Research Article Open Access
Optimisation of the Chitinase Production by Serratia Marcescens DSM 30121T and Biological Control of Locusts
Benine Mohamed Lamine1, Bendaha Mohammed Lamine1 and Abbouni Bouziane2*1Department of Biology, Faculty of Science, University Djillali Liabes of Sidi Bel Abbés 2200-Algeria
2Laboratory for the synthesis of the Environmental Information, Department of Biology, Faculty of Science, Sidi Bel Abbés University, 22000-Algeria
- Corresponding Author:
- Prof. Dr. Abbouni Bouziane
Laboratory for the synthesis of the Environmental Information
Department of Biology, Faculty of Science
Sidi Bel Abbés University, 22000-Algeria
E-mail: abbounibouziane@yahoo.de
Received date: March 12, 2012; Accepted date: March 27, 2012; Published date: March 29, 2012
Citation: Lamine BM, Lamine BM, Bouziane A (2012) Optimisation of the Chitinase Production by Serratia Marcescens DSM 30121T and Biological Control of Locusts. J Biotechnol Biomaterial 2:133. doi:10.4172/2155-952X.1000133
Copyright: © 2012 Lamine BM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Serratia marcescens DSM 30121T was tested for their chitinolytic activity on colloidal chitin agar. The production of the chitinase by Serratia marcescens DSM 30121Twas optimized by using of different parameters such as temperature, pH-value, colloidal chitin concentration and incubation time. The obtained results showed that the maximum chitinase production (0.556 U/ml) was observed at the pH-value of 6, at 30°C. Similarly, the recorded maximum chitinase production (0.368 U/ml) was obtained in the presence of 2% of colloidal chitin concentration in the culture medium after 144 h incubation time.
Furthermore, Serratia marcescens DSM 30121 T showed clear zones of hydrolysis of colloidal chitin on agar. Therefore, the present study showed that Serratia marcescens DSM 30121 T is good source for chitinase production. Moreover, the strain Serratia marcescens DSM 30121 T can be used for biological control of locusts.
Keywords
Colloidal chitin; Serratia marcescens DSM 30121T; production of chitinase; Optimisation
Introduction
Chitin, a polymer of N-acetyl-D-glucosamine ( Glc-NAc) residues linked by β (1-4) bonds and the second most widespread renewable natural resource following cellulose on the earth, with an annual recovery of 1-100 billion metric tonnes [1,2].
The main source of chitin is crustacean waste, which is also the main cell wall material in most fungi. Chitin and its derivatives have high economic value owing to their versatile biological activities and agrochemical applications [3-7].
Disposal by microbial degradation of chitin offers best solution to the problem leading to recycling of nutrients in the environment along with generating of useful products viz. Chitinase, chitooligosaccharides, N-Acethyl glucosamine and single cell protein [8,9].
Chitinase are extracellular inducible enzymes that catalyse the first step in chitin degradation, hydrolysis of β (1-4) linkages between the NAG molecules. Recently, chitinase production has received increased attention due to their wide range of biotechnological applications; especially in agriculture for biocontrol of fungi phytopathogenes and harmful insects as fugal cell and cuticle of insects contain chitin as essential component [10,11]. Moreover, they offer a potential additive or alternative to use of chemical fungicides [12].
The utilization of the shellfish waste solves not only environmental problems but also decreases the production costs of microbial chitinases. The production of inexpensive chitinolytic enzymes is an important element in the process [13].
From a technological point of view, it would be quite profitable to recover the by-products released from seafood processing because of its richness in compounds of high value added such as chitin products. Therefore, the present study was dealt with the colloidal chitin extraction from crustacean waste and their degradation by Serratia marcescens DSM 30121T. However, its economics for commercial exploitation has to be worked out.
Materials and Methods
Preparation of colloidal chitin
Colloidal chitin was prepared according the modified method described by Mathivanan [14]. Ten grams of practical grade crab shell chitin (Sigma Chemicals) were mixed with 150 ml 12 N HCl with continuous stirring for 2 h at 4°C. The suspension was repeatedly mixed with 1 litre water and filtered through a course filter paper. This step was followed four to five times and the pH of the suspension was adjusted to 7.0 by addition of 5 N NaOH and the colloidal suspension was washed several times with distilled H2O for desalting. After desalting, the suspension was centrifuged at 8000 rpm for 10 min and the precipitate was collected for further use as colloidal chitin.
Bacteria and culture conditions
Serratia marcescens DSM 30121T, was furnished from DSM (Deutsche Sammlung von Mikroorganismen-Germany). The ability of Serratia marcescens DSM 30121T to decompose colloidal chitin was tested on the following culture medium which was containing : peptone 5 g, iron gluconate 5 g, ammonium sulphate 0.1 g, iron sulphate 0.1 g, colloidal chitin 10 g, agar 15 g, water 1L, pH was adjusted between 7.2-7.4. Serratia marcescens DSM 30121T was pre-inoculated in 50 ml culture medium described above and incubated at 37ºC with agitation 150 rpm.
Effect of the temperature on chitinase production
In order to determine the optimum temperature for chitinase production, the growth of Serratia marcescens DSM 30121T was studied in the culture medium and incubated at different temperature (10, 20, 30 and 40°C) with agitation 150 rpm.
Effect of the pH-value on the chitinase production
The effect of the initial pH value on the chitinase production by Serratia marcescens DSM 30121T was investigated by varying of the initial pH of the culture medium from 5.0-9.0.
Primary screening for chitinase degradation
Primary screening of chitinase production was performed by spot inoculating Serratia marcescens DSM 30121T on the culture medium agar supplemented with colloidal chitin by using toothpick heads of 2 mm diameter and incubated at the temperature of 30°C. The apparition of the zone of clearance due to the colloidal chitin hydrolysis was recorded up to 5 days at 30°C.
Chitinase production assay
The chitinase production assay was determined by the method of Yanai [15]. 500 μl of culture supernatant was incubated with 300 μl of 1% colloidal chitin and 300 μl of 0.2 M acetate buffer (pH 4.0) at 37°C for 2 hours. The reaction product N-acetyl glucosamine was determined by the method of Reissig et al. [16], by using para-dimethylamino benzaldehyde reagent (DMAB) and was prepared as described by Kang [17]. Absorbance at 585 nm (A585) was taken against water as blank. One unit of chitinase production was defined as the amount of enzyme that produced 1 M of N- acetyl glucosamine per min under the above conditions. N-Acetyl glucosamine was taken as standard for all the enzyme assay calculations. Absorbance at 585 nm was observed and concentration of paranitro phenol was determined. One unit of exochitinase production was defined as the amount of enzyme that produced 1 M of Para-nitro phenol per min under the above conditions.
Estimation of protein content
Protein concentration in the processed shells was determined using the method described by Stoscheck et al. [18]. Bovine serum albumin (BSA) was used as a standard protein. Absorbance was determined spectrophotometrically at 260 and 280 nm (Milton Roy, New York, USA) and converted to protein concentration using the following equation:
Protein (mg/ml) = 1.55 A280 - 0.76 A260
Results and Discussion
Effect of temperature on chitinase production
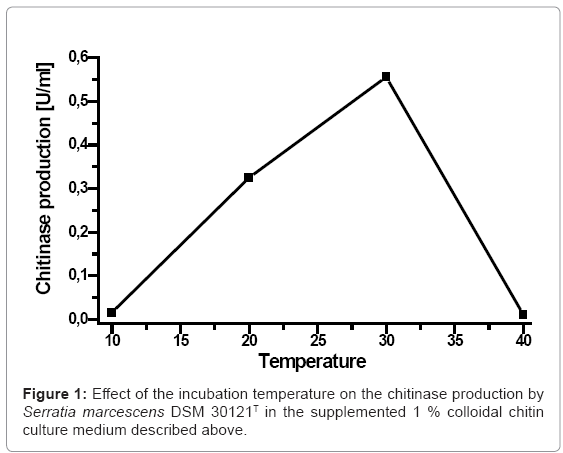
In order to display the influence of the temperature on the chitinase production by Serratia marcescens DSM 30121T, the enzyme production in the supplemented 1% colloidal chitin culture medium production was assayed at the following temperatures 10, 20, 30 and 40°C respectively. The maximum chitinase production (0,556 U/ml) was observed at temperature of 30°C (Figure 1). A considerable decrease of chitinase production was observed at elevated temperature up to 40°C (0.011 U/ml). The influence of temperature on the chitinase production by Serratia marcescens DSM 30121T, revealed that the enzyme was optimally active at the temperature of 30°C and was produced in a considerable amount at 20°C. These results revealed that the chitinase produced by Serratia marcescens DSM 30121T was not thermostable. Frandberg and Schnurer have reported that the optimum temperature for the enzyme production by Bacillus pabuli was 40°C [19].Whereas, by Vibrio alginolyticus TK-22 the optimum temperature was 30°C [20].
Furthermore, Shanmugalah et al. has demonstrated that the optimum temperature for chitinase production by Bacillus laterosporus was 35°C [21].
Effect of the ph-value on chitinase production
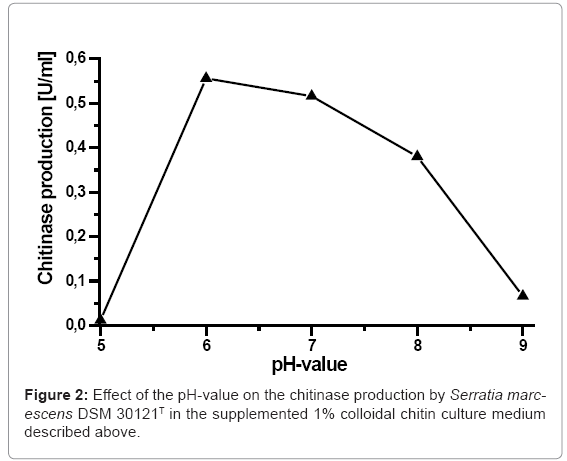
Normally, the effect of the initial pH of the supplemented 1% colloidal chitin culture medium influences the final yield of chitinase. In order to evaluate the effect of the pH-value on the chitinase production of Serratia marcescens DSM 30121T, the used culture media was adjusted with different levels of pH. The obtained results (Figure 2) presented that the maximum chitinase production (0.556 U/ml) by Serratia marcescens DSM 30121T was displayed at the pH-value of 6. Furthermore, a considerable amount of chitinase was produced by a broad pH range 5-8. However, the lowest chitinase production (0.012 U/ml) occurred at pH 5. An optimal chitinase production by Bacillus pabuli was reported to be 8.0 [19]. Shanmugalah et al. has even reported that an optimum pH-value for chitinase production by Bacillus laterosporus was 8.0 [21]. Furthermore, several workers have reported broad range of pH-value from 4.5 to 7.5 for chitinase production by Bacillus cereus [22], pH 5.0 to 8.0 for Aeromonas hydrophila H-2330 [23], pH 7.5 to 9.0 for Bacillus sp Bg-11 [24]. The chitinase of Serratia liquifaciens was produced by pH-value between 5.0 and 6.0 [25]. Whereas, the chitinase production Streptomyces erythraceus has an optimum pH 5.0 [26].
Effect of the substrate concentration on the chitinase production
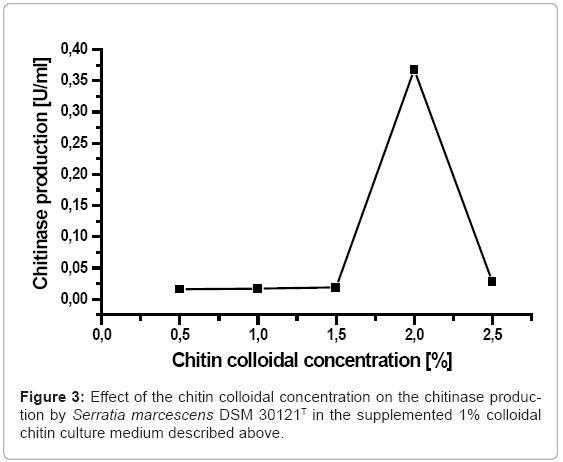
The colloidal chitin was used to determine the effect of substrate concentration on the chitinase production by Serratia marcescens DSM 30121T. The effect of different concentration of the colloidal chitin on the chitinase production by Serratia marcescens DSM 30121T was elucidated in (Figure 3). It is clear to see that the chitinase production grew along with the colloidal chitin concentration increase. The recorded maximum chitinase production (0.368 U/ml) was obtained in the presence of 2% of colloidal chitin concentration in the culture medium, whereas the chitinase production was constant at concentration between 0.5 and 1.5% up to a concentration of 1.6%.
Suzuki et al. has reported early that a minimum culture medium supplemented with different sources of carbon enhance the chitinase production [27]. Moreover, certain carbohydrates like glucose, starch, pectin have been identified for their adverse effect on the chitinase production when they are supplemented to chitin in the culture medium [28,29]. The present study on the influence of colloidal chitin concentration on chitinase production by Serratia marcescens DSM 30121T showed that chitin was correlated with substrate concentration and therefore the maximum chitin production was recorded as 2% chitin concentration in the medium culture. While analyzing chitinolytic enzyme production in Aeromonas sp found increased chitinase production along with chitin colloidal concentration growth to 1.5% [30]. Enzyme production was plumetted slightly at 2% chitin. A drop in chitinlytic production at 2.5% colloidal chitin concentration as found by Serratia marcescens DSM 30121T, may indicate a hampering reaction of colloidal chitin serving as substrate, or accumulation of intermediaries metabolites producing from chitin decomposition, which make up a synthesis inhibitor. Following the hypothesis of Michaelis Menten, one may anticipate that the enzymatic reaction rate is directly proportional to substrate concentration when at a low percentage. The greater the concentration the lesser influence of its growth on enzymatic reaction rate [31].
Effect of incubation times on the chitinase production
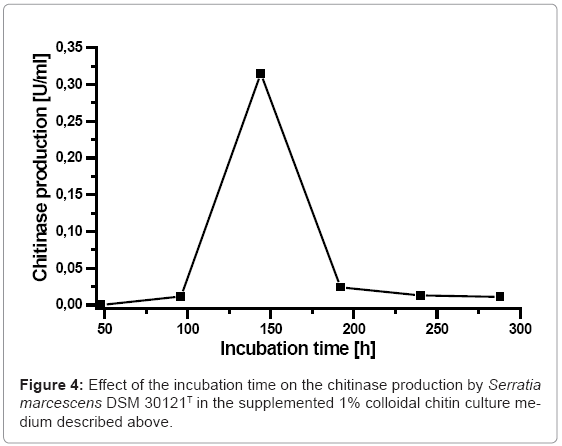
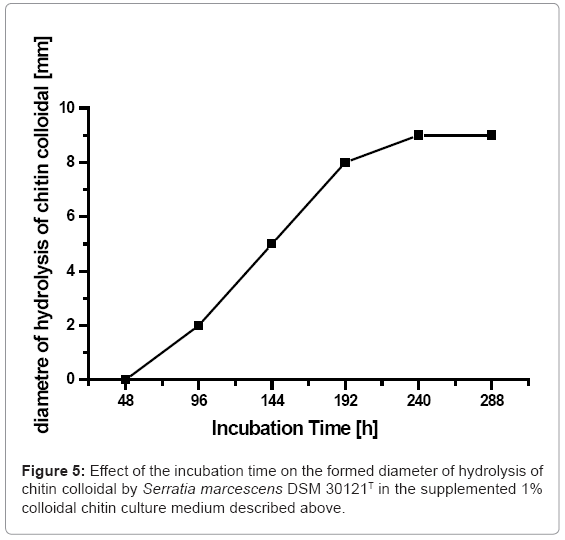
The effect of the incubation time on the chitinase production by Serratia marcescens DSM 30121T was presented in (Figure 4). It is clear to see that the chitinase production grew along with the incubation time increase. It evidenced no chinitinase production by Serratia marcescens DSM 30121T after 48 h incubation time. The chitinase production of Serratia marcescens DSM 30121T increased until it reached their maximum chitinase production (0,368 U/ml) after 144 hours incubation time and decreased drastically after that. The present study on the influence of the incubation time on the chitinase production appointed to the fact that Serratia marcescens DSM 30121T under investigation was able to decompose colloidal chitin most intensively first after 144 h incubation time. Most probably it results from the fact that colloidal chitin is a hard decomposable compound and Serratia marcescens DSM 30121T need a longer time to adapt to this substrate than to other high molecular compounds in terms to be able to start the chitinase production.
Production of zone hydrolysis for chitinase production
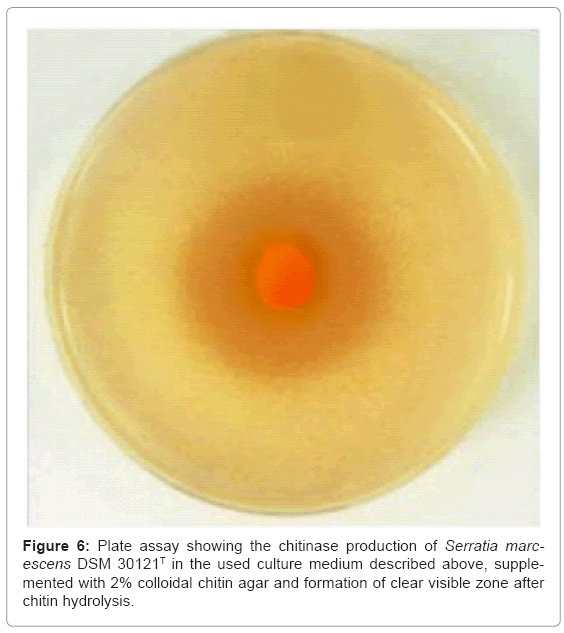
A rapid screening of chitinolytic bacteria was carried out by spread inocula of the colony of Serratia marcescens DSM 30121T on plates containing minimal salt medium supplemented with colloidal chitin as a sole source of carbon and energy. The chitin degrading bacteria formed colonies of 1-2 mm in diameter, surrounded by clear zone indicating chitinase production. Serratia marcescens DSM 30121T produced a clear zone of more than 0.5 cm by hydrolyzing the colloidal chitin, (Figure 5,6).
Conclusion
The invasions of the locusts contribute significantly to reduce the yield in agriculture. Conventionally, pesticides were used to control damage caused by locusts. However, their excessive use has led to several problems related to environmental degradation, pollution, development of resistant strains and lethal effect on the beneficial rhizobacteria. Therefore, it was necessary to develop news control strategies. Chitinolytic bacteria producing chitinase offer important potential alternate or additive to toxic chemical pesticide to reduce their charge in agriculture. The present study investigated the production of chitinase by Serratia marcescens DSM 30121T and the optimization of culture conditions for maximal chitinase production. The tested crude chitinase of Serratia marcescens DSM 30121T has an important activity in broad pH (6-8) range and temperature (20-30°C). Furthermore, the maximum chitinase production by Serratia marcescens DSM 30121T was obtained after 144 hours incubation times in the presence 2% colloidal chitin concentration. The investigation of further parameters for chitinase production can help in formulating a cheap medium for high chitinase production needed for field application.
Acknowledgements
The authors are thankful to the INRAA-Sidi Bel Abbes-Algeria for providing us with the necessary facilities for conducting this research. The authors would also like to acknowledge the excellent advice provided by Prof. Dr. Auling G. Institute for Microbiology Hannover-Germany).
References
- Jung BO, Roseman S, Park JK (2008) The central concept for chitin catabolic cascade in marine bacterium, Vibtios. Macromolecular research 16: 1-5.
- Rattanakit N, Plikomol A, Yano S, Wakayama M, Tachiki T (2002) Utilization of shrimp shellfish waste as substrate for solid-state cultivation of Aspergillus sp. S1-13: evaluation of a culture based on chitinase formation which is necessary for chitin-assimilation. J Biosci Bioeng 93: 550-556.
- Hirano S (1996) Chitin biotechnology application. Biotechnol Annu Rev 2: 237-258.
- Mathivanan N, Kabilan V, Murugesan K (1998) Purification, characterization, and antifungal activity of chitinase from Fusarium chlamydosporum, a mycoparasite to groundnut rust, Puccinia arachidis. Can J Microbiol 44: 646-651.
- Someya N, Nakajima M, Hirayae K, Hibi T, Akutsu K(2001) Synergistic antifungal activity of chinolytic enzymes and prodigiosin produced by the biocontrol bacterium Serratia marcescens strain B2 against the gray mold pathogen, Botrytis cinerea. Journal of General Plant Pathology 67: 312-317
- de la Vega LM, Barboza-Corona JE, Aguilar-Uscanga MG, Ram?rez-Lepe M (2006) Purification and caracterisation of an exochitinase from Bacillus thuringiensis subsp. aizawai and its action against phytopathogenic fungi. Can J Microbiol 52: 651-657.
- Chang WT, Chen YC, Jao CL (2007) Antifungal activity and enhancement of plant growth by Bacillus cereus grown on shellfish chitin wastes. Bioresour Technol. 98: 1224-1230.
- Gohel V, Maisura V, Chhatpar HS (2007) Utilization of various chitinous sources for production of mycolytic enzymes by Pantoea dispersa in bench top fermentor. Enzyme Microb Technol 40: 1608-1614.
- Pichyangkura R, Kudan S, Kuttiyawong K, Sukwattanasinitt M, Aiba S (2002) Quantitative production of 2-acetamido-2-deoxy-D-glucose from crystalline chitin by bacterial chitinase. Carbohydr Res 337: 557-559.
- Maisura V, Gohel V, Mehta AN, Patel RR, Chhatpar HS (2008) Biological control of Fusarium wilt of pigeonpea by Pantoea dispersa, a field assessment. Ann. Microbiol, 58: 411-419.
- Pinto AS, Barroto CC, Vainstein MH, Schrank,A, Ulhao CJ, (1997) Purification and characterization of an extracellular chitinase form the entomopathogen Metharizium anisopliae. Can J Microbiol 43: 322-327.
- Huang CJ, Chen CY (2008) Synergistic interactions between chitinase ChiCW and fungicides against plant fungal pathogens. J Microbiol Biotechnol 18: 784-787.
- Wang SL, Wang CY, Huang CY (2008) Microbial reclamation of squid pen for the production of a novel extracellular serine protease by Lactobacillus paracasei susp. Paracasei TKU012. Bioresour Technol 99: 3411-3417.
- Mathivanan N (1995) Studies on extracellular chitinase and secondary metabolites produced by Fusarium chlamdosporum, an antagonist to Puccinia arachidis, the rust pathogen of groundnut.
- Yanai K, Takaya N, Kojima M, Horiuchi H, Ohta A, et al. (1992) Purification of two chitinases from Rhizopus oligosporus and isolation and sequencing of the encoding genes. J Bacteriol 174: 7398?7406.
- Reissig JL, Storminger JL, Leloir LF (1955) A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem 217: 959-966.
- Kang SC, Park S, Lee DG (1999) Purification and Characterization of a Novel Chitinase from the entomopathogenic fungus, Metarhizium anisopliae. J Invertebr Pathol 73: 276?281.
- Stoscheck CM (1990) Quantitation of protein. Methods Enzymol. 182: 50-68.
- Frandberg E, Schnurer J (1994) Chitinolytic properties of Bacillus pabuliK1. J Appl Bacteriol 76: 361-367.
- Ohishi K, Yamagishi M, Ohta T, Suzuki M, Izumida H, et al. (1996) Purification and properties of two chitinase form Vibrio alginolyticus H-8. Journal of Fermentation Bioengineering 82: 598-600.
- Shanmugalah V, Mathivanan N, Balasubramanian N, Mancharan PT (2008) Optimization of cultural conditions for production of chitinase by Bacillus laterosporus MML2270 isolated from rice rhizosphere Soil. African Journal of Biotechnology 7: 2562-2568.
- Pleban S, Chernin L, Chet I (1997) Chitinolytic activity of an endophytic strain of Bacillus cereus. Lett Appl Microbiol 25: 284-288.
- Hiraga K, Shou L, Kitazawa M,Takahashi S, Shimada M, et al. (1997) Isolation and characterization of chitinase from a flake chitin degrading marine bacterium, Aeromonas hydrophila H-2330. Biosci Biotechnol Biochem 61: 174-176.
- Brushan B, Hoondal GS (1998) Isolation, purification and properties of a thermostable chitinase from an alkalophilic Bacillus sp. BG-11, Biotechnol Lett 20: 157-159.
- Brurberg MB, Nes IF, Eijsink VG (1996) Comparative studies of chitinases A and B from Serratia marcescens. Microbiology. 142: 1581-1589.
- Hara S, Yamamura Y, Fyjii Y, Mega T, Ikenaka T (1989) Purification and characterisation of chitinase produced by Streptomyces erythraeus. J Biochem 105: 484-489.
- ?Suzuki K, Suzuki M, Taiyoji M, Nikaidou N, Watanabe T (1998) Chitin binding protein (CBP21) in the culture supernatant of Serratia marcescens 2170. Biosci Biotechnol Biochem, 62: 128-135.
- Gupta R, Sexens RK, Chaturvedie P, Virdi JS (1995) Chitinase production by Streptomyces viridificans: It?s potential in fungal cell wall lyses. J Appl Bacterial 78: 378-383.
- Bhusan B (2000) Production and characterisation of a thermostable chitinase from a new alkalophilic Bacillus sp BG-11. J Appl Microbiol 88: 800-808.
- Huang JH, Chen CJ, SU YC (1996) Production of chinolytic enzymes from a novel species of Aeromonas. J Ind Microbiol 17: 89-95
- Stryer L (1997) Biochemia. PWN. Warszawa.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16754
- [From(publication date):
March-2012 - Nov 14, 2025] - Breakdown by view type
- HTML page views : 11810
- PDF downloads : 4944