Research Article Open Access
Optimization of Chitin Extraction from Chicken Feet
Aveen Faidhalla Jalal, Chinar Mohammed Risheed* and Bnar Mahmod Ibrahim
Department of Chemistry, Scientific-Education College, Salahaddin University, Iraq
- *Corresponding Author:
- Chinar Mohammed Risheed
Department of Chemistry
Scientific-Education College
Salahaddin University
Irbil, Kurdistan Region, Iraq
E-mail: chinar_jaf@yahoo.com
Received date: June 30, 2012; Accepted date: September 18, 2012; Published date: September 24, 2012
Citation: Jalal AF, Risheed CM, Ibrahim BM (2012) Optimization of Chitin Extraction from Chicken Feet. J Anal Bioanal Tech 3:145. doi: 10.4172/2155-9872.1000145
Copyright: © 2012 Jalal AF,, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The objective of present paper is to find optimal conditions for the extraction of chitin from chicken feet, so a chemical processing has been applied, to remove calcium carbonate (demineralization) and proteins (deproteinization). The optimum conditions included an acid concentration of 1.5 N, solid to acid ratio of 1:18 (w:v) at room temperature, the best yield and color were obtained with 1.0 N and 1:10 (w:v) of sodium hydroxide concentration and solid to base ratio respectively at 90°C. The yield of extracted chitin was found to be 15.2% with a DA of 91.66%. The FT-IR spectrum of the extracted chitin was compared with the standard chitin with a satisfactory result.
Keywords
Chitin; Chicken feet
Introduction
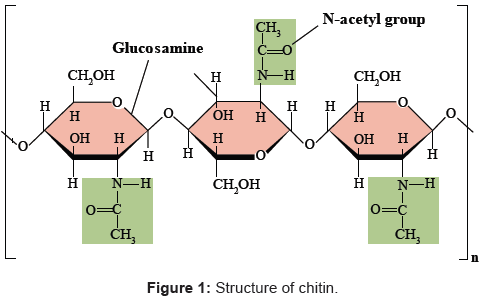
Chitin is an insoluble linear β-1,4-linked polymer of N-acetyl glucosamine [1,2]. It is the most abundant renewable natural resource after cellulose [3,4], a major constituent of the cell wall of many fungi, insect exoskeletons, and crustacean shells [3,5], and it is largely exists in wastes from the processing of marine food products (crab, shrimp and krill shells) [4,6,7].
α-, β-, and γ- forms exist with different chitin microfibril orientations. The α-form has antiparallel chains and is the most abundant in nature. It occurs in the shells of crustaceans (crabs, shrimp, lobsters, etc.), in the shells and skeletons of mollusks and krill, insects, etc., and in the cell walls of fungi (mushrooms, bakers’ yeast, etc.). The β-form has parallel chains and is rare in the nature. It occurs in squid pens, in the extracellular spines of the euryhaline diatom, and in pogonophore tubes. The γ-form has a mixture of anti parallel and parallel chains and is found in the cocoons of insects [8].
Chitin is a natural biopolymer with a chemical structure similar to that of cellulose and is a major component of the exoskeleton of invertebrates. Therefore, crustacean waste is ideal as raw material for chitin production. The extracted chitin can be used to produce chitin derived products, such as chitosans, chito-oligosaccharides and glucosamine, but also for bioplastic production. The interest to use chitinous products in foods and pharmaceuticals is increasing due to their broad range of industrial applications [9,10].
The name `chitin’ is derived from the Greek word chiton’, meaning a coat of mail. It is the second most abundant biopolymer on earth after cellulose named poly β (1→4)-2-acetamido-2-deoxy-D-glucose [6,11,12] as shown in Figure 1.
Chitin and its derivatives have many properties that make them attractive for a wide variety of applications, from food, nutrition and cosmetics to biomedicine, agriculture and the environment [13]. Their antibacterial, antifungal and antiviral properties make them particularly useful for biomedical applications [14] such as wound healing and dressings, weight loss agent, blood cholesterol control [15], surgical sutures, aid in cataract surgery and periodontal disease treatment [13,16]. In biotechnology, applications include wastewater treatment where chitin (as its derivative, chitosan) acts as an absorbent for heavy and radioactive metals, has use as food and feed additives [17], and in the manufacture of textile, paper, film and sponge sheet materials [5,12,18]. Chitin and its derivative chitosan are of commercial interest due to their excellent biocompatibility, biodegradability, nontoxicity, chelating and adsorption power [19,20].
Because of the importance of chitin, the present study has been aimed with the following objectives
- To extract the chitin from the chicken feet
- To find the optimum conditions of the extraction
- To quantify the yield of chitin
- To check the chitin extracted with standard chitin
Materials and Methods
Materials
Chicken feed materials were purchased from Erbil market. All the chemicals purchased from Merck were analytical grade.
Preparation of chitin
Chicken feet were washed with cold water and dried for two days at 45°C. Chitin was extracted from chicken feet following standard procedure [21]. The process mainly involved the following steps: Demineralization of feet; in this step, the feet were suspended in 1.0 N hydrochloric acid at room temperature with the ratio 1:15 (w/v). After 36 hours, the sample was quite squashy and was rinsed with water to remove acid and calcium chloride. Deproteinization of the sample; the demineralized sample was then treated with 1.25 N sodium hydroxide at 90°C for 24 hours with a ratio 1:12 (w/v). The residue was then collected and washed to neutrality in running tap water, and then it was dried. The extraction conditions used and optimum values (wrote with red color) are summarized in Table 1.
| Raw material | Conc. of demineralizer (HCl), N | Solid to acid ratio (w:v) | Conc. of deproteinizer (NaOH), N | Solid to base ratio (w:v) | Weight, g | Apperence | |
|---|---|---|---|---|---|---|---|
| Weight (g) | Color | ||||||
| 100 | Brown | 0.5 | 1:15 | 1.25 | 1:12 | 6.3 | |
| 1.0 | 8.7 | ||||||
| 1.5 | 10.4 | Light brown | |||||
| 2.0 | 9.6 | ||||||
| 2.5 | 9.2 | ||||||
| 1.5 | 1:10 | 8.9 | |||||
| 1:15 | 10.4 | ||||||
| 1:18 | 12.7 | yellow | |||||
| 1:20 | 12.1 | ||||||
| 1:25 | 11.6 | ||||||
| 1:18 | 0.1 | 8.5 | |||||
| 0.5 | 11.6 | ||||||
| 1.0 | 13.4 | white | |||||
| 1.5 | 11.2 | ||||||
| 2.0 | 10.3 | ||||||
| 1.0 | 1:5 | 10.8 | |||||
| 1:10 | 15.2 | Supper white | |||||
| 1:15 | 12.9 | ||||||
| 1:18 | 10.7 | ||||||
| 1:20 | 10.1 | ||||||
Table 1: Chemical parameters studied for extraction process.
Result and Discussion
The main purpose of present study was to find the optimum conditions for extraction of chitin from chicken feet. To our knowledge, this is the first paper presenting results on the extraction of chitin from Chicken feet samples.
The extraction of chitin from chicken feet involve two steps, demineralizations to remove calcium carbonate using hydrochloric acid and deproteinization to remove proteins with sodium hydroxide, as shown in Table 1, 100 g of chicken feet sample was used in each study, the best conditions of extraction process was as follow; 1.5 N of hydrochloric acid concentration was the best one between the concentrations(1.0 - 3.0 N) examined, while the solid to acid ratio was 1:18 (w:v), and the best yield and color of chitin were obtained with 1.0 N and 1:10 (w:v) of sodium hydroxide concentration and solid to base ratio tested respectively. The results are showed in Table 1. The extracted chitin yield with these conditions was 15.2%.
Hydrochloric acid is the most commonly used chemical in the demineralization of chitin sources. The reasons for use of strong acid are; (a) harms the physiochemical properties of chitin, (b) results in a harmful effluent wastewater and (c) increases the cost of chitin purification process. It was reported that using hydrochloric acid for the demineralization of chitin results in detrimental effects on the molecular weight and the degree of acetylation that negatively affects the intrinsic properties of the purified chitin [16].
It was reported that the more concentrated acid solution and larger reaction times would not show any improvement of the efficiency of the demineralization reaction. The persistence of minerals during demineralization reaction would be related not only to concentration and duration effects (in relation with an optimal swelling of chitin) but also to the process, either continuous or performed by stages [22].
Chicken feet contain calcium carbonate and organic macromolecules such as chitin and protein, and in major amounts, lipids. The importance of the optimization of the extraction process parameters (Conc. of hydrochloric acid, Solid to acid ratio, Conc. of sodium hydroxide and Solid to base ratio)in order to minimize chitin degradation and bring the impurity levels down to the satisfactory level for specific applications are quite essential. Therefore, a less harmful and cheaper demineralization process is needed for the chitin extraction. The crude chitin obtained was all insoluble.
Standard deviations and relative standard deviations were calculated for the results of the best values of the optimum conditions for four replicates, the results are shown in Table 2.
| Mean | SD | RSD |
|---|---|---|
| 10.4 | 0.3651 | 3.51 |
| 12.7 | 0.1414 | 1.113 |
| 13.4 | 0.2160 | 1.612 |
| 15.2 | 0.1825 | 1.201 |
Table 2: Statistical data of the method.
FT - IR Spectral Analysis
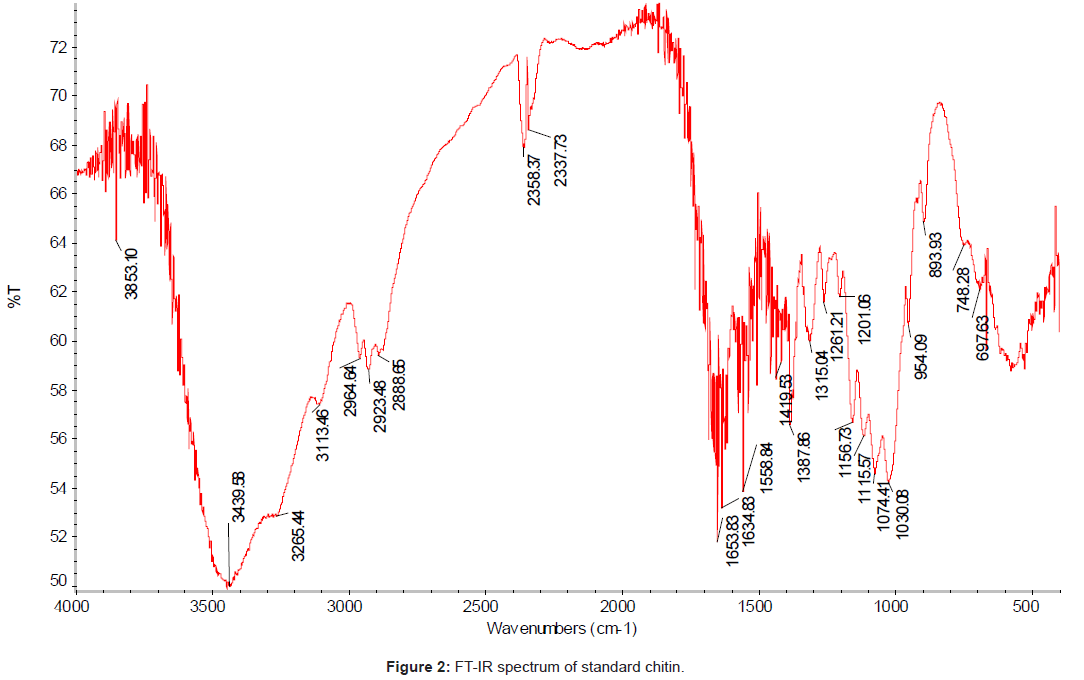
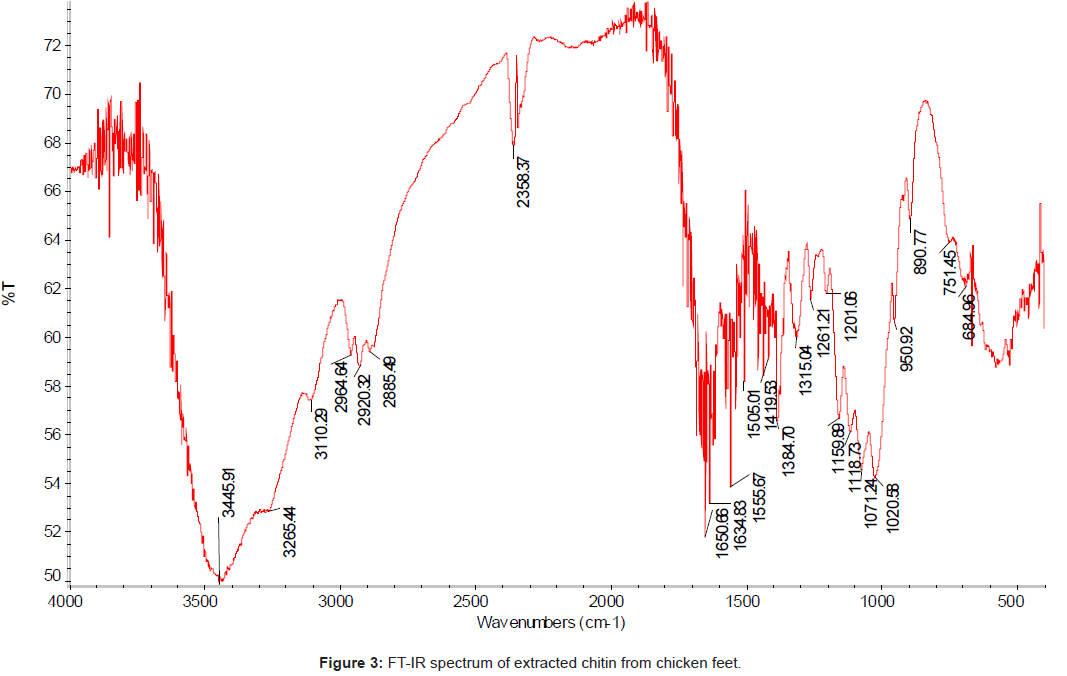
The FT-IR spectrum (using IR300 Spectrophotometer from Thermo Mattson by infrared spectrophotometer from 4,000 to 400 cm-1 with KBr pellets) of the chitin isolated from the chicken feet was compared with the standard chitin. The IR spectrum of the standard chitin contains 17 major peaks (Figure 2); and the IR spectrum of the sample (Figure 3). The major peaks of extracted chitin were compared with the standard one as shown in the Table 3.
| Vibration modes | Standard chitin (cm-1) | Chicken feet chitin |
|---|---|---|
| OH- out of Plane bending | 697.63 | 684.96 |
| NH-out of plane bending | 748.28 | 751.45 |
| Ring Stretching | 893.93 | 890.77 |
| CH3 wagging alone chain | 954.09 | 950.92 |
| CO-stretching | 1030.08 | 1020.58 |
| CO-stretching | 1074.41 | 1071.24 |
| Asymmetric in phase ring stretching | 1115.57 | 1118.73 |
| Asymmetric bridge O2 stretching | 1156.73 | 1159.89 |
| Amide III band and CH2 wagging | 1315.04 | 1315.04 |
| CH bending and symmetric CH3 deformation |
1387.67 | 1384.7 |
| CH2 bending and CH3 deformation | 1419.53 | 1419.53 |
| Amide II band | 1558.84 | 1555.67 |
| Amide I band | 1653.83, 1634.83 | 1650.66, 1634.83 |
| Symmetric CH3 stretching and asymmetric CH2 stretching | 2923.48 | 2920.32 |
| NH stretching | 3113.46 | 3110.29 |
| OH stretching | 3439.58 | 3445.91 |
| CH stretching | 2888.65 | 2885.49 |
Table 3: FT-IR spectral values of the main bands obtained for the standard chitin and extracted chitin from chicken feet.
The degree of acetylation; DA is the most important characteristic of chitin and its value depends on the source material and the preparation methods used. DA for chitin sample was calculated by infrared spectroscopy, using the baseline drawing for the intensity of the amide I band of the acetyl groups at 1650.66 cm-1 with that of the hydroxyl groups at 3445 cm-1 (reference band) following the Eq (1) [23-25].
DA = (A1655/A3450)×100
The DA values of chitin extracted from chicken feet calculated using the Eq (1) was 91.66%.
The chicken feet chitin showed an intense peak at 1558 cm-1 which corresponded to the N-H deformation of amide II. The bands at 1634 cm-1 and another at 1650 cm-1 are attributed to the vibrations of the amide I band, and the band at 1650 cm-1 corresponds to the amide I stretching of C=O. The sharp band at 1384 cm-1 corresponds to a symmetrical deformation of the CH3 group, and at 1555 cm-1 corresponds to the N–H deformation of amide II.
The amide I band shows a well defined peak at 1650 cm-1 with a minor shoulder at 1634 and 3445 cm-1, and the band at 1315 cm-1 characteristics of –OH, -NH2, - CO groups were chosen to measure the extent of N – acetylation. The stretching band of the OH groups involved in hydrogen bonds 0.3 – H…0-5 occurs at 3445 cm-1 [22].
The absorbance bands of 3445, 2885, 1419, 1650 and 1315 cm-1 indicated the hydroxyl stretching, C–H stretching, C–H deformations, C=O stretching in secondary amide (amide I) and C–N– stretching in secondary amide (amide III), respectively.
Conclusion
One of the major problems related to the preparation of pure chitins keeping a structure as close as possible than the native form is to minimize the partial deacetylation and chain degradation caused by demineralization and deproteinization applied during process of the raw materials. α-Chitin was isolated from chicken feet was accounting for 15.2% in the total weight of the dried chicken feet.
In the present study from the present investigation it is confirmed that the α-chitin has been extracted from chicken feet, with good yield. Furthermore, we have compared the IR chart process in order to be able to define the conditions for reaching the adequate chemical characteristics.
References
- Huang CJ, Wang TK, Chung SC, Chen CY (2005) Identification of an antifungal chitinase from a potential biocontrol agent, Bacillus cereus 28-9. J Biochem Mol Biol 38: 82-88.
- Ahmadi KJ, Yazdi MT, Najafi MF, Shahverdi AR, Faramarzi MA, et al. (2008) Optimization of Medium and Cultivation Condition for Chitinase Production by the Newly Isolated: Aeromonas sp. Biotechnology 7: 266-272.
- Yong T, Hong J, Zhangfu J, Li Z, Xiuqiong D, et al. (2005) Purification and Characterization of an Extracellular Chitinase Produced by Bacterium C4. Ann Microbiol 55: 213-218.
- Mejia-Saules JE, Waliszewski KN, Garcia MA, Cruz-Camarillo R (2006) The Use of Crude Shrimp Shell Powder for Chitinase Production by Serratia marcescens WF. Food Technol and Biotechnol 44: 95-100.
- Khanafari A, Marandi R, Sanatei Sh (2008) Recovery of Chitin and Chitosan from Shirmp Waste by Chemical and Microbial method. Iranian Journal of Environmental Health Science and Engineering 5: 1-24.
- Roy I, Mondal K, Gupta MN (2003) Accelerating enzymatic hydrolysis of chitin by microwave pretreatment. Biotechnol Prog 19: 1648-1653.
- Bolat Y, Bilgin S, Günlü A, Izci L, Koca SB, et al. (2010) Chitin-Chitosan Yield of Fresh Water Crab (Potamon potamios, Olivier 1804) Shell. Pakistan Veterinary Journal 30: 227-231.
- Zhang M, Haga A, Sekiguchi H, Hirano S (2000) Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int J Biol Macromol 27: 99-105.
- Tröger C, Niranjan K (2010) Sustainable Chitin Extraction and Chitosan Modification for Application in the Food Industry. International conference on food Innavation.
- Dutta PK, Dutta J, Tripathi VS (2004) Chitin and Chitosan: Chemistry, Properties and Application. JSIR 63: 20-31.
- Shahidi F, Arachchi JKV, Jeon YJ (1999) Food Applications of Chitin and Chitosans. Trends in Food Science & Technology 10: 37-51.
- Gamayurova VS, Kotlyar MN, Shabrukova NV, Khalitov FG (1998) Synthesis of Soluble Derivatives of Chtin-Glucan Complex.Chemistry and Computational Simulation. Butler Comm 1: 73-76.
- Naznin R (2005) Extraction of Chitin and Chitosan from Shrimp (Metapenaeus monoceros) Shell by Chemical Method. Pakistan Journal of Biological Sciences 8: 1051-1054.
- Taha SM, Swailam HM (2002) Antibacterial Activity of Chitosan against Aeromonas Hydrophila. Nahrung 46: 337-340.
- Rockway (2000) Absorbitol Fat Binding Report. Pharmanutrients 9: 15.
- Prameela K, Mohan CM, Hemalatha KPJ (2010) Extraction of Pharmaceutically Important Chitin and Carotenoids from Shrimp Biowaste by Microbial Fermentation Method. Journal of Pharmacy Research 3: 2393-2395.
- Thirunavukkarasu N, Shanmugam A (2009) Extraction of Chitin and Chitosan from Mud Crab Scylla Tranquebarica (FABRICIUS, 1798). International Journal on Applied Bioengineering 4: 31-33.
- Teng WL, Khor E, Tan TK, Lim LY, Tan SC (2001) Concurrent production of chitin from shrimp shells and fungi. Carbohydr Res 332: 305-316.
- Toan NV (2009) Production of Chitin and Chitosan from Partially Autolyzed Shrimp Shell Materials. The Open Biomaterials Journal 1: 21-24.
- Limam Z, Selmi S, Sadok S, Abed A (2011) Extraction and Characterization of Chitin and Chitosan from Crustacean by-Products: Biological and physicochemical Properties. African Journal of Biotechnology 10: 640-647.
- Islam MM, Masum SM, Rahman MM, Shaikh AA (2011) Preparation of Glucosamine Hydrochloride from Indigenous Shrimp Processing Waste. Bangladesh journal of scientific and industrial research 46: 375-378.
- Palpandi Ch, Shanmugam V, Shanmugam A (2009) Extraction of Chitin and Chitosan from Shell and Operculum of Mangrove Gastropod Nerita (Dostia) Crepidularia Lamarck. International Journal of Medicine and Medical Sciences 1: 198-205.
- Amaral IF, Granja PL, Barbosa MA (2005) Chemical modification of chitosan by phosphorylation: an XPS, FT-IR and SEM study. J Biomater Sci Polym Ed 16: 1575-1593.
- Majtan J, Bilikova K, Markovic O, Grof J, Kogan G, et al. (2007) Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int J Biol Macromol 40: 237-241.
- Liu S, Sun J, Yu L, Zhang Ch, Bi J, et al. (2012) Extraction and Characterization of Chitin from the Beetle Holotrichia parallela Motschulsky. Molecules 17: 4604-4611.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18030
- [From(publication date):
September-2012 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 13195
- PDF downloads : 4835