Research Article Open Access
Orchid Micropropagation: Regeneration Competence of Anther Culture
Vishal Sharma*Govt. P.G. College for Girls, Sector 11, Chandigarh (Panjab University), India
- Corresponding Author:
- Vishal Sharma
Govt. P.G. College for Girls, Sector 11
Chandigarh (Panjab University), India
E-mail: Vishal_2370@yahoo.com
Received date: August 10, 2012; Accepted date: September 25, 2012; Published date: September 28, 2012
Citation: Sharma V. (2012) Orchid Micropropagation: Regeneration Competence of Anther Culture. J Biotechnol Biomater 2:149. doi:10.4172/2155-952X.1000149
Copyright: © 2012 Sharma V, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The possibility of using anthers for initiating in vitro cultures of Rhynchostylis retusa (Orchidaceae), has been positively tested. The study was designed to study the effects of physiological status, stage of microspore development and pre-cold treatment on androgenic response in R.retusa. The anthers from open flowers (2 days of anthesis) in tetrad stage failed to respond despite variations in the chemical regime; whereas those from unopened buds (1.25-1.35cm long) with an intact operculum & pre-cold treatment with microspores in early & late-uninucleate decussate regenerated provided their nutritional complexities were satisfied through an exogeneous supply of Peptone (2mg/l) in the BAP (10mg/l) and NAA enriched Sharma medium. The best response was obtained when a 24hrs cold treatment was employed at 4°C in darkness. The complete plantlet (2-3 leaves &1-2 roots) was formed in 24wks.The plantlet was accilimatized & the survival rate is 70%.
Keywords
Orchids; Flowering plant; Anther culture; Callus; Pretreatment
Introduction
Androgenesis in flowering plants is a unique biological phenomenon. The principle of androgenesis is to arrest the development of the pollen grains and to force them towards a somatic pathway. Antherculture is the main technique for haploid induction in crop improvement.
Since Guha and Maheshwari [1,2] reported the induction of haploid plants from Datura innoxia, this technique became important for plant breeding and crop improvement [3]. Anther culture has become a powerful tool for the rapid production of haploid and inbred lines used for obtaining hybrid cultivars [4] and it has reduced the time required for breeding new cultivars by at least 3 to 5 years [5]. Hence, this system provides an unparalleled opportunity to shorten the breeding cycle and fix agronomic traits in the homozygous state and provides excellent material for research, plant breeding and plant transforma [6]. Production of haploids plants have been useful in providing access to recessive genes and for biotechnological manipulations, while using as a tool for cultivar development. Therefore, in vitro techniques are considered to be alternative tools of conventional method of plants improvement [7]. It is particularly useful in out breeders like orchids which generate a great deal of heterozygosity in the progenies.
Till now there is one report on anther culture in Orchids [8], but attempts to assess a similar competence of anther culture in monocots including Orchidaceae, have remained almost negligible because of the following reasons: (a) Little success in inducing callus and maintaining proper growth, (b) difficult to obtain suitable size of homogenous tissue from monocotyledonous plants [8].
In this paper, we report the possibility of using anthers for initiating in vitro cultures of Rhynchostylis retusa Bl. (Orchidaceae), a genus of fox tail orchid, is an important stem herb. The stem extract of R. retusa commonly known as ‘Rasna’ is used as expectorant for curing rheumatic diseases [9]. Besides being victim of its own beauty & utility R. retusa is progressively losing its natural habitat and heading towards extinction particularly in Sri Lanka [10].
Materials and Methods
Plant materials
Anthers of R. retusa were the experimental materials in the present investigations. The mother plants were collected in nature from Garhwal Himalayas Narendra Nagar (1000 m), grown in the pots in greenhouse, Panjab University, Chandigarh, using standard agronomic practices. The twigs with young inflorescence were harvested from stock plants were used as material for the present study.
Physiological status of the donor plants
The physiological status of the donor plants at the time of anther excision influences the sporophytic efficiency of microspores. Flowers from relatively young plants at beginning of flowering season were more responsive. Besides this use of any kind of pesticide, whether externally applied or systemic should be avoided 3-4 weeks preceding sampling. The donor plant should be taken care of from the time of flower induction to the sampling of pollen.
Study on relative of microspore development stage and flower bud shape
The flower buds of different size were took form Orchid plants at 6.00 A.M to 6.30 A.M and fixed in farmers fluid (1: 3: 6; Acetic acid: Chloroform: Alcohol) for 24 hrs and stored in 70% alcohol until use. More than 10 flower buds of each size were studied. The floral buds (1.0-2.0 cm) were taken and young anthers were squashed in 2% aceto-carmine solution to find out the exact stage of pollen development. After anther walls and residue removed, covered by cover slip, micro-slides were pressed and observed under microscope. Pollen was considered to be in certain microspore development stage if the number of microscopes in this stage was more than 50%. The relationship between length of flower buds and development stage of microspores were found.
Effect of Microspore Development Stage
It has been established that selection of anthers at an appropriate stage of pollen development is most critical. Anthers with microspores ranging from tetrad stage to the binucleate stage were inculated on BM [11] medium, Murashige & Skoog [12] and Sharma (Table 2) medium supplemented with NAA( α-naphthalene acetic acid), BAP (6-Benzyl amino purine) & Peptone.
Pre-treatment of Anthers
(i) Effect of pre-cold treatment: Anthers of flower bud were pre-treated under 4ºC for 0 h, 8 h, 24 h and 48 h were inoculated on various combinations with NAA (α-naphthalene acetic acid), BAP (6-Benzyl amino purine), KN (Kinetin) & Peptone. It is possible that a pre-cold treatment retards ageing of the anther wall, allowing high proportion of microspores to change their developmental patterns from gametophytic to sporophytic.
(ii) Hot treatment: Flower buds after inoculation were transferred to culture room at 25 ± 2°C.This temperature shock appears to cause dissolution of microtubules and dislodging of the spindle which causes abnormal division of the microspore nucleus.
Culture media
Effects of medium composition on callus induction was done in present studies. On the basis of preliminary experimental results, a refined experiment was designed where Murashige and Skoog [20], Mitra et al. [4], medium, was compared with Sharma (Table 2). Callus induction rates of the cultured anthers were determined on day 30 and are shown in table 1. It is clear that present medium (Table 2) medium was more conducive to the induction of callus than Murashige and Skoog [20] and Mitra et al. [4], medium when supplemented with the BAP, NAA and peptone. The composition of medium is one of the important factors determining not only the success of anther culture but also the mode of development. Since explants failed to regenerate despite repeated subcultures on different medium, the effect of CuSO4.5H2O on the anther regenerative competence, was tested by varying the CuSO4.5H2O level (0.08, 0.20, 0.80, 1.2, 1.8 and 2.2 mg/l) in the different media. (Table 2) and the concentration (2.2 mg/l) favours androgenesis in compliance to earlier reports in cereals.
| Medium | Number of Anthers inoculated | Number of Anthers responded |
|---|---|---|
| MS(1962)#+BAP*+NAA#+Peptone | 300 | - |
| BM (1976)#+ BAP*+NAA#+Peptone | 300 | - |
| Sharma#+BAP*+NAA#+Peptone | 300 | 8.3± 4.16 |
BAP (1-10 mg/l); NAA (1-10 mg/l); Peptone (1-2 mg/l); #medium in present study with increased concentration of CuSO4.5 H2O(2.2 mg/l)
Table 1: Effects of medium composition on callus induction in Orchid anther culture.
| S.NO | CONSTITUENTS | Sharma medium (mg/l) |
|---|---|---|
| 1 | Macronutrient Ca(NO3)2.4H2O | 200 |
| 2 | KNO3 | 180 |
| 3 | (NH4)2.SO4 | 100 |
| 4 | NaH2PO4. 2H2O | 150 |
| 5 | MgSO4.7 H2O | 250 |
| 6 | Micronutrient KI | 0.03 |
| 7 | MnCl2. 4H2O | 0.4 |
| 8 | ZnSO4. 7 H2O | 0.05 |
| 9 | H3BO3 | 0.6 |
| 10 | CuSO4.5 H2O | 2.2 |
| 11 | Na2MoO4 | 0.05 |
| 12 | CO(NO3)2.6H2O | 0.05 |
| 13 | MnSO4. 7 H2O | |
| 14 | CoCl2 6 H2O | |
| 15 | Na2FeEDTA | Separate stock solution of Na2FeEDTA is preparedby disolving 0.745g of Na2EDTA and 0.557g of FeSO4in100ml ofdistilled water.3 ml/l of this solution is used. |
| 16 | Vitamins Thiamine - HCL | 0.3 |
| 17 | Pyridoxine - HCL | 0.3 |
| 18 | Nicotinic acid | 1.25 |
| 19 | Riboflavin | 0.05 |
| 20 | Biotin | 0.05 |
| 21 | Folic acid | 0.3 |
| 22 | Sucrose | 20 g/l |
| 23 | Agar | 9 g/l |
| 24 | BAP | 10 mg/l |
| 25 | NAA | 1 mg/l |
| 26 | Peptone | 2 mg/l |
Table 2: Composition of Induction basal media commonly used in Orchids anther culture.
Inducing callus from orchids anther method
All operations were carried out in laminar air-flow cabinet under aseptic conditions. The flower buds were dipped in Ethanol (70%, 30 seconds) and Streptomycin (0.1%, 20 min) and immersed in Sodium hypochlorite (4%, 15 min), with periodic agitation for 5 minutes and washed with sterile distilled water for five times. After filament removed, the intact anthers were inoculated on inducing medium. The unopened floral buds were given pre-cold treatment for 0-72 hrs at 4°C. The anthers from sterilized floral buds (1-2 cm)were inoculated on sucrose (2%) supplemented and agar (0.9%) gelled Sharma (Table 2) basal medium, and its various combinations with NAA (α-naphthalene acetic acid), BAP (6-Benzyl amino purine), KN (Kinetin) & Peptone. The pre-inoculation medium pH was adjusted at 5.6. In parallel set of experiments 0.2% activated charcoal (AC) was used in the medium. Sixteen replicates for each treatment and the experiments were repeated several time. The physical environmental conditions in which the cultures are placed can enhance differentiation.The cultures incubated at 25 ± 2°C (23-27°C) in dark for 10 days in the initial stages of induction of morphogenesis, darkness is normally more effective. After induction, kept under 16 hrs illumination with 3500 lux light intensity.
Accilimatization of the Plantlet
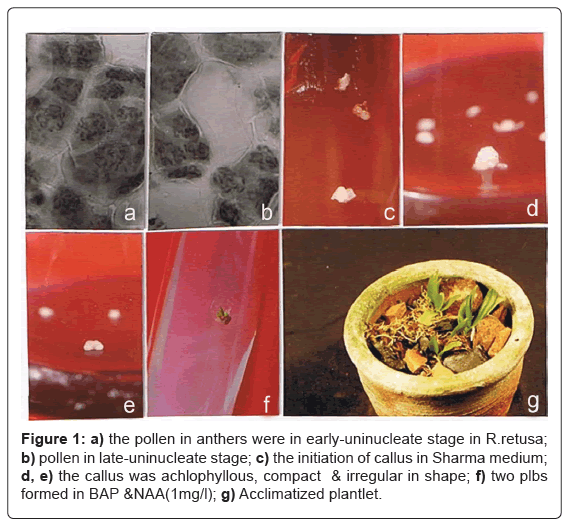
After well-developed shoot and root formation the plantlets (3 cm tall) were transferred to semisolid medium containing only half strength macro & micro salts of BM(4) medium; sucrose and vitamins were elimated. The plantlets were kept in this condition until they are 4-5cm tall, and washed with luke warm water before transfering to moss, pinebark, brick and char - coal pieces (1:1:1:1) mixture. Humidity was maintained by covering each pot with transparent polythene bag. Holes of increasing size were made in the bags to reduce the humidity level gradually. The bags were removed after 4 weeks and small plants in the pots were transferred from 90% shade to the sunlight. Survival rate was 70%. Spraying with fungicide (Bavistin 1%) twice a week was necessary to keep fungus off from the young plants. Figure 1g shows an acclimatized plantlet.
Data collection and analysis
A randomized complete block design was used for callus induction experiments. 300 anthers were cultured (6 anthers per conical flask and 16 replicates per treatment) and the experiments are repeated several time. Cultures were recorded at regular intervals for 24 weeks.
Results
Relativity of flower bud and microspore stage
The microspore stage is a main factor for induction of androgenesis. In R.retusa early to late- uninucleate stage optimal for induction (Table 4). The relativity of flower bud and microspore stage was summarized. When floral bud length was <1.25 cm, it was in tetrad stage. When floral bud length was 1.25-1.30 cm, the pollen in anthers were in earlyuninucleate stage (Figure 1a). When floral bud length was 1.30-1.35 cm, they were in late-uninucleate stage (Figure 1b) and when floral bud length was more than 1.35 cm, it started binucleate stage, even become mature microspore.
Effect of pre-cold treatment on the callus formation from anthers: The pre-treatment of floral buds with low temperature is well tried technique for enhancing androgenesis in cultured anthers of many species [13]. It is also an essential step for enhancing the ratio of responding anther (Table 3). Thus, maintaining anthers for periods ranging from 24-48 hrs at 4°C prior to culture stimulates androgenesis in R.retusa. Analysis of variance of the callus induction rate indicated that callus formation was significantly affected by pre-cold treatment and callus formation rate of R. retusa increased after anthers pre-treated under 4°C condition for 24 hrs. On contrary, when cold treatment (4°C) was 0hrs, 8hrs, 48hrsand 78hrs either no anthers formed callus or anthers become necrotic within one week.
| Pre-Cold treatment time | Callus formation rate(%) Rhyncostylisretusa |
Remarks |
|---|---|---|
| 0hrs | - | Anthers failed to respond & become necrotic . |
| 8hrs | - | Anthers failed to respond & become necrotic . |
| 24hrs | 12.5% | Friable, acholophyllous and organogenetic callus formed |
| 48hrs | 12.5% | Anthers become necrotic within one week within one wk of culture. |
| 72hrs | - | Anthers failed to respond & become necrotic . |
Table 3: Effect of Pre-Cold treatment on Callus induction of Anthers.
Effect of microspore development stage on callus formation from anthers
The Effect of microspore development stage on callus formation from anthers of Rhyncostylisretusa was statistically significant (Table 4). The early to late uninucleate stage of microspores were most suitable for androgenic response and highest inductivity of callus was observed when anthers were in late –uninucleate stage, while lowest occurred in anthers of tetrad stage (Table 4).
| Microspore development stage | No. of Proliferative Loci/Explant | Pathway | Callus formation rate(%) | No.of Plantlet obtained/ explant |
Remarks |
|---|---|---|---|---|---|
| Tetrad | - | - | - | - | - |
| Early-uinucleate | 4 | Callus mediated | 12.5 | - | Callus is non-organogenetic |
| Late- uninucleate | 4 | Callus mediated Plb development | 12.5 | 4 | Callus is achlorophyllous, friable&organogenetic |
Table 4: Effect of Microspore Development Stage on Callus induction of Rhyncostylis retusa Anthers in BM+BAP(10mg/l)+Peptone(2mg/l).
Effect of BAP,auxin and peptone on the callus formation
The callus formation rate of anthers reached the highest when medium added with cytokinin (BAP), Auxin (NAA) and Peptone. The callus formation was obligatory to 10 mg/l treatment of cytokinin and 1 mg/l auxin and Peptone (2 mg/l), unless the CuSO4.5H2O concentration was increased (2.2 mg/l) in the nutrient pool. In R.retusa 12.5% explants responded to a treatment with 10mg/l BAP and 1 mg/l NAA, hence the BAP and NAA was essential for induction in anthers, when microspores in late-uninucleate stage. The callus was achlophyllous, compact & irregular in shape & organonogenetic (Figures 1c-1e), whereas the callus formed in early-uninucleate stage is non-organogenetic (Table 4).
Regeneration of plants
Calluses of about 4-5 mm in size were transferred from the callus induction media and cultured onto Sharma (Table 2) medium supplemented with 1% sucrose, 1 mg/l each of BAP and NAA for the induction of shoots under a 16 hrs light photoperiod with a light intensity of about 3500 lux at 25 ± 2°C.
Discussion
The genotype, culture media, physiological status of donor plant, anther wall factor, stage of pollen development, and effect of temperature and light basically influence callus induction and plant regeneration frequently from immature anther. Anther culture and subsequent plant regeneration offer an alternative and efficient technique to conventional breeding method and enable production of several plants from single anther.
The anthers of R.retusa, from open flowers (2days of anthesis) failed to respond despite variations in chemical regime due to enhanced production of ethylene in the flower from anthesis onwards ,is another factor favouring regenerative competenent nature of unopened floral buds, as ethylene is inhibitory to regeneration [14], whereas, those from unopened buds (1.25 - 1.35 cm long; 14 days prior to anthesis) with an intact operculum & provided their nutritional complexities were satisfied through an exogeneous supply of Peptone (2 mg/l) in the BAP (10 mg/l) and NAA enrichedSharma (Table 2) medium supplemented with higher dose of CuSO4.5H2O. A similar promotory role of higher doses of CuSO4.5H2O has already recorded in several dicots and monocot plants [15]. Since Cu2+ is a component and/or factor of many important enzymes involved in electron transport, and protein and carbohydrate biosynthesis. In this connection, it is worthwhile to mention that zygograms of certain enzymes are considered as useful markers of morphogenesis in tissue raised cultures. In most cases, the early to late uninucleate stage of microspores were most suitable for androgenic response in Maize, Barley, Triticum [6]. However, in most plants, it was easier to form callus for pollen in late-uninucleate stage.The present results also showed that anthers of late-nucleate stage were induced the organogenetic callus in R.retusa.
A perusal of literature reveals that studies related to embryogenetic competence of anthers has been few and far between in orchids. Infact, prior to the present studies Dendrobium ’Tomie White’ represents only taxon where the totipotency of pollen tetrads were realized in vitro [8]. However, in Dendrobium ’Tomie White’ requires Coconut water in Vacin & Went [16], medium was obligatory for the anther regeneration [13]. The organic supplement coconut water is replaced with peptone in present studies.
Application of cold treatment has become an essential measure to increase the efficiency of androgenesis in many species [14]. Literature reveals operculum was necessary to production of embroids in anther culture because operculum extracted some chemical substances which promoted the developing structure [17].
While the morphogenetic changes leading to plantlet development is not clearly described in Dendrobium‘Tomie White’ [8], these were callus mediated in present studies. The callus was achlophyllous, friable, & irregular in shape. About 30 days later, some large calluses reached 5-7 mm were ready for transfer for the induction of shoots and shoots regenerated were excised from the mother tissues and cultured on only auxin enriched medium (NAA; 3 mg/l) for initiation of roots.
The embryogenetic potential of callus was obligatory to a subculture in BAP & NAA (1 mg/l) & Sucrose (1%) supplemented medium, for the induction of shoots under a 16 hrs light photoperiod with a light intensity of about 3500 lux at 25 ± 2°C. Two PLBs were obtained from each of the responding explant in 12 wks (Figure 1f). The complete plantlet (2-3 leaves & 1-2 roots) was formed in 24 weeks.
References
- Guha S, Maheshwari SC (1964) In vitro production of embryos from anthers of Datura. Nature 204: 497
- Guha S, Maheshwari SC (1966) Cell division and differentiation of embryos in the pollen grains of Datura in vitro. Nature 212: 97-98
- Nitsh JP, Nitsh C (1969) Haploid plants from pollen grains. Science163: 85-87.
- Sopory SK, Munshi M (1996) Anther Culture In vitro Haploid Production in Higher Plants, eds. SM Jain, Sopory SK, Veilleus RE ) 145-176 Kluwer Academic Publishers, Netherlands.
- Tai TH (2003) Rice Biotechnology, In: Rice; Origin, History, Technology and Production, eds. Smith CW, Dilda RH 203-220
- Datta SK (2005) Androgenic haploids: Factors controlling development and its application in crop improvement. Current Science.89th ed.vol.11: 870-1878
- Sayem MA, Siddique SS, Al-Amin M (2010) In vitro shoot regeneration through anther culture of Brassica spp. Bangladesh J Agril Res 35: 331-341.
- Suryowinoto M, Somaryo L (1985) Totipotency of Pollen tetrads in Dendrobium Tomiewhite .In:Proc.4thASEAN Congress Philippines’, ed. HL Valmayor, pp.185-Phillipine Council for Agriculture and Resource Research and Development and the Ministry of foreign affairs, Phillipines
- Lawler LJ (1984) Ethanobotany of the orchidaceae, Orchid Biology: Reviews and Prespectives. 27-149. Cornell Univ. Press, Ithaca, London.
- Wicramasinghe RH (1992) Lanka’s orchids under threat. Malayan Orchid Rev 26: 23-27
- Mitra GC, Prasad RN, ROY Chowdhary A (1976) Inorganic salts and differentiation of protocorms in seed callus of an orchid and correlated changes in its free amino acid content. Indian J Exp Biol 14: 350-351.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497
- Maheshwari SC, Tyagi AK, Malhotra K, Sopory SK (1980) Induction of haploid from pollen grains in angiosperms - the current status. TAG Theoretical and Applied Genetics 58: 193-206
- Khalid M, Chraibi B, Latche A, Raustan JP, Fallot J (1991) Stimulation of shoot regeneration from cotyledons of Helianthus annuus by ethylene inhibitors silver and cobalt. Plant Cell Reports 10: 204-207.
- V Sharma, SP Vij (1997) Effect of CuSO4.5H2O on in vitro regenerative capacity on foliar explants excised from mature Vanda cristata Lindl.plants. Phytomorphology 47: 2: 203-208
- Vacin EF, Went F (1949) Some pH changes in nutiernt solutions. Bot Gaz 110: 605-613.
- Letarte J, Simion E, Miner M, Kasha KJ (2006) Arabinogalactans and arabinogalactan-proteins induce embryogenesis in wheat (TriticumaestivumL.) microspore culture. Plant Cell Rep 24: 691-698.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 17043
- [From(publication date):
October-2012 - Dec 06, 2025] - Breakdown by view type
- HTML page views : 12027
- PDF downloads : 5016