Editorial Open Access
Photodynamic Therapy as an Emerging Treatment Modality for Cancer and Non-Cancer Diseases
Zhiwei Hu1*, Nancy Oleinick2 and Michael R Hamblin3,4,5
1The Ohio State University College of Medicine, Department of Surgery and The James Comprehensive Cancer Center, Columbus, OH, USA
2Case Western Reserve University, Department of Radiation Oncology, and the Case Comprehensive Cancer Center, Cleveland, OH, USA
3The Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA, USA
4Department of Dermatology, Harvard Medical School, Boston, MA, USA
5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, USA
- *Corresponding Author:
- Dr. Zhiwei Hu
Department of Surgery and The James Comprehensive Cancer Center
The Ohio State University College of Medicine, Columbus, OH 43210, USA
Tel: 614-685-4606
E-mail: Zhiwei.hu@osumc.edu
Received date: March 29, 2014; Accepted date: April 01, 2014; Published date: April 04, 2014
Citation: Hu Z, Oleinick N, Hamblin MR (2014) Photodynamic Therapy as an Emerging Treatment Modality for Cancer and Non-Cancer Diseases. J Anal Bioanal Tech S1:e001. doi: 10.4172/2155-9872.S1-e001
Copyright: © 2014 Hu Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
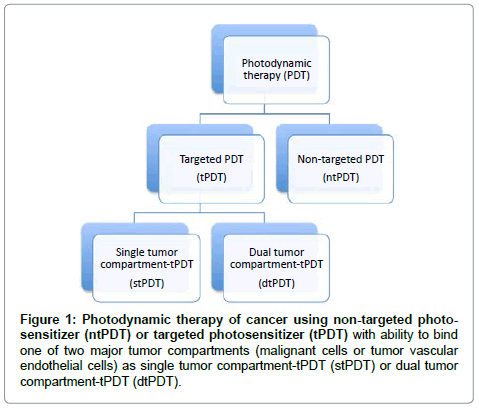
Photodynamic therapy (PDT) is a treatment modality involving photoactivatable chemicals (called photosensitizers), light and tissue oxygen [1-6]. PDT has clinical applications in the treatment of a variety of solid cancers [4,7-21], including but not limited to those of lung, skin, breast, head and neck, digestive tract, pancreas, liver, bladder, ovary, prostate and brain. In addition, there are many clinical applications of PDT for treatment of a wide range of non-cancerous conditions, such as bacterial and fungal infections; hyperproliferative or inflammatory conditions, such as macular degeneration or psoriasis; and premalignant conditions, such as actinic keratosis [22] and Barrett’s esophagus [23]. To achieve better therapeutic efficacy, new photosensitizers and novel light sources are continuously being developed, and the mechanisms of action are becoming better understood [24,25]. To achieve improved tumor selectivity and to reduce side effects in the treatment of cancer, the concept of targeted photodynamic therapy has been successfully developed by attaching specific functionalities to the photosensitizer, such as antibodies recognizing tumor antigens [26,27], or ligands and peptides to recognize receptors [28], which could be selectively expressed on one of the two major tumor compartments, either on the malignant cells or on tumor neovasculature. To achieve better efficacy than PDT that is targeted to a single tumor compartment (stPDT), a recent editorial [29] in this Journal summarized a new PDT approach (Figure 1), which was designed for dual targeting of photosensitizers (dtPDT) to both malignant cells and neovasculature [30,31] by conjugating photosensitizers to a protein, factor VII, the natural ligand for tissue factor. This approach allows for dual targeting of malignant cells and of tumor neovasculature, both of which either overexpress or selectively express tissue factor [30-33], respectively.
Figure 1: Photodynamic therapy of cancer using non-targeted photosensitizer (ntPDT) or targeted photosensitizer (tPDT) with ability to bind one of two major tumor compartments (malignant cells or tumor vascular endothelial cells) as single tumor compartment-tPDT (stPDT) or dual tumor compartment-tPDT (dtPDT).
In this special issue on PDT-Cancer, PDT scientists and experts worldwide contributed seven peer-reviewed articles. These review and research articles broadly cover the use and applications of PDT for cancer, for infections and for inflammatory conditions.
Menon and Stafinski [34] in Canada thoroughly reviewed reports of clinical applications and clinical trials of PDT for the treatment of different types of cancers that were published in English between January 1997 and June 2011. They summarized a total of 266 studies, which involved 11,427 patients and 34 different types of cancer in diverse organs, such as anal and perianal cutaneous cancers, as well as cancers of the bile duct, bladder, brain, breast, cervical, esophageal, eye, gastrointestinal, head and neck, lung, peritoneal cavity, colorectal, liver, ovary, pancreas, prostate, skin, vulva, etc.
Tsipursky, Churgin, Conway and Peyman [35] in the United States reviewed PDT for treatment of intraocular tumors published between 1995 and 2011. Their review article showed that PDT with Verteporfin® has proven successful in the clinic for treatment of benign and malignant intraocular tumors, including choroidal and retinal capillary hemangioma, vasoproliferative tumors of the retina, astrocytoma, choroidal metastasis and choroidal melanoma, with potential to treat retinoblastoma.
Hirakawa, Yamanaka, Matsumoto and Yasuda [36] in Japan examined protein-damaging activity and mechanisms of action of phosphorous (V) porphyrin. Based on the results from in vitro testing, they believe that the activity of this photosensitizer may be preserved under a lower oxygen concentration such as a tumor through an oxygenindependent electron transfer mechanism. If this observation could be repeated in vivo in tumors, this photosensitizer may be suitable for PDT of cancer, in which tumor microenvironment could be hypoxic.
Rugani, Truschnegg, Acham, Kirnbauer and Jakse [37] in Austria reviewed clinical applications of PDT and low-level laser therapy in treatment of bisphosphonate-related osteonecrosis of the jaw (BRONJ), a serious side effect of the use of bisphosphonate agents in the treatment of cancer and osteoporosis. They report their case studies of PDT in 12 patients with BRONJ (5 patients at stage 0 and 7 at stage 2), in which methylene blue as photosensitizer was applied topically to the surface of affected tissues followed immediately by irradiation with a 680 nm diode laser twice a week for two weeks. After treatment for two weeks, mucosal healing was observed in all 5 patients with stage 0 and 5 of 7 patients with stage 2 disease. They concluded that PDT could be used as an adjuvant therapy before or after surgery, or even as the primary treatment option for very early BRONJ and for those patients in whom the option of surgery is not indicated.
Blake, Allen and Curnow [38] in the UK studied the effect of iron chelation and oxygen concentration on the accumulation, photobleaching and cytotoxicity of protoporphyrin IX (PpIX) during PDT of human glioma cells in vitro. They tested three PpIX precursors, aminolevulinic acid (ALA) and its esters, methyl aminolevulinate (MAL) and hexyl aminolevulinate (HAL), with an iron chelator CP94. They report that lower oxygen concentration (5% vs. 20% and 40%) and the addition of the iron chelator CP94, which can cross the bloodbrain barrier, enhanced accumulation of PpIX (derived from either ALA or MAL) in human glioma U87MG cells. This finding could have translational potential since the tumor environment is usually hypoxic.
Nowak-Sliwinska, Weiss, Sickenberg, Griffioen and van den Bergh [39] from Switzerland and the Netherlands extensively summarized current clinical applications of PDT as a monotherapy or as a combination therapy in non-malignant and malignant eye disorders. This review article discusses the clinical use of Verteporfin-PDT for treatment of non-malignant eye disorders, including choroidal neovascularization (CNV), age-related macular degeneration (AMD), pathologic myopia, polypoidal choroidal vasculopathy (PCV), inflammation, angioid streaks, central serous chorioretinopathy, and ophthalmic tumors, including benign tumors, choroidal hemangioma, retinal vasoproliferative tumors, malignant tumors (choroidal melanoma, squamous cell carcinoma and choroidal metastasis). Nowak- Sliwinska et al. [39] also indicate the limitations of current Verteporfin- PDT (poor selectivity of non-targeted Verteporfin which leads to side effects, such as damage to retinal pigment epithelial cells, and made some suggestions, such as combination therapy with anti-angiogenic inhibitors and antibodies and/or anti-inflammatory hormones, targeted PDT, RNA interference (to inhibit VEGF production), to improve PDT in the future for treatment of eye disorders.
Alemany-Ribes, Garcia-Diaz, Acedo, Agut, Nonell, Sagrista, Mora, Canete, Villanueva, Stockert and Semino [40] in Spain propose to use three-dimensional (3D) cell culture techniques as a novel and emerging in vitro model for screening and testing new photosensitizers and drugdelivery systems in PDT of cancer. Ideally, a 3D model can mimic in vivo gene and molecular expression patterns and the particular cellular and tissue structure not found in standard 2D tissue culture. They summarize the currently available 3D model systems, including tissue explants, cellular spheroids, scaffold-based cultures, whole perfused organs and hollow-fiber bioreactors, of which the most commonly used platforms are cellular spheroids and scaffold-based cultures. The 3D models could be conveniently applied in PDT and other drug screening tests, and even more so when 3D biomaterial printing becomes available in research laboratories.
In conclusion, the papers in this special issue further expand on the many potential roles of photodynamic therapy in the treatment of human disease and the many novel approaches to expand the applications and efficacy of PDT. Clearly, PDT has therapeutic potential as monotherapy or combination therapy with chemotherapy, radiation therapy, immunotherapy and/or other neovascular-targeting therapy for the treatment of cancer and non-cancer diseases.
Acknowledgements
This work was partly supported by an institutional start-up fund from The Ohio State University College of Medicine and The OSU James Comprehensive Cancer Center (to Z.H.). M.R.H. is supported by US NIH grant AI050875.
References
- Gomer CJ (1989) Photodynamic therapy in the treatment of malignancies. Semin Hematol 26: 27-34.
- Oleinick NL, Evans HH (1998) The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res 150: S146-156.
- Castano AP, Mroz P, Hamblin MR (2006) Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer 6: 535-545.
- Dougherty TJ (1984) Photodynamic therapy (PDT) of malignant tumors. Crit Rev OncolHematol 2: 83-116.
- Henderson BW, Dougherty TJ (1992) How does photodynamic therapy work? PhotochemPhotobiol 55: 145-157.
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, et al. (1998) Photodynamic therapy. J Natl Cancer Inst 90: 889-905.
- Guyon L, Ascencio M, Collinet P, Mordon S (2012) Photodiagnosis and photodynamic therapy of peritoneal metastasis of ovarian cancer. PhotodiagnosisPhotodynTher 9: 16-31.
- Allison R, Moghissi K, Downie G, Dixon K (2011) Photodynamic therapy (PDT) for lung cancer. PhotodiagnosisPhotodynTher 8: 231-239.
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, et al. (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61: 250-281.
- Allison RR, Sheng C, Cuenca R, Bagnato VS, Austerlitz C, et al. (2010) Photodynamic therapy for anal cancer. PhotodiagnosisPhotodynTher 7: 115-119.
- Juarranz A1, Jaén P, Sanz-Rodríguez F, Cuevas J, González S (2008) Photodynamic therapy of cancer. Basic principles and applications. ClinTranslOncol 10: 148-154.
- Fan BG, Andrén-Sandberg A (2007) Photodynamic therapy for pancreatic cancer. Pancreas 34: 385-389.
- Dolmans DE, Fukumura D, Jain RK (2003) Photodynamic therapy for cancer. Nat Rev Cancer 3: 380-387.
- Biel MA (2002) Photodynamic therapy in head and neck cancer. CurrOncol Rep 4: 87-96.
- Ost D (2000) Photodynamic therapy in lung cancer. Oncology (Williston Park) 14: 379-386, 391.
- Greenwald BD (2000) Photodynamic therapy for esophageal cancer. Update. Chest SurgClin N Am 10: 625-637.
- Gossner L, Ell C (2000) Photodynamic therapy of gastric cancer. GastrointestEndoscClin N Am 10: 461-480.
- Kato H (1998) Photodynamic therapy for lung cancer--a review of 19 years' experience. J PhotochemPhotobiol B 42: 96-99.
- Schuitmaker JJ, Baas P, van Leengoed HL, van der Meulen FW, Star WM, et al. (1996) Photodynamic therapy: a promising new modality for the treatment of cancer. J PhotochemPhotobiol B 34: 3-12.
- Marcon NE (1994) Photodynamic therapy and cancer of the esophagus. SeminOncol 21: 20-23.
- Delaney TF, Glatstein E (1988) Photodynamic therapy of cancer. ComprTher 14: 43-55.
- Zane C, Facchinetti E, Rossi M, Specchia C, Calzavara-Pinton P (2014) A randomized clinical trial of photodynamic therapy with methylaminolevulinate versus 3% diclofenac plus hyaluronic acid gel for the treatment of multiple actinic keratoses of the face and scalp. Br J Dermatol .
- Qumseya BJ, David W, Wolfsen HC (2013) Photodynamic Therapy for Barrett's Esophagus and Esophageal Carcinoma. ClinEndosc 46: 30-37.
- Yoo JO, Ha KS (2012) New insights into the mechanisms for photodynamic therapy-induced cancer cell death. Int Rev Cell MolBiol 295: 139-174.
- Firczuk M, Winiarska M, Szokalska A, Jodlowska M, Swiech M, et al. (2011) Approaches to improve photodynamic therapy of cancer. Front Biosci (Landmark Ed) 16: 208-224.
- Mayo GL, Melendez RF, Kumar N, McKinnon SJ, Glickman RD (2003) Antibody-targeted photodynamic therapy. Am J Ophthalmol 136: 1151-1152.
- Schmidt S (1993) Antibody-targeted photodynamic therapy. Hybridoma 12: 539-541.
- Sharman WM, van Lier JE, Allen CM (2004) Targeted photodynamic therapy via receptor mediated delivery systems. Adv Drug Deliv Rev 56: 53-76.
- Hu Z (2012)Dual targeting of tumor neovasculature and tumor cells by tissue factor-targeted photodynamic therapy. J Anal Bioanal Techniques 3: e105.
- Hu Z, Rao B, Chen S, Duanmu J (2011) Selective and effective killing of angiogenic vascular endothelial cells and cancer cells by targeting tissue factor using a factor VII-targeted photodynamic therapy for breast cancer. Breast Cancer Res Treat 126: 589-600.
- Hu Z, Rao B, Chen S, Duanmu J (2010) Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice. BMC cancer 10: 235.
- Duanmu J, Cheng J, Xu J, Booth CJ, Hu Z (2011) Effective treatment of chemoresistant breast cancer in vitro and in vivo by a factor VII-targeted photodynamic therapy. Br J Cancer 104: 1401-1409.
- Cheng J, Xu J, Duanmu J, Zhou H, Booth CJ, et al. (2011) Effective treatment of human lung cancer by targeting tissue factor with a factor VII-targeted photodynamic therapy. Curr Cancer Drug Targets 11: 1069-1081.
- Menon D, Stafinski T (2011) An Evidence Scoping Review of Photodynamic Therapy (PDT) in the Treatment of Cancers. J Anal Bioanal Tech S1:002.
- Tsipursky MS, Churgin DS, Conway MD, Peyman GA (2011) A Review of Photodynamic Therapy for Intraocular Tumors. J Anal Bioanal Tech S1:001.
- Hirakawa K, Yamanaka T, Matsumoto J, Yasuda M (2013) Examination of Protein-Damaging Activity of Phosphorus (V) Porphyrin Photosensitizer for Photodynamic Therapy. J Anal Bioanal Tech S1:003.
- Rugani P, Truschnegg A, Acham S, Kirnbauer B, Jakse N (2013) Use of Photodynamic Therapy in Treatment of Bisphosphonate-related Osteonecrosis of the Jaws: Literature Review and Case Series. J Anal Bioanal Tech S1:006.
- Blake E, Allen J, Curnow A (2013) The effects of protoporphyrin IX-induced photodynamic therapy with and without iron chelation on human squamous carcinoma cells cultured under normoxic, hypoxic and hyperoxic conditions. PhotodiagnosisPhotodynTher 10: 575-582.
- Nowak-Sliwinska P, Weiss A, Sickenberg M, Griffioen AW, van den Bergh H (2013) The Role of Photodynamic Therapy in Non-malignant and Malignant Eye Disorders. J Anal Bioanal Tech S1:007.
- Alemany-Ribes M, García-Díaz M, Acedo P, Agut M, Nonell S, et al. (2013) Why Not Introducing the Third Dimension in Photodynamic Therapy Research? J Anal Bioanal Tech S1: 004.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15995
- [From(publication date):
specialissue-2013 - Dec 06, 2025] - Breakdown by view type
- HTML page views : 11134
- PDF downloads : 4861