Our Group organises 3000+ Global Conferenceseries Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Open Access Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Google Scholar citation report
Citations : 14
Journal of Pharmacokinetics & Experimental Therapeutics peer review process verified at publons
Indexed In
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- ICMJE
Useful Links
Share This Page
Editorial Board
Submit Manuscript
Journal Impact Factor 0*
Submit manuscript at or send as an e-mail attachment to the Editorial Office at manuscript@omicsonline.org
If you are interested in publishing with us or have any questions, please feel free to contact us directly on WhatsApp .
Table of Contents
About the Journal
Journal of Pharmacokinetics & Experimental Therapeutics is an open access journal that focuses on pharmacokinetic properties of drugs, including Absorption, Distribution, Metabolism and Excretion (ADME) while underlining the importance of experimental therapeutics in pharmacokinetic research. Journal of Pharmacokinetics & Experimental Therapeutics accepts manuscripts in clinical pharmacokinetics, population pharmacokinetics, antibody pharmacokinetics, pharmacokinetics review, vancomycin pharmacokinetics, tacrolimus pharmacokinetics, pharmacokinetic drugs, pharmacokinetics intravenous, pharmacokinetics oral, pharmacokinetics renal, pharmacokinetics human, pharmacokinetics rat, pharmacokinetics mice, pharmacokinetics for children, pharmacokinetics research to promote healthy life are welcome.
DMPK Studies
DMPK or Drug Metabolism and Pharmacokinetics is a major part of studies related to drugs often referred to as ADME (Absorption, Distribution, Metabolism and Elimination). It is concerned with the study of aspects of absorption, distribution, metabolism and elimination of drug compounds which are administered through any route of administration.
Journals Related to DMPK Studies:
Journal of Pharmacokinetics & Experimental Therapeutics, Drug Metabolism & Toxicology, Research & Reviews: Journal of Pharmaceutical Analysis, Journal of Drug metabolism & Toxicology, European Journal of Drug Metabolism and Pharmacokinetics, Drug Metabolism and Pharmacokinetics.
ADME Studies
ADME is an abbreviation in the fields of pharmacokinetics and pharmacology for absorption, distribution, metabolism and excretion of the particular pharmaceutical compound or the drug and describes the disposition of that specific drug within an organism.
Journals Related to ADME Studies:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Physiobiochemical Metabolism, Journal of Pharmacokinetics and Pharmacodynamics, Journal of Drug metabolism & Toxicology, Journal of Drug metabolism & Toxicology.
Linear Pharmacokinetics
Linear Pharmacokinetics ,the characteristic of drugs that indicates the instantaneous rate of change in drug concentration depends only on the current concentration. The half-life will remain constant, irrespective of how high the concentration.
Journals Related to Linear Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Nonlinear Pharmacokinetics
Nonlinear pharmacokinetics is the characteristic of drugs that briefs that the absorption and bioavailability can cause increases in drug concentrations that are disproportionately high or low relative to the change in dose. This characteristic of drugs only alters with changes in dosage of drugs.
Journals Related to Nonlinear Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Pharmacokinetics
The formal study of the movement of drugs in the body, including the processes of absorption, distribution, localization in tissues, biotransformation and excretion commonly termed as (ADME) of the drugs from the body.
Journals Related to Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, Drug Metabolism & Toxicology, Research & Reviews: Journal of Pharmaceutical Analysis, Journal of Drug metabolism & Toxicology, European Journal of Drug Metabolism and Pharmacokinetics, Drug Metabolism and Pharmacokinetics.
Pharmacokinetics Modelling
Pharmacokinetic modeling provide sets of equations that implies the time courses of Compounds and their metabolites in various tissues throughout the body. The interest of pharmacokinetic modeling in pharmacology and toxicology has begun from the need to relate internal concentrations of active compounds at their target sites with doses of the compound given to an animal or human being.
Journals Related to Pharmacokinetics Modelling:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Morphine Pharmacokinetics
Morphine is a narcotic pain reliever used to treat moderate to severe pain. Morphine is easily absorbed and after absorption, it is rapidly distributed within the body, including the brain and even highly distributed and most commonly excreted through urine.
Journals Related to Morphine Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, Drug Metabolism & Toxicology, Research & Reviews: Journal of Pharmaceutical Analysis, Journal of Drug metabolism & Toxicology, European Journal of Drug Metabolism and Pharmacokinetics, Drug Metabolism and Pharmacokinetics.
Atorvastatin Pharmacokinetics
Atorvastatin is a cholesterol-lowering medication that blocks the production of cholesterol in the body. The main one of the pharmacokinetic aspect of Atorvastatin is that it has rapid oral absorption with an approximate time to maximum plasma concentration of 1–2 hours.
Journals Related to Atorvastatin Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Clopidogrel Pharmacokinetics
Clopidogrel is an oral, thienopyridine-class antiplatelet agent used to inhibit blood clots in coronary artery disease, peripheral vascular disease and such arterial or vascular associated disorders. The pharmacokinetics study of Clopidogrel active metabolite and its antiplatelet effects are measured by ex vivo platelet aggregation assays.
Journals Related to Clopidogrel Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Physiobiochemical Metabolism, Journal of Pharmacokinetics and Pharmacodynamics, Journal of Drug metabolism & Toxicology, Journal of Drug metabolism & Toxicology.
Fluticasone Pharmacokinetics
Fluticasone, an oral inhalation is used to prevent difficulty breathing, chest tightness, wheezing, and coughing caused or observed generally in the patients suffering from asthma. The aim of the pharmacokinetic studies was to determine the systemic bioavailability of intranasal fluticasone.
Journals Related to Fluticasone Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Esomeprazole Pharmacokinetics
Esomeprazole is used to treat gastro esophageal reflux disease (GERD), a condition in which backward flow of acid from the stomach causes heartburn and possible injury of the esophagus and sometimes even causes severe acidity. The pharmacokinetics of esomeprazole is generally not affected differently by intrinsic or extrinsic factors after intravenous administration compared to oral administration.
Journals Related to Esomeprazole Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Rosuvastatin Pharmacokinetics
Rosuvastatin is used together with diet, weight-loss, and exercise to reduce the risk of heart attack and stroke and to decrease the chance that heart surgery in people who need it and having heart disease or who are at risk of developing heart disease or ready to get the stroke. Rosuvastatin pharmacokinetics was studied in an open-label and parallel-group. The plasma concentrations of rosuvastatin and metabolites were determined by HPLC-mass spectrophotometry.
Journals Related to Rosuvastatin Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Quetiapine Pharmacokinetics
Quetiapine is an atypical antipsychotic medicine. An antipsychotic medicine helps to adjust the levels of dopamine and other chemicals in human brain. It is rapidly absorbed, distributed throughout the body, highly metabolized and eliminated as mostly unchanged drug.
Journals Related to Quetiapine Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Adalimumab Pharmacokinetics
Adalimumab is used to treat people with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis, and children with juvenile idiopathic arthritis. Pharmacokinetic parameters were analyzed for adalimumab and MTX during both phases of the study.
Journals Related to Adalimumab Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Etanercept Pharmacokinetics
Etanercept is used to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and juvenile idiopathic arthritis. There are no particular or formal pharmacokinetic studies have been conducted to examine the effects of renal or hepatic impairment on etanercept disposition.
Journals Related to Etanercept Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Olanzapine Pharmacokinetics
Olanzapine is a drug used for treating patients with schizophrenia and manic disorders. Pharmacokinetic studies showed that olanzapine, orally disintegrating tablets dosage forms of olanzapine are bioequivalent.
Journals Related to Olanzapine Pharmacokinetics:
Journal of Pharmacokinetics & Experimental Therapeutics, International Journal of Drug Development and Research, Drug Metabolism & Toxicology, Journal of Pharmacokinetics and Biopharmaceutics, Journal of Pharmacokinetics and Pharmacodynamics, Asian Journal of Pharmacodynamics and Pharmacokinetics.
Journal Highlights
Fast Editorial Execution and Review Process (FEE-Review Process):
Journal of Pharmacokinetics & Experimental Therapeutics is participating in the Fast Editorial Execution and Review Process (FEE-Review Process) with an additional prepayment of $99 apart from the regular article processing fee. Fast Editorial Execution and Review Process is a special service for the article that enables it to get a faster response in the pre-review stage from the handling editor as well as a review from the reviewer. An author can get a faster response of pre-review maximum in 3 days since submission, and a review process by the reviewer maximum in 5 days, followed by revision/publication in 2 days. If the article gets notified for revision by the handling editor, then it will take another 5 days for external review by the previous reviewer or alternative reviewer.Acceptance of manuscripts is driven entirely by handling editorial team considerations and independent peer-review, ensuring the highest standards are maintained no matter the route to regular peer-reviewed publication or a fast editorial review process. The handling editor and the article contributor are responsible for adhering to scientific standards. The article FEE-Review process of $99 will not be refunded even if the article is rejected or withdrawn for publication.
The corresponding author or institution/organization is responsible for making the manuscript FEE-Review Process payment. The additional FEE-Review Process payment covers the fast review processing and quick editorial decisions, and regular article publication covers the preparation in various formats for online publication, securing full-text inclusion in a number of permanent archives like HTML, XML, and PDF, and feeding to different indexing agencies.
h-index
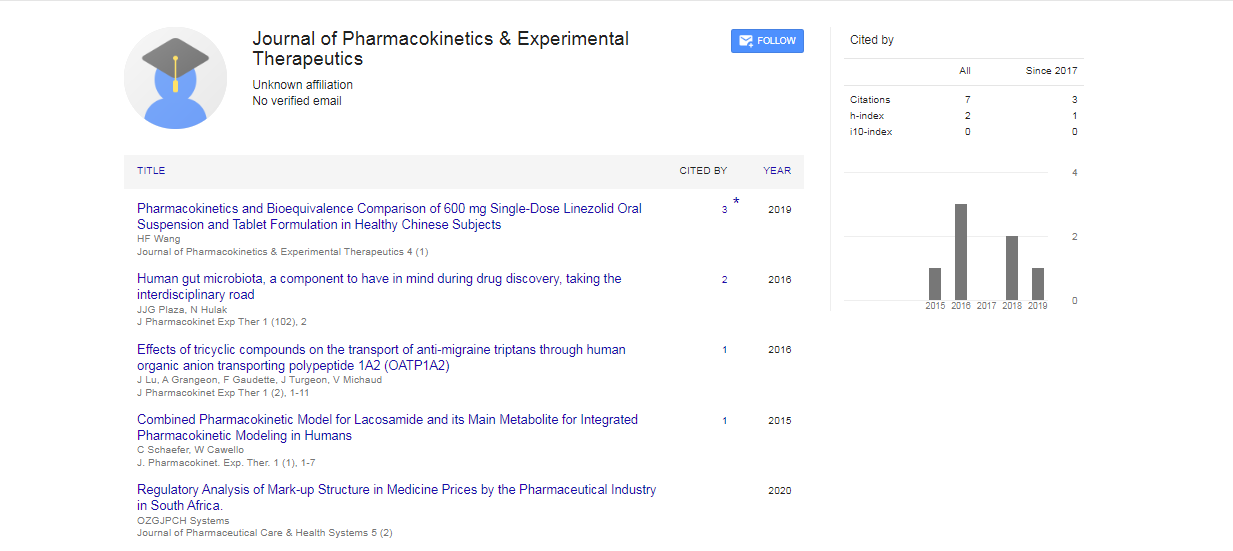
Articles published in Journal of Pharmacokinetics & Experimental Therapeutics have been cited by esteemed scholars and scientists all around the world. Journal of Pharmacokinetics & Experimental Therapeutics has got h-index 2, which means every article in Journal of Pharmacokinetics & Experimental Therapeutics has got 2 average citations.
Recently Published Articles
-
Acute parotitis secondary to herpes zoster ophthalmicus: Case report of two patients
-
Utility of Imunohistochemical expression of smoothelin in staging of urothelial carcinoma in transurethral resection specimen of bladder tumours
-
Does the coronavirus spread through food
-
REVIEW OF THE BENEFIT OF RADIOTRACER IN BONE METASTASES
-
Zero-Inflated Models for Assessing the Effects of Supplements on Reducing Prostate Cancer Incidence
-
Assessment of oxidative stress parameters in the kidney after the administration of nitrate in drinking water : an experimental study

 Spanish
Spanish  Chinese
Chinese  Russian
Russian  German
German  French
French  Japanese
Japanese  Portuguese
Portuguese  Hindi
Hindi