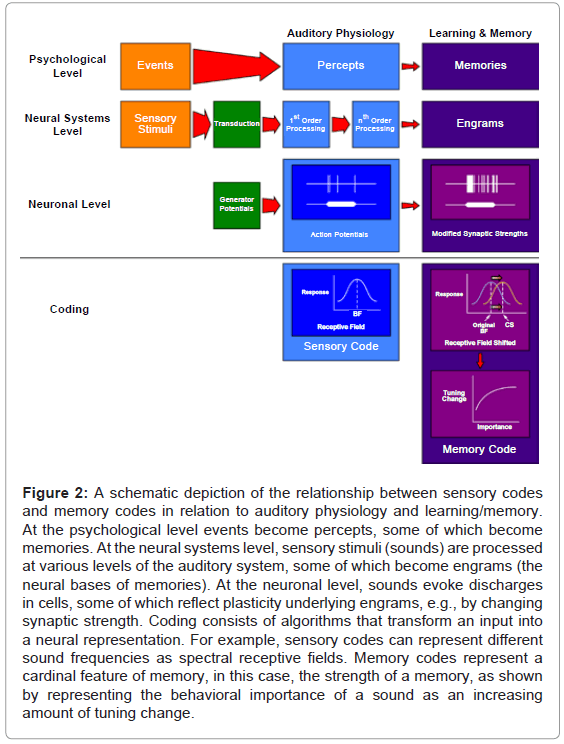
Such findings raised a novel question about neural codes. Sensory codes are known to exist for numerous stimulus parameters, e.g., for many cells, an increase in the number of spikes is proportional to increased acoustic loudness, yielding a neural rate code for acoustic level: the greater the level, the greater the rate of discharge. Is it possible that the brain also employs “memory codes” for cardinal features of stored experience? Figure 2 presents a diagrammatic summary of how a memory code might parallel a sensory code. In the example given, a memory code for the “behavioral importance” of memories of tones is suggested: “the greater the learned importance, the greater the shifting of frequency tuning”. Thus, if a tone became extremely important, the prediction would be that more neurons would shift to its frequency (Figure 2).
Figure 1: Associative learning produces tuning shifts. An example of a complete shift of frequency tuning of a single cell in A1 of the guinea pig from a pre-training best frequency (BF) of 0.75 kHz to the CS frequency of 2.5 kHz after 30 trials of tone – shock pairing, during which the guinea pig developed a cardiac conditioned response. Inset shows pre- and post-training cellular responses (rasters) for the pre-training BF and the CS frequencies. Note the marked increase in neuronal discharges to the signal frequency of 2.5 kHz and the pronounced decrease in neuronal responses to the pre-training best frequency of 0.75 kHz.
Figure 2: A schematic depiction of the relationship between sensory codes and memory codes in relation to auditory physiology and learning/memory. At the psychological level events become percepts, some of which become memories. At the neural systems level, sensory stimuli (sounds) are processed at various levels of the auditory system, some of which become engrams (the neural bases of memories). At the neuronal level, sounds evoke discharges in cells, some of which reflect plasticity underlying engrams, e.g., by changing synaptic strength. Coding consists of algorithms that transform an input into a neural representation. For example, sensory codes can represent different sound frequencies as spectral receptive fields. Memory codes represent a cardinal feature of memory, in this case, the strength of a memory, as shown by representing the behavioral importance of a sound as an increasing amount of tuning change.
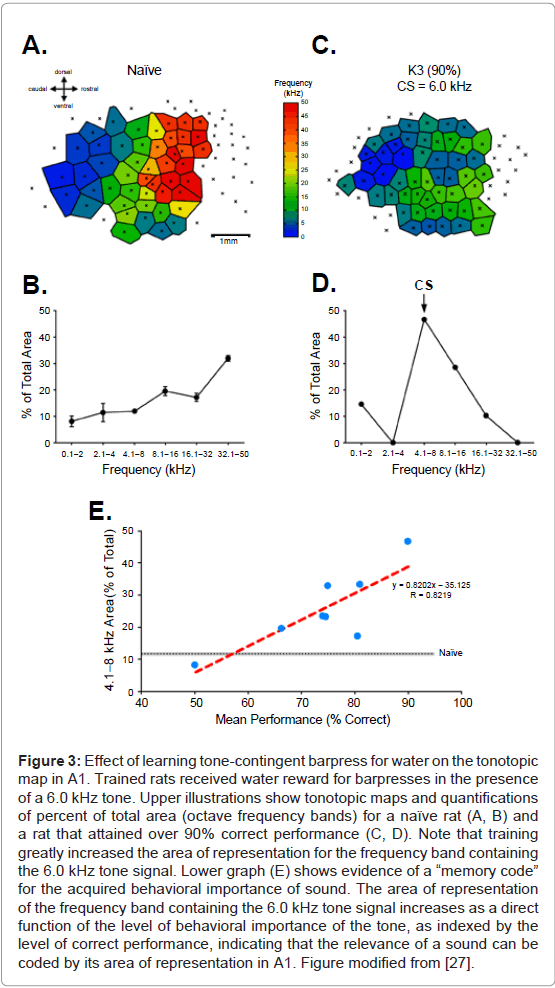
The search for such a memory code began with a study in which rats learned to press a bar to receive water reward, but only in the presence of a 6.0 kHz tone. The behavioral importance of this tone was varied across animals by different levels of water restriction, thus varying the value of the reward across animals. After completion of training, maps of A1 showed an expanded representation for the 6.0 kHz frequency band, as expected. To test for the hypothetical memory code, the amount of expanded tone cue area was compared to the level of tone importance, which was reflected in the level of correct performance: the greater the value of the reward and the greater its importance to the subjects [27]. The findings revealed that the greater the level of behavioral importance, the greater the gain in the tone’s representational area (Figure 3). This relationship supports the proposal that the brain uses memory codes as well as sensory codes, in general, and indicates that the amount of gain in representational area is a likely candidate as a memory code for the acquired importance of sound.
Gain in Representational Area and the Strength of Memory
Support for the presence of a memory code for the learned importance of sound in the primary auditory cortex raised another issue. We know that memories vary in their strength; some experiences are quickly forgotten (like the telephone number of a pizza parlor) while others stay with us for life (like our mother’s name). Memory strength is usually greater for memories that are more important. Could A1 be involved in conferring the strength of auditory memory for more important experiences of sounds?
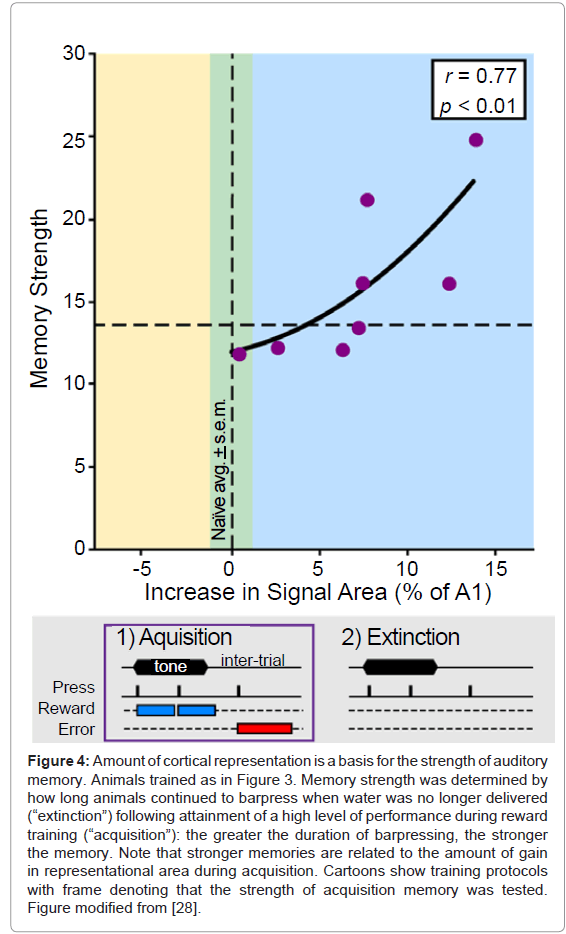
To test this possibility, rats were first trained to press a bar for water only in the presence of a tone. All animals received the same amount of water and were equally thirsty, but, as with humans, rats form memories that differ in strength. After achieving high levels of correct performance, the strength of their memories for the tone was assessed by continuing the standard training protocol, except that no water was given for correct responses during a single test session. This procedure, known as “experimental extinction”, yields an estimate of the strength of the original memory: the more times animals continued to bar press to the (now unrewarded) tone, the stronger their memory that the tone had predicted reward. In short, they continued to expect (believe) that water would be forthcoming.
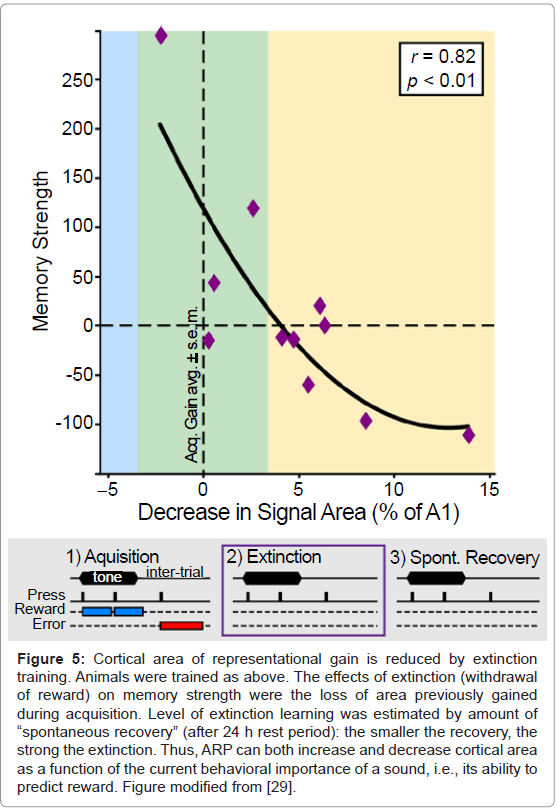
Determination of tonotopic maps after extinction revealed that the slower the extinction (i.e., the stronger the memory), the greater the area representing the frequency of the training tone [28] (Figure 4). The complementary finding was also obtained in another study: the faster the extinction (i.e., the weaker the memory), the greater the loss of area that had previously been gained [29] (Figure 5). Thus, it appears that the primary auditory cortex may be a substrate of memory strength. Although it may seem almost too simple, the findings support the hypothesis that the greater the number of cells that shift their tuning to better emphasize a sound, the stronger will be the memory of that sound. Conversely, memories can be made weaker by reducing the amount of an auditory cue’s representational area.
Figure 3: Effect of learning tone-contingent barpress for water on the tonotopic map in A1. Trained rats received water reward for barpresses in the presence of a 6.0 kHz tone. Upper illustrations show tonotopic maps and quantifications of percent of total area (octave frequency bands) for a naïve rat (A, B) and a rat that attained over 90% correct performance (C, D). Note that training greatly increased the area of representation for the frequency band containing the 6.0 kHz tone signal. Lower graph (E) shows evidence of a “memory code” for the acquired behavioral importance of sound. The area of representation of the frequency band containing the 6.0 kHz tone signal increases as a direct function of the level of behavioral importance of the tone, as indexed by the level of correct performance, indicating that the relevance of a sound can be coded by its area of representation in A1. Figure modified from [27].
Figure 4: Amount of cortical representation is a basis for the strength of auditory memory. Animals trained as in Figure 3. Memory strength was determined by how long animals continued to barpress when water was no longer delivered (“extinction”) following attainment of a high level of performance during reward training (“acquisition”): the greater the duration of barpressing, the stronger the memory. Note that stronger memories are related to the amount of gain in representational area during acquisition. Cartoons show training protocols with frame denoting that the strength of acquisition memory was tested. Figure modified from [28].
Associative Representational Plasticity Depends on “How” an Auditory Problem is Solved
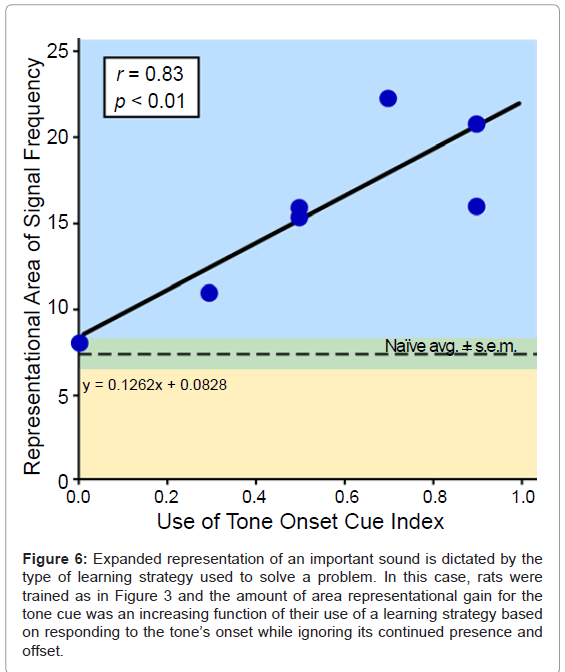
The amount or level of learning, whether about sound or something else, is the “gold standard” for assessing the effectiveness of a training regimen. However true this may be for psychological or behavioral impact, it appears not so for the auditory cortex. While one would assume that auditory learning will involve plasticity in A1, surprisingly, it is not so. Rather, the learning strategy used to solve an auditory problem appears to be critical. Thus, ARP does not form unless animals learn to bar press to a tone for water reward by attending only to the onset of the tone while ignoring its continued presence and its offset [30-32]. In fact, there is a significant relationship between the amount of onset cue use and the representational area of the cue frequency: the greater the onset cue use, the greater the gain in area of the cue tone (Figure 6).
An explanation of this counterintuitive finding is not yet known. However, one possibility is that sensory neurons develop plasticity only for that stimulus parameter to which they respond best, i.e., for the parameter they best encode. It seems that neurons in A1 are particularly sensitive to onset transients [33]. Therefore, as the onset of the training tone contains onset transients, the animals’ use of tone onset could contribute to ARP in A1. However, regardless of the involved mechanism, that learning strategy strongly determines cortical plasticity has considerable implications for clinical remediation of problems of auditory comprehension. We will return to this point later.
Associative Plasticity in the Auditory Cortex: Not What You Think It Is
Campbell’s “sensory analytic” model of A1 appears to be wrong. Unfortunately, most workers still assume that the function of the primary auditory cortex is that of acoustic analysis devoid of acoustic comprehension. How can this position be held in the face of the types of findings summarized above? The answer is simple. Learning-induced plasticity is assumed to be the basis of “perceptual learning”, which is an improvement in perceptual acuity due to experience. Therefore, amending it to include the improvement of auditory perception by experience would save the sensory-analytic model. This belief, in actuality an unconscious assumption that is concordant with common sense and traditional concepts, is so ingrained as to merit little if any discussion.
Figure 5: Cortical area of representational gain is reduced by extinction training. Animals were trained as above. The effects of extinction (withdrawal of reward) on memory strength were the loss of area previously gained during acquisition. Level of extinction learning was estimated by amount of “spontaneous recovery” (after 24 h rest period): the smaller the recovery, the strong the extinction. Thus, ARP can both increase and decrease cortical area as a function of the current behavioral importance of a sound, i.e., its ability to predict reward. Figure modified from [29].
Figure 6: Expanded representation of an important sound is dictated by the type of learning strategy used to solve a problem. In this case, rats were trained as in Figure 3 and the amount of area representational gain for the tone cue was an increasing function of their use of a learning strategy based on responding to the tone’s onset while ignoring its continued presence and offset.
But is the sensory-analytic model saved? Can we avoid recognizing the need for a re-conceptualization of A1? Is it possible to avoid the complications that would ensue if primary auditory cortex also performs cognitive functions such as auditory comprehension and the assignment of meaning to sound?
The evidence indicates “No”. ARP in A1 far transcends perceptual learning, and is rather a likely basis of the auditory signal component of genuine associative learning, i.e., learning that a sound has acquired behavioral relevance. As such, it can serve as a predictor of forthcoming events, such as that aim pending aversive stimulus can be avoided when its occurrence is correctly predicted.
While perceptual learning certainly can develop during training, such increased acuity usually requires increasingly difficult discrimination training [26,34]. Moreover perceptual learning cannot explain the findings. Major findings include: (a) evidence of a memory code for stimulus importance (increased representational area can encode acquired importance of a tone), (b) evidence that A1 is a substrate of and determinant for the strength of auditory memory (the greater the gain in representational area, the more difficult it is to extinguish a memory and the greater the loss of area, the weaker is the memory) and (c) the dependence of the formation of cortical plasticity upon the learning strategy used to solve the auditory problem (A1 develops ARP only if subjects use a learning strategy that involves responding to tone onset while ignoring tone offset).
Therefore, genuine learning, memory and cognitive processes involved in problem solving develop in the primary auditory cortex. The plasticities that develop are in no sense substrates of perceptual learning. Campbell’s assumptions can finally be laid to rest. Primary auditory cortex is not merely an auditory analyzer. We need to reconceptualize A1 as “cognitive auditory cortex”. This will be a challenge but will lead to a more valid and helpful understanding of auditory cortex.
Some implications of this paradigmatic innovation are addressed next.
Implications for Basic Auditory Neuroscience
The involvement of the primary auditory cortex in cognitive functions, particularly learning and memory as reviewed here, has implications for a basic understanding of auditory processing in particular, as well as for the functional organization of the cerebral cortex in general.
Auditory processing
Regarding auditory processing, Campbell [7] was clearly wrong. Although the first studies of the effects of learning on auditory coding in A1 had to wait some 80 years, it is now indisputable that the primary auditory cortex is not confined to the analysis of sounds. Neither can one still maintain that the interpretation, comprehension or acquired meaning of sounds is the domain of “higher” auditory cortical fields only. Therefore, we are faced with the fact that whatever may prove to be the differences in function between primary and so-called secondary/higher auditory cortices, they are not delineated along the lines of the traditional division of labor so elegantly encapsulated by Campbell’s “analytic-meaning” dichotomous distinction.
We are now confronted with the realization that the responses of neurons in A1 reflect both the physical parameters of acoustic stimuli (as assumed in the traditional model) and also the acquired behavioral relevance or meaning of sounds, i.e., their psychological parameters. This state of affairs implies an inherent ambiguity in the interpretation of cellular response. For example, the same amount of sound-evoked discharge could be produced by a loud tone that had no particular behavioral meaning, or by a quiet tone that had acquired behavioral significance. Understanding how the auditory cortex apparently solves this problem is an important challenge for auditory neuroscience.
Functional organization of cortex
Implications for the functional organization of the cerebral cortex are no less important. The traditional model of sensory cortex as espoused by Campbell is part of a larger conception of the cerebral cortex. In its simplest, yet dominant, form this model assigns three major stages to the cortex: sensory analysis, association and motor output. The second stage has been thought to involve “higher” sensory fields (per Campbell) plus “association” fields that were intercalated between and among sensory fields, where multisensory information was integrated. This model bears a striking resemblance to a reflex arc, although on a more complex level. In any event, as the first purely sensory analytic stage is no longer tenable, so the model as a whole is untenable. Consideration of alternatives, however, is beyond the scope of this article.
Implications for Clinical Auditory Neuroscience
Post-traumatic stress disorder
The fact of ARP in A1 may also impact the treatment of certain auditory and related disorders. The unwelcome intrusiveness of “flashback” sounds in post-traumatic stress disorder (PTSD) may reflect normal learning and memory neural processes pushed to the extreme. Recall that a behaviorally important tone can gain representational area in A1, and that the greater the strength of memory, the greater the gain in area (Figure 4). Intrusive images and memories in PTSD, in fact, are extraordinarily strong. Although the original experience may have been unique and brief, intrusive images may last a lifetime. Therefore, it is conceivable that they are based on greatly extended overrepresentations in the cerebral cortex. In particular, auditory flashbacks may reflect an extreme increase in their area of representation, even in A1. If so, then appropriate imaging studies should reveal an extended area of representation in auditory cortex. Consequently, therapeutic interventions might target reducing representational area, although the treatments might have to be more sophisticated than the use of extinction training as reported above (Figure 5).
Cholinergic intervention
Treatments of auditory processing disorders, as opposed to hearing loss per se, might benefit from knowledge of neurotransmitter involvement in the mechanisms of learning the meaning of sounds. While several neuromodulators have been implicated, such as dopamine and nor-epinephrine, the cholinergic system has been studied most intensively [25]. Increased release of endogenous acetylcholine (ACh) in animal models (by electrical stimulation of the source of cortical cholinergic innervation) induces ARP in A1, which is blocked by muscarinic antagonists [35,36]. Moreover, ARP induced by cholinergic activation has the same features as natural memory [37]. Perhaps of even greater potential importance, the same direct activation of the cholinergic system in animals actually implants specific, behavioral associative memory that has the same characteristics as natural memory (e.g., [38]). Moreover, the level of brain stimulation can control the amount of detail about an auditory experience that an animal remembers; greater release of ACh produces greater memory for acoustic detail [39]. Collectively, such findings suggest that appropriate use of cholinergic agents, perhaps combined with the use of tone-onset learning strategies, may be able to treat some auditory processing disorders by reorganization of the primary auditory cortex.
Conclusions
The major goal of this review has been to explain how a synthesis of the fundamental experimental approaches of two disciplines, auditory neurophysiology and the neurobiology of learning and memory have been mutually supportive in revealing that learning systematically modifies auditory coding in the primary auditory cortex. Such research also underscores the dangers of accepting assumptions about brain function, no matter how reasonable they may seem. Although it seems eminently reasonable that the first stage of auditory processing in the cortex should be the analysis of sounds, while later, “higher” stages do the work of association, interpretation and comprehension of sound, the auditory system does not conform to such common sense. The relationship between auditory analysis and auditory comprehension is far more intimate than realized. The challenge is now to begin to apply insights gained from this new understanding to the clinic.
Acknowledgements
We thank Gabriel K. Hui and Jacquie Weinberger for assistance. This research was supported by research grants from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (NIDCD), DC-02938 and DC-010013 to NMW.
References
- Edeline JM (2003) The thalamo-cortical auditory receptive fields: regulation by the states of vigilance, learning and the neuromodulatory systems. Exp Brain Res 153: 554–572.
- Ohl FW, Deliano M, Scheich H, Freeman WJ (2003) Analysis of evoked and emergent patterns of stimulus-related auditory cortical activity. Rev Neurosci 14: 35–42.
- Rauschecker JP (2003) Functional organization and plasticity of auditory cortex. In: Peretz I (ed) The Cognitive Neuroscience of Music. Oxford University Press, London. Pp. 357–365.
- Scheich H, Brechmann A, Brosch M, Budinger E, Ohl FW, et al. (2011) Behavioral semantics of learning and crossmodal processing in auditory cortex: the semantic processor concept. Hear Res 271: 3–15.
- Weinberger NM (2007) Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem 14: 1–16.
- Weinberger NM (2011) Reconceptualizing the primary auditory cortex: learning, memory and specific plasticity. In: Winer JA, Schreiner CE (eds) The Auditory Cortex. Springer, New York. Chap. 22: 465–491.
- Campbell AW (1905) Histological Studies on the Localisation of Cerebral Function. University Press, Cambridge.
- Diamond IT (1985) A history of the study of the cortex: changes in the concept of the sensory pathway. In: Kimble GA, Schlesinger K (eds) Topics in the History of Psychology, vol. 1. Lawrence Erlbaum Associates, Hillsdale, NJ. Chap. 8, pp. 305–387.
- Kaas JH, Hackett TA (2000) Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci U S A 97: 11793–11799.
- Tunturi AR (1944) Audio frequency localization in the acoustic cortex of the dog. Am J Physiol 141: 397–403.
- GALAMBOS R, SHEATZ G, VERNIER VG (1956) Electrophysiological correlates of a conditioned response in cats. Science 123: 376–377.
- Weinberger NM, Diamond DM (1987) Physiological plasticity in auditory cortex: rapid induction by learning. Prog Neurobiol 29: 1–55.
- Weinberger NM (2004) Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci 5: 279–290.
- Morris JS, Friston KJ, Dolan RJ (1998) Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc Biol Sci 265: 649–657.
- Thiel CM, Bentley P, Dolan RJ (2002) Effects of cholinergic enhancement on conditioning-related responses in human auditory cortex. Eur J Neurosci 16: 2199–2206.
- Thiel CM, Friston KJ, Dolan RJ (2002) Cholinergic modulation of experience-dependent plasticity in human auditory cortex. Neuron 35: 567–574.
- Kluge C, Bauer M, Leff AP, Heinze HJ, Dolan RJ, et al. (2011) Plasticity of human auditory-evoked fields induced by shock conditioning and contingency reversal. Proc Natl Acad Sci U S A 108: 12545–12550.
- Weinberger NM, Diamond DM, McKenna TM (1984) Initial events in conditioning: plasticity in the pupillomotor and auditory systems. In: Lynch G, McGaugh JL, Weinberger NM (eds) Neurobiology of Learning and Memory. Guilford Press, New York. Chap. 12, pp. 197–227.
- Diamond DM, Weinberger NM (1986) Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res 372: 357–360.
- Diamond DM, Weinberger NM (1989) Role of context in the expression of learning-induced plasticity of single neurons in auditory cortex. Behav Neurosci 103: 471–494.
- Gonzalez-Lima F, Scheich H (1986) Neural substrates for tone-conditioned bradycardia demonstrated with 2-deoxyglucose. II. Auditory cortex plasticity. Behav Brain Res 20: 281–293.
- Bakin JS, Weinberger NM (1990) Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res 536: 271–286.
- Ohl FW, Scheich H (1996) Differential frequency conditioning enhances spectral contrast sensitivity of units in auditory cortex (field Al) of the alert Mongolian gerbil. Eur J Neurosci 8: 1001–1017.
- Ohl FW, Scheich H (1997) Learning-induced dynamic receptive field changes in primary auditory cortex of the unanaesthetized Mongolian gerbil. J Comp Physiol A 181: 685–696.
- Weinberger NM (1998) Tuning the brain by learning and by stimulation of the nucleus basalis. Trends Cogn Sci 2: 271–273.
- Recanzone GH, Schreiner CE, Merzenich MM (1993) Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci 13: 87–103.
- Rutkowski RG, Weinberger NM (2005) Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A 102: 13664–13669.
- Bieszczad KM, Weinberger NM (2010) Representational gain in cortical area underlies increase of memory strength. Proc Natl Acad Sci U S A 107: 3793–3798.
- Bieszczad KM, Weinberger NM (2012, in press) Extinction reveals that primary sensory cortex predicts reinforcement outcome. Eur J Neurosci.
- Berlau KM, Weinberger NM (2008) Learning strategy determines auditory cortical plasticity. Neurobiol Learn Mem 89: 153–166.
- Bieszczad KM, Weinberger NM (2010) Learning strategy trumps motivational level in determining learning-induced auditory cortical plasticity. Neurobiol Learn Mem 93: 229–239.
- Bieszczad KM, Weinberger NM (2010) Remodeling the cortex in memory: Increased use of a learning strategy increases the representational area of relevant acoustic cues. Neurobiol Learn Mem 94: 127–144.
- Heil P, Irvine DR (1998) The posterior field P of cat auditory cortex: coding of envelope transients. Cereb Cortex 8: 125–141.
- Brown M, Irvine DR, Park VN (2004) Perceptual learning on an auditory frequency discrimination task by cats: association with changes in primary auditory cortex. Cereb Cortex 14: 952–965.
- Bakin JS, Weinberger NM (1996) Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A 93: 11219–11224.
- Miasnikov AA, McLin D 3rd, Weinberger NM (2001) Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. Neuroreport 12: 1537–1542.
- Weinberger NM (2003) The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem 80: 268–284.
- McLin DE 3rd, Miasnikov AA, Weinberger NM (2002) Induction of behavioral associative memory by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A 99: 4002–4007.
- Weinberger NM, Miasnikov AA, Chen JC (2006) The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiol Learn Mem 86: 270–285.
Citation: Weinberger NM (2012) Plasticity in the Primary Auditory Cortex, Not What You Think it is: Implications for Basic and Clinical Auditory Neuroscience. Otolaryngol S3:002. DOI: 10.4172/2161-119X.S3-002
Copyright: © 2012 Weinberger NM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15462
- [From(publication date): 1-2012 - Apr 06, 2025]
- Breakdown by view type
- HTML page views: 10648
- PDF downloads: 4814