Research Article Open Access
Pectinmethylesterase Production from mixed agro- wastes by Penicillium notatum NCIM. 923 in Solid-State fermentation
| Soumi Gayen* and Uma Ghosh | |
| Department of Food Technology & Biochemical Engineering Jadavpur University, West Bengal, Kolkata, India | |
| Corresponding Author : | Soumi Gayen Department of Food Technology & Biochemical Engineering Jadavpur University, West Bengal, Kolkata, India, E-mail: ssoumigayen@yahoo.co.in |
| Received April 16, 2011; Accepted June 15, 2011; Published June 17, 2011 | |
| Citation: Gayen S, Ghosh U (2011) Pectinmethylesterase Production from mixed agro- wastes by Penicillium notatum NCIM. 923 in Solid-State fermentation. J Bioremed Biodegrad 2:119. doi:10.4172/2155-6199.1000119 | |
| Copyright: © 2011 Gayen S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The enzyme pectinmethylesterase (PME; EC 3.1.1.11) catalyzes the hydrolysis of the methyl ester groups from pectin and has been found in plants as well as in pathogenic fungi and bacteria. PME is of significance to the citrus industry because it has been established as the causative agent for juice clarification and gelation of frozen concentrates. The fruit processing industries produce a large amount of waste material, which poses considerable disposal problems and ultimately leads to pollution. Dried citrus peel is rich in carbohydrates, proteins and pectin; pectin acts as the inducer for production of pectinolytic enzymes by microbial systems. Thus, in the present study, dried citrus peel and wheat bran was used as substrate for the production of pectin methyl esterase (PME; EC 3.1.1.11) by fungus Penicillium notatum . Maximum enzyme activity was obtained with 1:1(w/w) substrate ratio, which gives a solid mass of initial pH 5.5, when incubated at 30°C for 120 h at 1:1 (w/v) initial moisture content ratio under static condition.
| Keywords |
| Pectin methyl esterase; Penicillium notatum; Solid mass, Moisture; Static condition |
| Introduction |
| In last few years there has been an increasing trend towards efficient utilization and value-addition of agro-industrial residues such as wheat bran, coffee pulp, sugarcane bagasse, apple pomace and others. Biotechnological processes, especially the solid substrate fermentation, have contributed enormously to such reutilization. The application of agro-industrial residues in solid substrate fermentation bioprocesses not only provides an alternative substrate but also helps to solve some of the pollution problems caused by their accumulation. Pectic substances (pectins) in plant tissues and enzymes that degrade them are of major importance to the food industry because of their effect on the texture of foods, such as apples, peaches, and tomatoes, and in the preparation of wines and fruit juices. Microorganisms have long played a major role in the production of food (dairy, fish and meat products) and alcoholic beverages. In addition, several products of microbial fermentation are also incorporated into food as additives and supplements (antioxidants, flavours, colourants, preservatives, sweeteners, . . .). There is great interest in the development and use of natural food and additives derived from microorganisms, since they are more desirable than the synthetic ones produced by chemical processes. Solid substrate fermentation is defined as any fermentation process performed on a non-soluble material that acts both as physical support and source of nutrients in absence of free flowing liquid [1]. Solid substrate fermentation offers numerous advantages for the solid substrate fermentation offers numerous advantages for the production of bulk chemicals and enzymes [2-4]. The nature of the solid substrate employed is the most important factor affecting solid substrate fermentation processes and its selection depends upon several factors mainly related with cost and availability and, thus, may involve the screening of several agro-industrial residues. In solid substrate fermentation process the solid substrate not only supplies the nutrients to the culture but also serves as an anchorage for the microbial cells. Among the several factors, which are important for microbial growth and activity in a particular substrate, particle size and moisture level/ water activity are the most critical [5-14]. Research on the selection of suitable substrates for solid substrate fermentation has mainly been centered around agro-industrial residues due to their potential advantages for filamentous fungi, which are capable of penetrating into the hardest of these solid substrates, aided by the presence of turgor pressure at the tip of the mycelium. In addition, the utilization of these agro-industrial wastes, on the one hand, provides alternative substrates and, on the other, helps in solving pollution problems, which otherwise may cause their disposal [15]. Pectinase enzymes include pectin methylesterase (pectin esterase) and depolymerising enzyme (polygalacturonase and lyases). Pectinolytic enzymes, capable of degrading pectin and leading tomaceration of planttissues, are the first enzymes secreted by most fungal pathogens when attacking plant cell walls [16-17]. Pectin degradation can be attained by the combined action of several enzymes such as pectinmethylesterases and pectin depolymerases, including hydrolases and lyases, such as polymethylgalacturonase and pectinlyase. Degradation involves the breakdown of polygalacturonic acid through two enzymatic processes: lyases split the ∞-1-4 glycosidic bond between galacturonic acid residues by trans-limitation, while polygalac- turonases catalyze a hydrolytic cleavage. Pectinases find extensive applications in fruit processing industries including clarification of fruit juices, wines, extraction of fruit juice, in the manufacturing of pectin free starch, or curing of coffee. Solid substrate fermentation was carried out by Thermoascus aurantiacus 179-5 using carbon containing wastes of orange bagasse, sugar cane bagasse and wheat bran for production of pectic enzymes of pectin lyase (Pl) and polygalacturonase(Pg) [18]. Alkaline and thermotolerant pectinase enzyme production was studied by Kashyap et al. by Bacillus sp. DT7 using solid substrate fermentation. Production of this enzyme was affected by nature of solid substrate, level of moisture content, presence or absence of carbon, nitrogen, mineral and vitamin supplements [19]. Comparison of the production of pectin methylesterase (PME) by Aspergillus niger from apple pomace in solid- substrate and submerged fermentation was made for higher PME production. Overall, the solid substrate fermentation gave 2.3 times higher PME activity than submerged fermentation, using optimized parameters of fermentation [20]. Utilization of orange peels as an agroindustrial waste for production of pectin lyase (PL) [E.C.4.2.2.10] by Curvularia inaequalis (Shear) Boedijn NRRL 13884 was investigated by Afifi et al using solid state culture [21]. |
| Materials and Methods |
| Microorganisms |
| The present investigation was started with the organisms of Penicillium notatum NCIM. 923 which were collected from National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory, Pune (India) were maintained on Czapek Dox medium at 30°C for 6 days. A spore inoculum was prepared by adding a loopful of spores from the slant to 5 mL of sterile distilled water and shaken vigorously. The inoculums was used to inoculate the fermentation medium [22]. |
| Raw materials |
| Agro-industrial residues of wheat bran and orange peel are collected from the local market; the orange peel was thoroughly washed with distilled water and oven dried at 60°C for 24h up to final moisture content 8-10%. Dried orange peel was ground into fine powder and both separately packed in polyethylene pouches for further studies [23]. |
| Extraction of enzyme |
| For enzyme assay [24] of PME pectin, water and sodium hydroxide (NaOH) were required. The amount of acid produced was neutralized by 0.02 (N) NaOH solutions. One unit of PME was defined as the amount of enzyme that released 1 µmol of carboxyl groups/min. PME activity was calculated by using the following formula [25]. |
 |
| Enzyme Activity then calculated to gds (gram dry solid). |
| Determination of cell growth |
| Cell growth was determined as dry cell mass. The mat was collected by filtration, and dried at 65-70°C till constant weight was obtained. |
| Specific activity |
| Specific activity is defined as the amount of substrate converts by the enzyme (reactions catalyzed), per mg protein in the enzyme preparation, per unit of time. Specific activity is a term used for measuring enzyme kinetics (rate of reaction of an enzyme with a particular substrate). That is the amount of product formed by an enzyme in a given amount of time under given conditions per milligram of total protein. The significance or importance of measuring specific activity is that specific activity is a measure of enzyme purity. The value becomes larger as an enzyme preparation becomes more pure, since the amount of protein (mg) is typically less, but the rate of reaction stays the same (or may increase due to reduced interference or removal of inhibitors). In the present study specific activity of crude enzyme was found 128 U/g of biomass. |
| Analytical methods |
| Moisture content of substrate was determined by AOAC method (AOAC, 1985) [26]. |
| Solid substrate fermentation process |
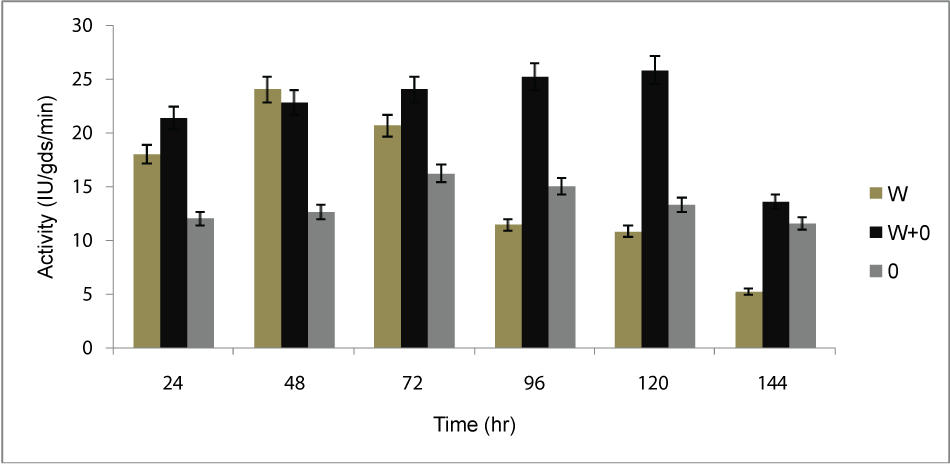
| Inoculation time: Partially dried orange peel (OP, 5g), wheat bran (WB, 5g) and mixture of OP and WB (1:1 w/w) was taken separately into three different conical flasks, and fermentation was carried out after inoculation with the organism, all flasks were incubated at 30°C for 6 days. Assay was done after 24 hr of interval. Optimum time was observed in the 120 hr (Figure 1) for the maximum production of PME by Penicillium notatum NCIM.923 in solid substrate fermentation under static conditions. |
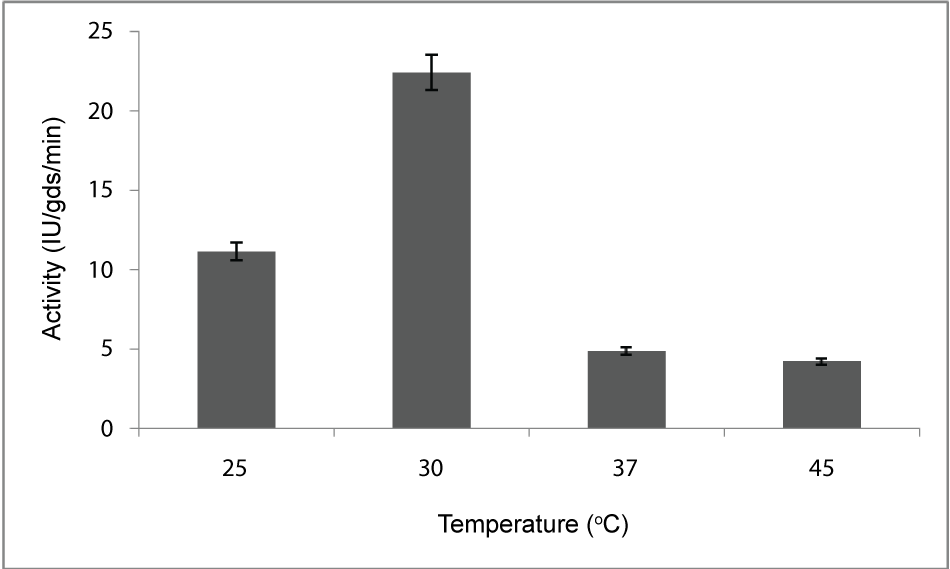
| Effect of temperature: To find out the optimum temperature of enzyme production, the organism was allowed to grow at different temperatures ranging from 20 to 45°C. Optimum temperature observed in this study was 30°C (Figure 2) for the maximum production of PME by Penicillium notatum NCIM. 923 in solid substrate fermentation conditions. |
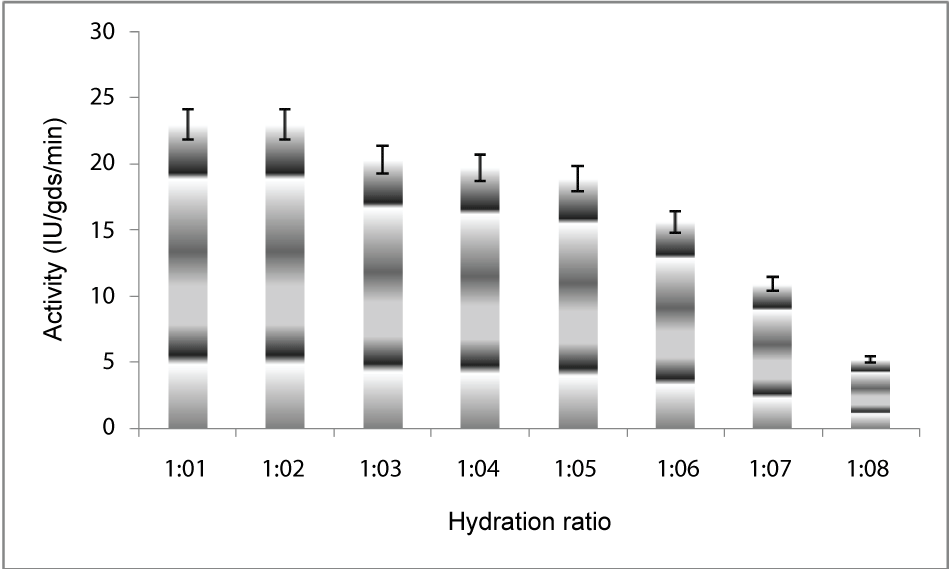
| Effect of hydration levels: To determine the optimum hydration ratio for maximum PME production in solid substrate fermentation, the substrate was supplemented with different quantities of water(1:1,1:2,1:3,1:4,1:5,1:6,1:7,1:8) and then inoculated at 30°C for 5 days of fermentation. Substrate to water ratio that gave the highest PME production was selected for further studies (Figure 3). |
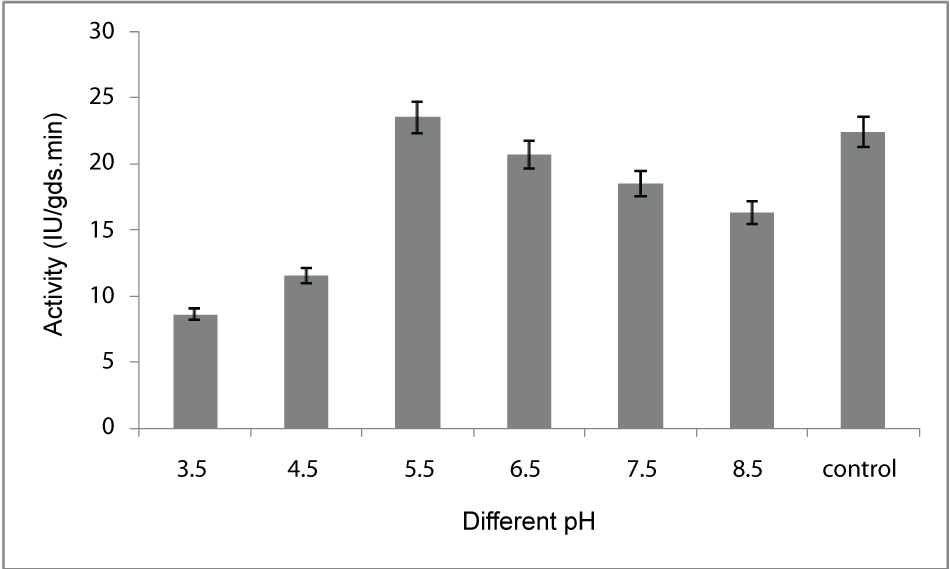
| Effect of pH: For optimization of pH the fermentation media was adjusted to 3.5, 4.5, 5.5, 6.5, 7.5, 8.5 using 1(N) HCl and 1(N) NaOH solution. After inoculation, all flasks were incubated at 30°C for 5 days of fermentation and the enzyme production was measured as described earlier. Among the various pH tested, maximum production of enzyme (23.5 IU/gds/min) was observed at pH 5.5 (Figure 4). |
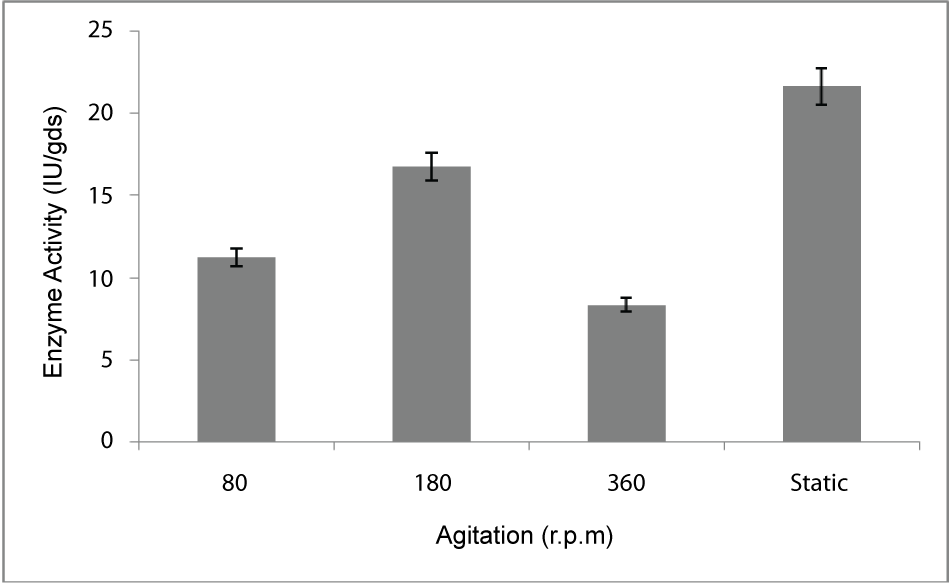
| Effect of agitation: PME production in batch culture was conducted under three different agitation speeds (80, 180, 360 rev/min and stationary) under constant air supply throughout the fermentation batch. The effect of agitation speed on PME production was shown on Figure 5. Highest PME production was 21.6 U/gds/min which was obtained at stationary condition |
| Result and Discussion |
| The PME conditions optimized for the strain Penicillium notatum NCIM. 923 selected in the present investigations included type of medium, its temperature, incubation time, hydration, pH and agitation. During the present investigations, maximum production of PME at 120h of incubation has been observed. Aspergillus heteromorphus showed maximum production of PME at 144h of incubation has been observed which was reported by [27]. Maximum production of pectic enzyme from different moulds varies from 1-6 days [28]. Penicillium frequentans [29] showed maximum pectinase activity at the 60th hour. Temperature is another very important factor which is known to influence the metabolic rate of the organism involved in the process, which in turn determines the amount of the end product. Temperature has been observed to be one of the major process variables affecting the production of PME in solid substrate fermentation conditions. Environmental temperature is one of the most important factors affecting the growth rate of microbes. These reactions are mostly enzyme catalyzed. However, a point is reached the optimum temperature when there is also a very rapid increase the rate of inactivation of heat sensitive cell components, like enzymes, ribosomes, DNA, membranes etc. Above an optimum temperature, this heat denaturation will occur so rapidly that there is a corresponding rapid drop in the rate of growth to give a maximum temperature for growth for that particular microorganism. Optimum temperature of 30°C for maximum production observed in present case falls within the range of 21°C [30] to 35°C [31] already reported. Inter particle mass transfer of oxygen, nutrients and enzymes depend on nature of solid substrates taken and moisture content of solid substrate fermentation media. Maximum yield was obtained at the dilution ratio of 1:1 and 1:2 in the mixed (OP: WB, 1:1, w/w) fermentation media. Fungi are well known to favor a moist environment for their growth. An optimum moisture level has to be maintained, as lower moisture tends to reduce nutrient diffusion, microbial growth, enzyme stability and substrate swelling [32,33]. Higher moisture levels leads to particle agglomeration, gas transfer limitation and competition from bacteria [34]. When grown in medium with different pH values, maximum production of enzyme was observed at pH 5.5. On either side of this optimum pH, the PME producing ability declined. The variation in PME production due to change in pH may be because of maximum availability of nutrients at that particular pH, as has been reported by [30]. Semenova et al. (2003) [35] also reported that the production of pectinase by A. japonicum was maximum in acidic medium. The influence of wide range of pH from 2.3 [36] to 7.2 [37] on the production of microbial pectinase from different substrates has been reported. Maximum production of PME was obtained at static condition which was 21.6 IU/gds/min. |
| References |
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14593
- [From(publication date):
June-2011 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 10164
- PDF downloads : 4429
