Short Communication Open Access
Peritoneal Cytology in the Staging Process of Gastric Cancer: Do or Don’t?
Chin W Ang* and Lam Chin Tan
Department of General and Upper Gastrointestinal Surgery, University Hospital, Clifford Bridge Road, Coventry, West Midlands, UK CV2 2DX, UK
- Corresponding Author:
- Chin W Ang
Department of General and Upper Gastrointestinal Surgery
University Hospital, Clifford Bridge Road
Coventry, West Midlands, UK CV2 2DX, UK
Tel: 00447846710148
E-mail: acwee@doctors.org.uk
Received Date: September 28, 2013; Accepted Date: November 23, 2013; Published Date: November 30, 2013
Citation: Ang CW, Tan LC (2013) Peritoneal Cytology in the Staging Process of Gastric Cancer: Do or Don’t? J Gastroint Dig Syst 3:156. doi: 10.4172/2161-069X.1000156
Copyright: © 2013 Ang CW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Rigorous cancer staging protocols are paramount in assessing the extent of a patient’s cancer. This will determine an appropriate treatment strategy for the individual patient, improve survival prognosis, allow healthcare providers and researchers to exchange information in common terminology, and enable recruitment of patients into clinical trials. Staging systems for gastric cancer evolves with time as we continue to learn more about it.
After curative resection, the commonest pattern of gastric cancer relapse is peritoneal metastasis, which is the most frequent cause of death with gastric cancer [1]. Despite advances in radiological techniques, staging laparoscopy continues to contribute substantially in staging patients with gastric cancer. Studies have shown that up to 30% of gastric cancer beyond T1 disease on EUS and absent metastatic disease on CT scan will have peritoneal disease evident on laparoscopy [2,4]. PET scan is superior to CT scan for the detection of distant metastases [5,6] but owing to its sensitivity of only ~50% for detecting peritoneal carcinomatosis [7], PET cannot replace laparoscopy which clearly offers the advantage of direct visualisation of peritoneal cavity. In addition, peritoneal washings can be readily performed during staging laparoscopy to detect intraperitoneal free cancer cells (IPFCC).
IPFCC are exfoliated free cancer cells from the primary lesion. Patients with radiologically resectable gastric cancer but positive IPFCC have been shown to be significantly associated with early disease recurrence and poor survival, despite R0 resection [8,9]. Therefore, IPFCC is a marker of peritoneal micrometastatic development even in patients with no visible evidence of peritoneal disease on laparoscopy. In this context, peritoneal washing cytology provides invaluable staging information which predicts the possibility of peritoneal recurrence or treatment failure and allows targeted therapeutic approach to gastric cancer. The Japanese Classification of Gastric Carcinoma (JCGC) recommended peritoneal wash cytology as part of staging for gastric cancer since 1990s and patients with positive IPFCC have been regarded as Stage IV disease [10]. While the Society of American Gastroenterologists and Endoscopic Surgeons (SAGES) values peritoneal washings during staging laparoscopy [11], the European Society for Medical Oncology (ESMO) perceives peritoneal washings as non-obligatory [12]. Only recently, the American Joint Committee on Cancer (AJCC) staging system in 2010 had included peritoneal cytology into the TNM 7th Edition, with a positive peritoneal cytology staged as M1 disease [13]. Despite these internationally renowned classification systems, the practice of peritoneal washings in the Western countries is not uniform or routine due to several unresolved concerns.
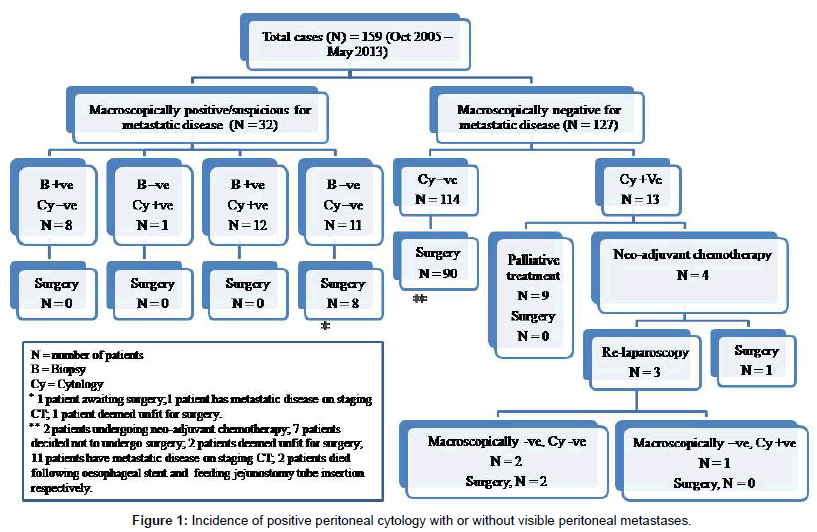
In our institution, the author (Tan LC) routinely performs staging laparoscopy and peritoneal washing cytology on patients with radiologically resectable gastric cancer (Figure 1). One of the main criticisms of peritoneal washing cytology is its low sensitivity. The positive cytology rate ranged from 14-70% reported in the literature [14-16] which reflects the heterogeneity of patient cohorts with variable disease severity (those patients with more advanced disease correlate to higher positive cytology rate), the experience of pathologists, interobserver variability and differences in diagnostic criteria [17]. More specifically, in patients with potentially curative resections, the rate of cytology positivity ranged from 4.4-11% [18,19], consistent with our patient cohort where there were 10.2% of patients with negative radiological and staging laparoscopy for metastatic disease but positive IPFCC. In vice versa, our series also showed that a significant proportion of patients (8/20) have biopsy-proven peritoneal disease but with a negative IPFCC, this observation has also been described by Wang et al. [20]. These results further attest that peritoneal washing cytology has low sensitivity for IPFCC.
One of the strategies investigated to improve the sensitivity of peritoneal washing cytology is to perform multi-cavities washings. The majority of gastric surgeons perform peritoneal washings only in pouch of Douglas, as recommended by the JCGC. Interestingly, Homma et al. [21] demonstrated higher sensitivity rate when washings were performed in multiple cavities-the right and left subphrenic space, inside the omental bursa and pouch of Douglas.
However, the much investigated and debatable improvement strategies lie in histopathology using immunoassay and advanced molecular biology methods. The conventional Papanicolaou staining cytological technique has been the current standard of practice, as in our institution, despite its widely reported low sensitivity rate. The immunoassay technique directed against cancer-associated antigen CEA has been shown to increase detection rate by up to 14% over conventional cytology [22]. Unfortunately this technique has largely remained in the research arena and not been adopted into routine clinical practice due to the application of the highly reliable reverse transcriptase polymerase chain reaction technique (RT-PCR). Many studies have demonstrated the superiority of RT-PCR in terms of sensitivity rate compared to immunoassays and conventional cytology in detecting IPFCC [17,23-26]. This molecular biology technique, however, is costly, time consuming and associated with a relatively low specificity rate [27,28]. Despite these limitations, the Japanese has been using molecular biology technique as the standard of clinical practice since 2006 in several large cancer centres and university hospitals for peritoneal washings from staging gastric cancer patients [28].
The most controversial concern regarding peritoneal washings in staging gastric cancer is perhaps its implications in planning an appropriate treatment algorithm for positive IPFCC, which is largely unknown. As demonstrated in our series (Figure 1), the management of patients with positive IPFCC identified during the staging process evolves with time and has resulted in several pathways [29]. Traditionally, most patients have been treated with a palliative approach rather than curative intent, in view of the fact that there are tremendous evidence in the published literature showing high rates of early peritoneal recurrence disease and death despite R0 resection. Therefore, it is highly unlikely that these patients will achieve any survival benefit and their quality of life is significantly compromised by the gastric resection. However, there is emerging evidence that these patients treated more aggressively with neo-adjuvant chemotherapy [30] or intraperitoneal chemotherapy [31] in combination with surgical resection with curative intent have much improved survival. More impressively, Kuramoto et al. [32] demonstrated in a randomised controlled trial that patients with positive IPFCC without overt peritoneal metastasis and treated with the combination of surgery and extensive intraoperative peritoneal lavage followed by intraperitoneal chemotherapy have overall 5-year survival rate of 43.8%. On the other hand, some institutions [29,30] adopt a much more pragmatic approach and have also shown favourable survival outcome, with aggressive neoadjuvant chemotherapy followed by re-staging process – re-imaging, re-laparoscopy and repeat peritoneal washings. Surgery is performed if there has been no disease progression radiologically and patients have become IPFCC-negative, with a respectable 2.5 year of median disease-free survival. However, all these treatment modalities are not standardised or uniform, and mostly remain in the research or clinical trial phase.
Undeniably, peritoneal washings and the identification of IPFCC do provide invaluable information in the staging process of gastric cancer. It is therefore not a question of ‘to do it or not’, but to do it right with evidence-based approach in obtaining the specimen and pathological analysis. Although survival prognosis of patients with positive IPFCC is dismal, it should not be a death sentence for these patients in view of the emergence of several promising treatment modalities associated with improved survival. Therefore, these patients should be managed with individualised treatment pathways and enrolled into clinical trials. Hopefully this will lead to the development of a much awaited treatment algorithm which is uniform and universally accepted.
References
- Kodera Y (2008) Disseminated cancer cells in the peritoneal cavity: what can we do when we detect them? Gastric Cancer 11: 192-193.
- Lowy AM, Mansfield PF, Leach SD, Ajani J (1996) Laparoscopic staging for gastric cancer. Surgery 119: 611-614.
- Watt I, Stewart I, Anderson D, Bell G, Anderson JR (1989) Laparoscopy, ultrasound and computed tomography in cancer of the oesophagus and gastric cardia: a prospective comparison for detecting intra-abdominal metastases. Br J Surg 76: 1036-1039.
- Sarela AI, Lefkowitz R, Brennan MF, Karpeh MS (2006) Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg 191: 134-138.
- Kinkel K, Lu Y, Both M, Warren RS, Thoeni RF (2002) Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR imaging, PET): a meta-analysis. Radiology 224: 748-756.
- Smyth E, Schöder H, Strong VE, Capanu M, Kelsen DP, et al. (2012) A prospective evaluation of the utility of 2-deoxy-2-[(18) F]fluoro-D-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. 118: 5481-5488.
- Yoshioka T, Yamaguchi K, Kubota K, Saginoya T, Yamazaki T, et al. (2003) Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med 44: 690-699.
- Bentrem D, Wilton A, Mazumdar M, Brennan M, Coit D (2005) The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol 12: 347-353.
- Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, et al. (1999) Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 72 : 60-64.
- Japanese Gastric Cancer Association (1998) Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer 1: 10-24.
- Hori Y (SAGES Guidelines Committee) (2008). Diagnostic laparoscopy guidelines: this guideline was prepared by the SAGES Guidelines Committee and reviewed and approved by the Board of Governors of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), November 2007. Surg Endosc. 22: 1353-1383.
- Okines A, Verheij M, Allum W, Cunningham D, Cervantes A, et al. (2010) ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21 Suppl 5: v50-54.
- Sobin L, Gospodarowicz M, Wittekind C(2009) TNM classification of malignant tumours. 7th ed. New York: Wiley; 2009.
- Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, et al. (1999) Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg 178: 256-262.
- Li JK, Zheng M, Miao CW, Zhang JH, Ding GH, et al. (2005) Peritoneal lavage cytology and carcinoembryonic antigen determination in predicting peritoneal metastasis and prognosis of gastric cancer. World J Gastroenterol 11: 7374-7377.
- Cetin B, Atalay C, Aslan S, Babacan B, Hatipoqlu C, et al. (2005) Peritoneal carcinoembryonic antigen level for predicting locoregional and distant spread of gastric cancer. Surg Today 35: 919-924.
- Kodera Y, Nakanishi H, Yamamura Y, Shimizu Y, Torii A, et al. (1998) Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer. 79: 429-433.
- Boku T, Nakane Y, Minoura T, Takada H, Yamamura M, et al. (1990) Prognostic significance of serosal invasion and free intraperitoneal cancer cells in gastric cancer. Br J Surg 77: 436-439.
- Suzuki T, Ochiai T, Hayashi H, Hori S, Shimada H, et al. (1999) Peritoneal lavage cytology findings as prognostic factor for gastric cancer. Semin Surg Oncol 17: 103-107.
- Wang Z, Zhang X, Xu H, Zhou X, Jiang L, et al. (2005) Detection of peritoneal micrometastasis by reverse transcriptase-polymerase chain reaction for heparanase mRNA and cytology in peritoneal wash samples. J Surg Oncol 90: 59-65.
- Homma Y, Ushida S, Yamada M, Kobayashi H, Suzuki K (2010) Positive peritoneal washing cytology in multiple cavities can predict poor prognosis of advanced gastric cancer patients. Ann Surg Oncol 17: 455-460.
- Benevolo M, Mottolese M, Cosimelli M, Tedesco M, Giannarelli D, et al. (1998) Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. J Clin Oncol 16: 3406-3411.
- Yonemura Y, Fujimura T, Ninomiya I, Kim BS, Bandou E, et al. (2001) Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase-polymerase chain reaction for matrix metalloproteinase-7 mRNA. Clin Cancer Res 7: 1647-1653.
- Wang JY, Lin SR, Lu CY, Chen CC, Wu DC, et al. (2005) Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett 223: 129-135.
- Yonemura Y, Endou Y, Fujimura T, Fushida S, Bandou E, et al. (2001) Diagnostic value of preoperative RT-PCR-based screening method to detect carcinoembryonic antigen-expressing free cancer cells in the peritoneal cavity from patients with gastric cancer. ANZ J Surg 71: 521-528.
- Sugita Y, Fujiwara Y, Taniguchi H, Mori T, Ishii T, et al. (2003) Quantitative molecular diagnosis of peritoneal lavage fluid for prediction of peritoneal recurrence in gastric cancer. Int J Oncol 23: 1419-1423.
- Nakanishi H, Kodera Y, Yamamura Y, Ito S, Kato T, et al. (2000) Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer 89: 411-417.
- Fujiwara Y, Doki Y, Taniguchi H, Sohma I, Takiguchi S, et al. (2007) Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer 10: 197-204.
- Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, et al. (2010) Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol 17: 3173-3180.
- Lorenzen S, Panzram B, Rosenberg R, Nekarda H, Becker K, et al. (2010) Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Ann Surg Oncol 17: 2733-2739.
- Yonemura Y, Endou Y, Shinbo M, Sasaki T, Hirano M, et al. (2009) Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol 100: 311-316.
- Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, et al. (2009) Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 250: 242-246.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 16371
- [From(publication date):
December-2013 - Oct 27, 2025] - Breakdown by view type
- HTML page views : 11620
- PDF downloads : 4751