Zanthoxylum piperitum Activates Thermogenic Gene Expression and Induces Beige Adipogenesis in White Adipose Tissues of Mice
Received: 15-Dec-2020 / Accepted Date: 08-Jan-2021 / Published Date: 15-Jan-2021 DOI: 10.4172/2165-7904.1000421
Abstract
Background: Zanthoxylum piperitum (ZP) is a spice that is widely used in Asia. It is uniquely flavored and creates a tingling sensation in the mouth and tongue. An anti-obesity effect from ZP has been reported, but the mechanism is not well understood.
Objective: To evaluate the effects of ZP on adiposity.
Methods: Fresh fruits of ZP were dried and its pericarp was crushed into powder. ZP-powder was orally administered to mice fed with either a standard diet (STD) or high-fat diet (HFD) for eight weeks. Food intake and body weight were measured every day. At the end of the study, organs were excised. Gene expression analysis and immunohistochemistry of white adipose tissue were performed to evaluate the effects of ZP on adiposity.
Results: ZP markedly decreased body weight and fat mass and improved glucose–lipid metabolism in STD-fed mice, but there was no significant decrease in food intake. We therefore suspected ZP-induced activation of energy expenditure in adipocytes. ZP reduced food intake of HFD-fed mice, so we hypothesized that there may be inhibition of HFD-induced hedonic eating. We also analyzed thermogenic gene expressions in inguinal white adipose tissue (iWAT) to see if ZP induced beige adipogenesis. ZP notably increased expression levels of uncoupling protein-1 (UCP- 1), peroxisome proliferator–activated receptor gamma coactivator 1-alpha (PGC1α and cell death–inducing DFFAlike effector a (Cidea) in iWAT from both STD- and HFD-fed mice. Immunohistochemistry of the iWAT revealed the presence of multilocular adipocytes that were stained with UCP-1, which suggests induction of beige adipogenesis.
Conclusion: ZP activates thermogenic gene expressions and induces beige adipogenesis in inguinal white adipose tissue of mice. Moreover, ZP was shown to inhibit hedonic eating in mice fed with a high fat diet. These effects of ZP could be utilized in complementary treatment of obesity.
Keywords: Beige adipocyte; Obesity; UCP-1, White adipose tissue; Zanthoxylum piperitum
Introduction
Metabolic syndrome is a cluster of conditions including abdominal obesity, increased blood pressure, elevated fasting blood glucose, and abnormal cholesterol or triglyceride levels [1]. It increases the risk of cardiovascular disease, of strokes, and of diabetes mellitus, Treatment of obesity is therefore necessary to prevent the various endocrine diseases that are associated with metabolic syndrome. Behavioral approaches, such as exercise and diet therapy, are necessary for the management of obesity. When such lifestyle modification failed, pharmacotherapy must also be considered; However, anti-obesity drugs can have associated adverse effects [2]. Alternative agents that could be used to support diet therapy with fewer or no side effects would be therefore clinically valuable.
Mammals have two different types of adipose tissue: white adipose tissue (WAT) which stores energy as fat, and brown adipose tissue (BAT), which maintains core body temperature through non-shivering thermogenesis. Recently, a novel thermogenic cell type, beige adipocyte, has been cloned from WAT in mice and humans [3]. This adipocyte is stimulated by cold exposure, sympathomimetic agents, peroxisome proliferator–activated receptor γ (PPARγ) agonist, and other factors [4]. In humans, positron emission tomography (PET) scans can show activation of BAT around the neck, the supraclavicular region, and the chest [5]. The gene expression pattern of adipocytes isolated from this region has been suggested to be similar to beige adipocytes in mice [3,4,6]. Activation of BAT has reported association with lower BMI and decreased abdominal fat area in humans [7]. Activation of brown and beige adipocytes is therefore an attractive alternative therapeutic target.
Zanthoxylum piperitum (ZP) is a uniquely flavored spice that creates a tingling sensation in the mouth and tongue. ZP has been reported to inhibit food consumption in mammals and to suppress fat accumulation in adipocytes; However, the precise mechanism is not unclear [8,9]. We explore the effects of ZP on adipogenesis.
Materials and Methods
Reagent
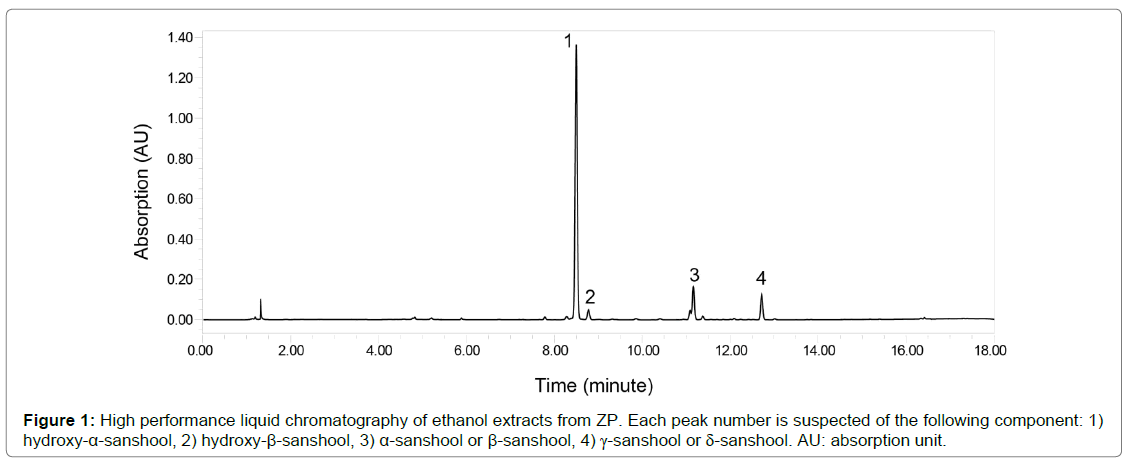
Ripe fruit of the Japanese pepper ZP was harvested in Wakayama Prefecture between June and August. Fresh fruits of ZP were then dried at 60°C for about 12 hours, followed by separation into pericarp and seeds; approximately 18 kg of dried pericarp was obtained from 100 kg of fresh fruits. The dried ZP pericarp was then crushed into powder by Wonder Crush/Mill WDL-1 (Osaka Chemical Co., Ltd., Osaka, Japan), and provided to the authors as ZP-powder (Nakano BC, Japan). ZPpowder was subdivided into sealed aliquots and stored at -20°C before use, it was mixed with powdered diet when performing experiments. Figure 1 shows high performance liquid chromatography (HPLC) of ethanol extracts from ZP.
Animals
Male C57BL/6J mice weighing 16-17 g (initial weight) were maintained at a constant temperature (23°C), with a fixed 12 h light/12 h dark cycle. All experiments were performed strictly in accordance with an animal procedures protocol that was specifically approved by the Wakayama Medical University Committee on the Ethical of Animal Experiments (permit number 643). After a period of habituation to the laboratory for one week, mice were randomly assigned to one of six groups (n=8 per group): Standard diet (STD, AIN-93M, Research Diets, New Brunswick, USA) alone, STD+1%ZP, STD+3%ZP, and high fat diet (HFD, HFD-60, Oriental Yeast Co. Ltd, Tokyo, Japan) alone, HFD+1% ZP and HFD+3%ZP. These groups were assessed for the effect of ZP on diet-induced obesity. They were maintained on the diet plan assigned to their group for eight weeks. We measured change in daily food intake and weekly body weight (BW). At the end of the study, the mice were anesthetized under isoflurane and euthanized by cervical dislocation. All efforts were made to minimize suffering. The following organs were excised and weighed: liver, inguinal white adipose tissue (iWAT), epididymal WAT (eWAT), mesenteric WAT (mWAT), and BAT. Harvested organs were immediately preserved in RNAlater (Thermo Fisher Scientific, Lithuania) or directly frozen in liquid N2 and stored at -80°C until further use. Fasting blood glucose and lipids were assessed in the overnight fasted state.
Metabolic assays
Blood samples were taken from the tail vein and were collected in heparinized capillary tubes, followed by separation of plasma by microhematocrit centrifugation. Whole blood glucose was measured by glucometer and plasma insulin was measured using the Ultra-high Sensitivity Insulin Measurement kit (Takara, MS303, Japan). Plasma total cholesterol (TCH) and triglyceride (TG) levels were determined using the Cholesterol E-Test and Triglyceride E-Test (Wako Pure Chemical Industries, Japan), respectively.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Tissue samples were stored overnight in ribonucleic acid (RNA) at 4°C, followed by total RNA isolation using the RNeasy Lipid Tissue Mini Kit (QIAGEN, Germany). Total RNA (1.0 μg) was converted into cDNA with the TaqMan Reverse Transcription Reagents (Invitrogen, Lithuania), and RT-qPCR was carried out on an Applied Biosystems 7500 with TaqMan Master Mix (Applied Biosystems, USA). Gene expression levels were calculated by the comparative Ct method and normalized to β-actin.
Western blotting
Proteins were extracted from frozen tissues, promptly processed, and homogenized in T-PER Tissue Protein Extraction Reagent (Thermo Scientific Pierce, USA) with 1% protease inhibitor cocktail (P8340, Sigma-Aldrich, USA). Protein concentrations were quantified by Bradford assay. Twenty micrograms of protein per lane from each sample was separated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE). The resolved proteins were transferred to the polyvinylidene difluoride (PVDF) membranes (#162-0714, Bio-Rad, USA) and blocked for an hour with 5% nonfat dry milk in water at room temperature. Primary antibodies were incubated overnight at 4°C. Western blotting was visualized using HRP-conjugated secondary antibodies and ECL chemiluminescent substrate.
Histology
Adipose tissues were fixed overnight in 10% buffered formalin and embedded in paraffin, the liver was embedded in optimal cutting temperature (OCT) compound. Each section was stained with hematoxylin and eosin (H&E). The liver was also stained with oil red O to evaluate hepatic steatosis. Unstained paraffin sections were dewaxed and immunostained as follows: antigen was retrieved in a 97°C water bath for 1 hour. Endogenous peroxide was then inactivated with 0.3% hydrogen peroxide in methanol and blocked with serumfree protein block (Dako, USA, X0909). It was then incubated with rabbit anti-uncoupling protein-1 (UCP-1) antibody (Abcam; 1:1000 dilution) overnight at 4°C and incubated with biotinylated anti–rabbit IgG (H+L) secondary antibody (Vector laboratories, USA; 1:200). ABC complex (Vectastain ABC kit, Vector Laboratories, USA) was used for detection, and then cell nuclei were stained with hematoxylin.
Computed tomography (CT)
Abdominal fat mass and lean body mass were quantified on a small-animal CT (CosmoScan GX, Rigaku, Tokyo, Japan). Each mouse was anesthetized with isoflurane, placed in the supine position, and a whole-body scan was taken. CT images were analyzed using CT Atlas Metabolic Analysis Ver. 2.03 software (Rigaku, Japan). Visceral and subcutaneous fat volumes were measured separately. At the end of CT scanning, mice were anesthetized under isoflurane and euthanized, and each organ was excised and weighed.
Statistical Analyses
All statistical analyses were performed with SPSS software. Statistically significant differences between the two groups were determined by two-tailed Student’s paired or unpaired t tests. For multiple comparisons, analysis of variance (ANOVA) was used, with the Bonferroni test for post-hoc analysis. Data are expressed as means ± standard error of the mean (SE); p<0.05 was considered significant.
Resultss
ZP decreases body weight and abdominal fat mass in STD-fed mice
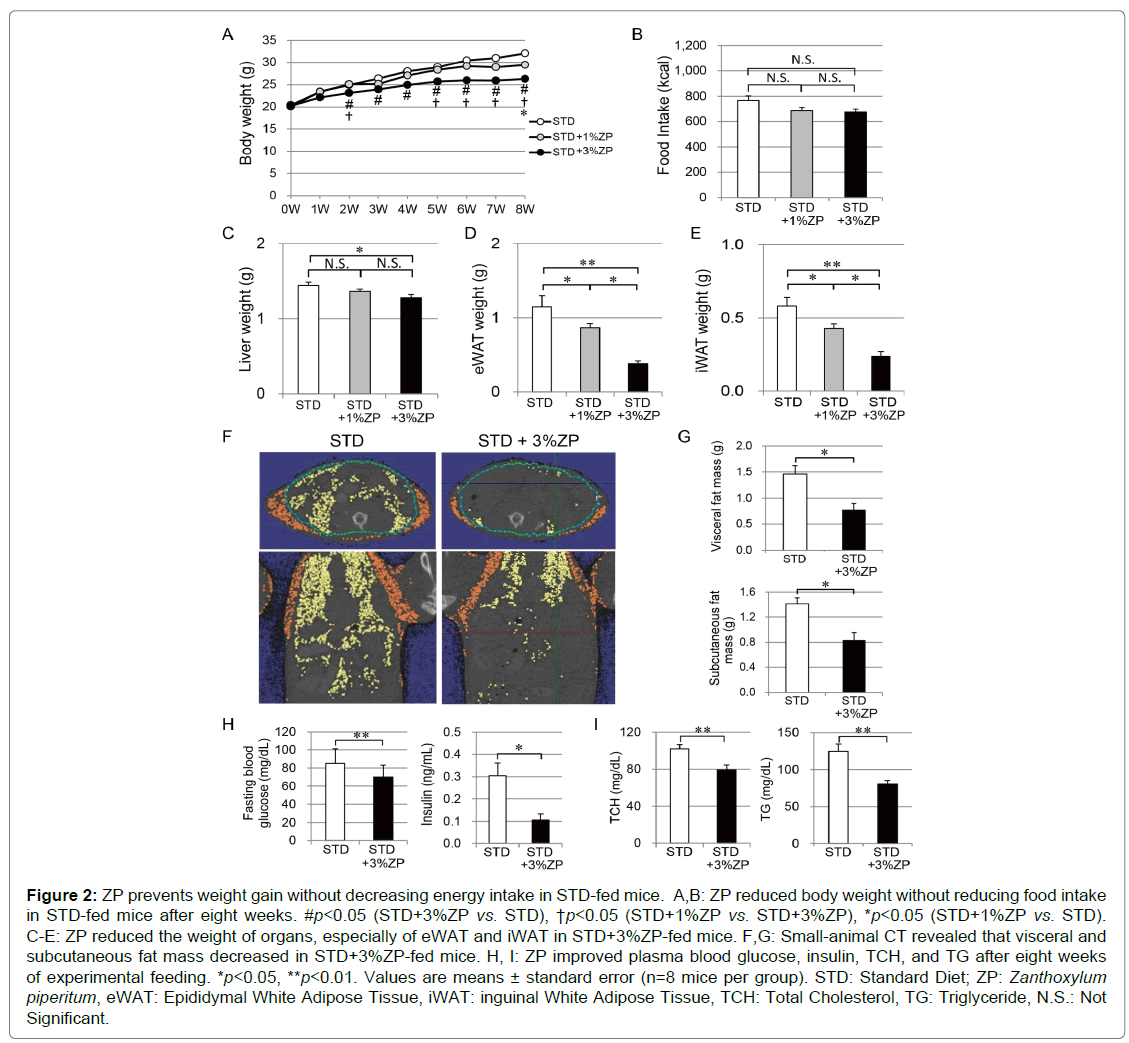
Figure 2A shows body weight changes in the mice fed for eight weeks with STD, STD+1%ZP, and STD+3%ZP diets. Mice administered ZP gradually lost weight. After two weeks of feeding, STD+3%ZP-fed mice had a significant decrease in weight compared with those without ZP. There was a more moderate reduction in the STD+1%ZP-fed mice. After five weeks, the reduction of body weight in STD+3%ZP-fed mice was more remarkable than in STD+1%ZP-fed mice.
Total energy intake after eight weeks did not differ significantly between the groups (Figure 2B). Total energy intake was similar between STD+1%ZP-fed mice and STD+3%ZP-fed mice, but there was significantly greater weight loss in STD+3%ZP-fed mice. ZP therefore appears to suppress weight gain, regardless of total energy intake. This effect was more prominent in mice that were fed a higher dose of ZP.
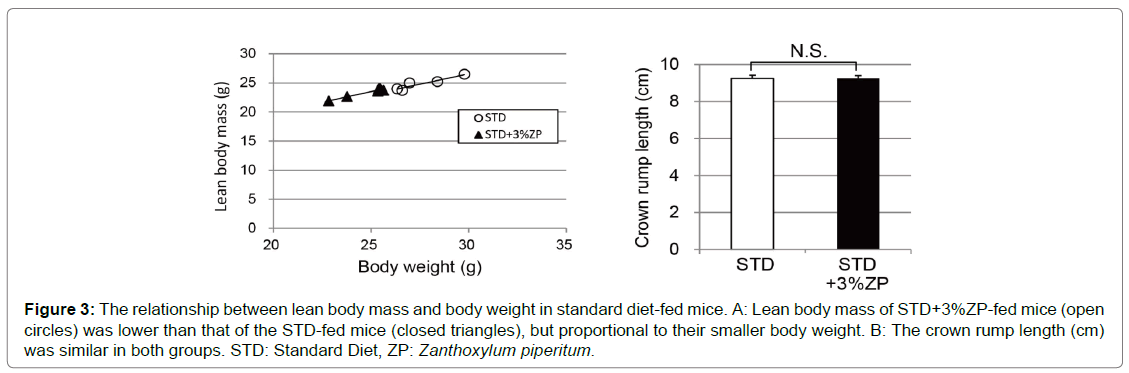
At the end of the eight-week study, we compared the weights of each organ between the groups. Liver weight was slightly reduced in STD+3%ZP-fed mice (Figure 2C). The mass of eWAT and iWAT was significantly reduced in STD-fed mice that were administered ZP, especially STD+3%ZP-fed mice (Figures 2D, E). In addition, fat analysis using small-animal CT revealed that abdominal fat mass of STD+3%ZP-fed mice was half that of STD-fed mice without ZP (Figure 2F, G). Lean body mass of STD+3%ZP-fed mice was slightly reduced, but proportional to their smaller body weight (Figure 3). No growth failure associated with malnutrition was observed in either of the ZPadministered groups; they had equal crown-rump lengths (Figure 3B). The weight-reducing effect of ZP appears to mainly be attributed to a decrease in adipose tissue mass.
Figure 2: ZP prevents weight gain without decreasing energy intake in STD-fed mice. A,B: ZP reduced body weight without reducing food intake in STD-fed mice after eight weeks. #p<0.05 (STD+3%ZP vs. STD), †p<0.05 (STD+1%ZP vs. STD+3%ZP), *p<0.05 (STD+1%ZP vs. STD). C-E: ZP reduced the weight of organs, especially of eWAT and iWAT in STD+3%ZP-fed mice. F,G: Small-animal CT revealed that visceral and
subcutaneous fat mass decreased in STD+3%ZP-fed mice. H, I: ZP improved plasma blood glucose, insulin, TCH, and TG after eight weeks of experimental feeding. *p<0.05, **p<0.01. Values are means ± standard error (n=8 mice per group). STD: Standard Diet; ZP: Zanthoxylum piperitum, eWAT: Epididymal White Adipose Tissue, iWAT: inguinal White Adipose Tissue, TCH: Total Cholesterol, TG: Triglyceride, N.S.: Not
Significant.
Figure 3: The relationship between lean body mass and body weight in standard diet-fed mice. A: Lean body mass of STD+3%ZP-fed mice (open circles) was lower than that of the STD-fed mice (closed triangles), but proportional to their smaller body weight. B: The crown rump length (cm) was similar in both groups. STD: Standard Diet, ZP: Zanthoxylum piperitum.
ZP improves glucose and lipid metabolism in STD-fed mice
To assess the metabolic effects of ZP, we measured plasma blood glucose, insulin, TCH, and TG levels under fasting conditions. Fasting blood glucose and insulin levels were significantly lower in STD+3%ZPfed mice than in those without ZP (p<0.01, p<0.05) (Figure 2H). Consequently, the homeostasis model assessment of insulin resistance index (HOMA-R), calculated as fasting insulin (U/L) × fasting glucose (mg/dL)/405, was lower in those without ZP (p<0.01). Urine glucose tests were negative in both groups (data not shown). Plasma TCH and TG levels were also lower in STD+3%ZP-fed mice than in those without ZP (p<0.01) (Figure 2I). ZP modifies glucose metabolism and insulin sensitivity in addition to improving lipid profiles.
ZP activates thermogenic gene expressions and induces beige adipogenesis in STD-fed mice
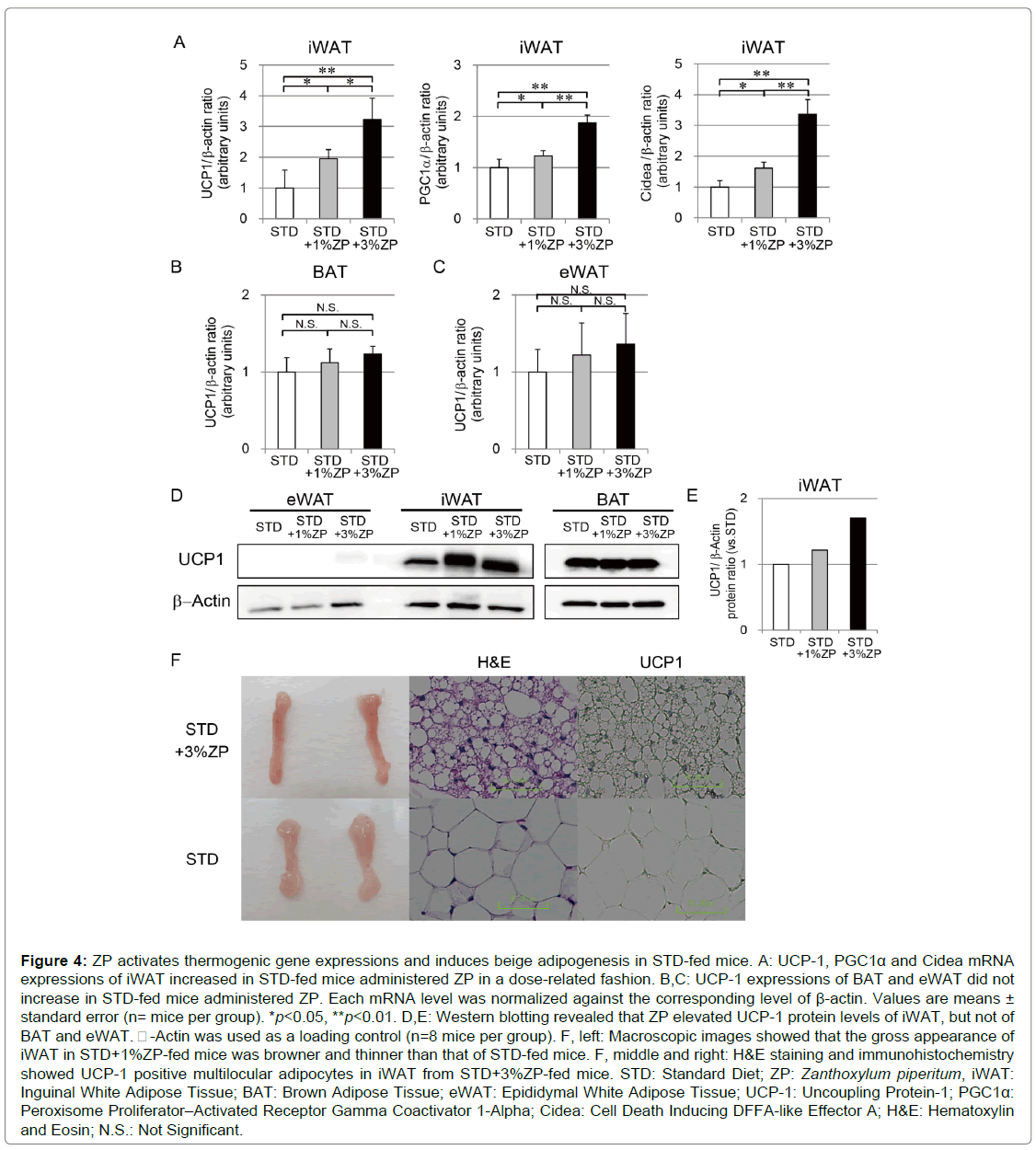
The weight-reducing effect of ZP was independent of total energy intake. We therefore examined beige adipogenesis as one mechanism of energy consumption acceleration. RT-qPCR analysis revealed that UCP-1 expression in iWAT was significantly higher in STD-fed mice with ZP than in those without ZP in a dose-related fashion (Figure 4A). On the other hand, UCP-1 expression in eWAT and BAT did not differ between the groups (Figures 4B, C). Beige adipocytes are often induced in iWAT by cold exposure and beta-adrenergic stimuli, so we analyzed thermogenic gene expression in iWAT in association with beige adipogenesis. Expression levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) and cell death-inducing DFFA-like effector a (Cidea) were significantly elevated in STD+3%ZPfed mice. Western blotting analysis confirmed elevation of UCP-1 protein expression in iWAT of STD-fed mice with ZP (Figures 4D, E).
The gross appearance of iWAT in STD+3%ZP-fed mice was browner and thinner than that of STD-fed mice without ZP (Figure 4F, left). To evaluate adipocyte morphology, adipose tissue sections were stained with H&E and visualized using optical microscopy (Figure 4F, center). Microscopic images of iWAT from STD-fed mice showed unilocular adipocytes containing large lipid droplets typical of WAT. By contrast, iWAT from STD+3%ZP-fed mice had multilocular adipocytes containing smaller lipid droplets, similar to those observed in BAT. Immunohistochemistry showed that iWAT from STD+3%ZPfed mice was mildly UCP-1–positive (Figure 4F, right), which is consistent with the elevated mRNA and protein expression levels of UCP-1. ZP appears to activate thermogenic gene expression and induces beige adipogenesis in iWAT.
Figure 4: ZP activates thermogenic gene expressions and induces beige adipogenesis in STD-fed mice. A: UCP-1, PGC1α and Cidea mRNA expressions of iWAT increased in STD-fed mice administered ZP in a dose-related fashion. B,C: UCP-1 expressions of BAT and eWAT did not increase in STD-fed mice administered ZP. Each mRNA level was normalized against the corresponding level of β-actin. Values are means ± standard error (n= mice per group). *p<0.05, **p<0.01. D,E: Western blotting revealed that ZP elevated UCP-1 protein levels of iWAT, but not of BAT and eWAT. -Actin was used as a loading control (n=8 mice per group). F, left: Macroscopic images showed that the gross appearance of iWAT in STD+1%ZP-fed mice was browner and thinner than that of STD-fed mice. F, middle and right: H&E staining and immunohistochemistry showed UCP-1 positive multilocular adipocytes in iWAT from STD+3%ZP-fed mice. STD: Standard Diet; ZP: Zanthoxylum piperitum, iWAT: Inguinal White Adipose Tissue; BAT: Brown Adipose Tissue; eWAT: Epididymal White Adipose Tissue; UCP-1: Uncoupling Protein-1; PGC1α: Peroxisome Proliferator–Activated Receptor Gamma Coactivator 1-Alpha; Cidea: Cell Death Inducing DFFA-like Effector A; H&E: Hematoxylin and Eosin; N.S.: Not Significant.
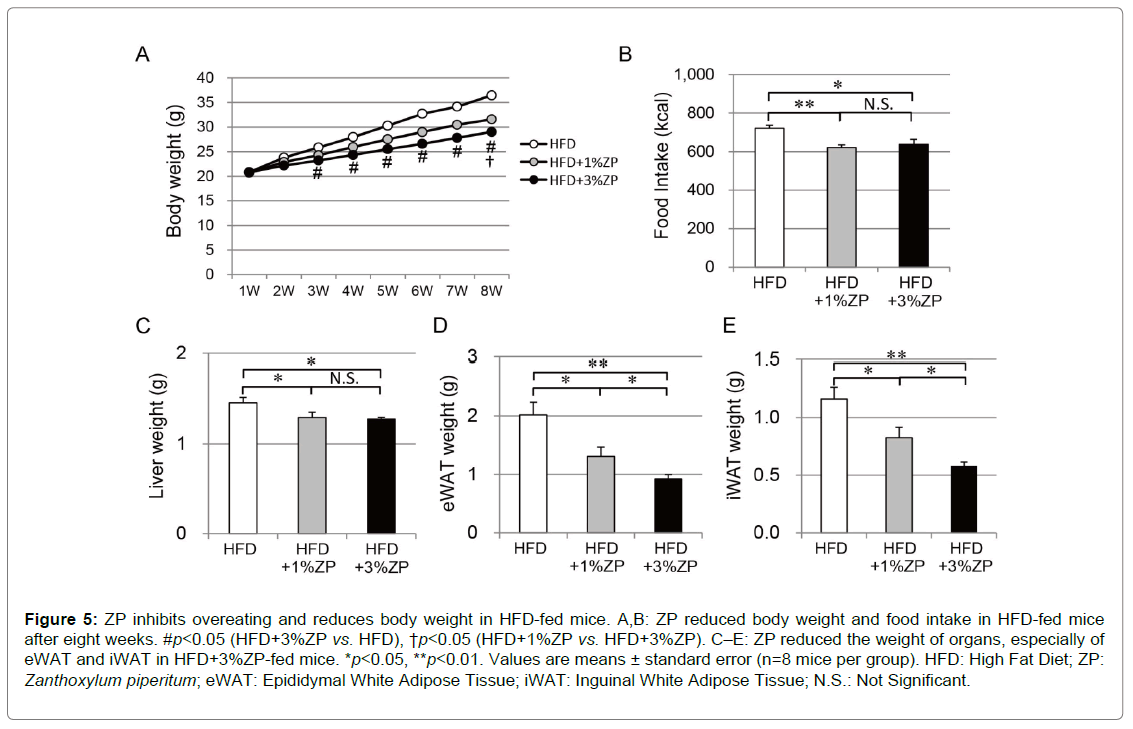
ZP inhibits overeating and reduces body weight in HFD-fed mice
We next investigated the effect of ZP on HFD-fed mice; changes in bodyweight are shown in Figure 5A. Mice administered ZP gradually lost weight: HFD+3%ZP-fed mice exhibited a significant decrease in weight compared with those without ZP after three weeks of feeding. The reduction of weight in HFD+1%ZP-fed mice with was more moderate than in HFD+3%ZP-fed mice. The bodyweight of HFD+3%ZP-fed mice was lower than in HFD+1%ZP-fed mice, but not significantly.
Total energy intake was reduced in HFD-fed mice that were administered ZP (Figure 5B), but it was similar between those with HFD+1%ZP-fed and HFD+3%ZP-fed mice. We therefore considered that the weight loss in HFD-fed mice that were administered ZP is primarily attributed to the reduction in energy intake and, at least in part, to increase in energy expenditure.
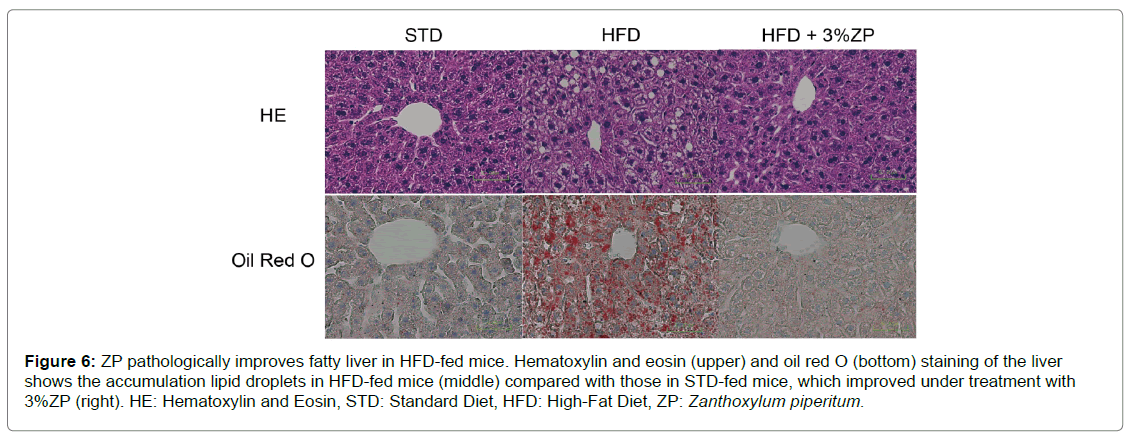
HFD-fed mice administered with ZP also had lower liver weight and fat mass, concomitant with amelioration of fatty liver (Figures 5C–E & Figure 6). The improvement of fatty liver was more remarkable in HFD+3%ZP-fed mice than in HFD+1%ZP-fed mice.
Figure 5: ZP inhibits overeating and reduces body weight in HFD-fed mice. A,B: ZP reduced body weight and food intake in HFD-fed mice after eight weeks. #p<0.05 (HFD+3%ZP vs. HFD), †p<0.05 (HFD+1%ZP vs HFD+3%ZP). C–E: ZP reduced the weight of organs, especially of eWAT and iWAT in HFD+3%ZP-fed mice. *p<0.05, **p<0.01. Values are means ± standard error (n=8 mice per group). HFD: High Fat Diet; ZP: Zanthoxylum piperitum; eWAT: Epididymal White Adipose Tissue; iWAT: Inguinal White Adipose Tissue; N.S.: Not Significant.
Figure 6: ZP pathologically improves fatty liver in HFD-fed mice. Hematoxylin and eosin (upper) and oil red O (bottom) staining of the liver shows the accumulation lipid droplets in HFD-fed mice (middle) compared with those in STD-fed mice, which improved under treatment with 3%ZP (right). HE: Hematoxylin and Eosin, STD: Standard Diet, HFD: High-Fat Diet, ZP: Zanthoxylum piperitum.
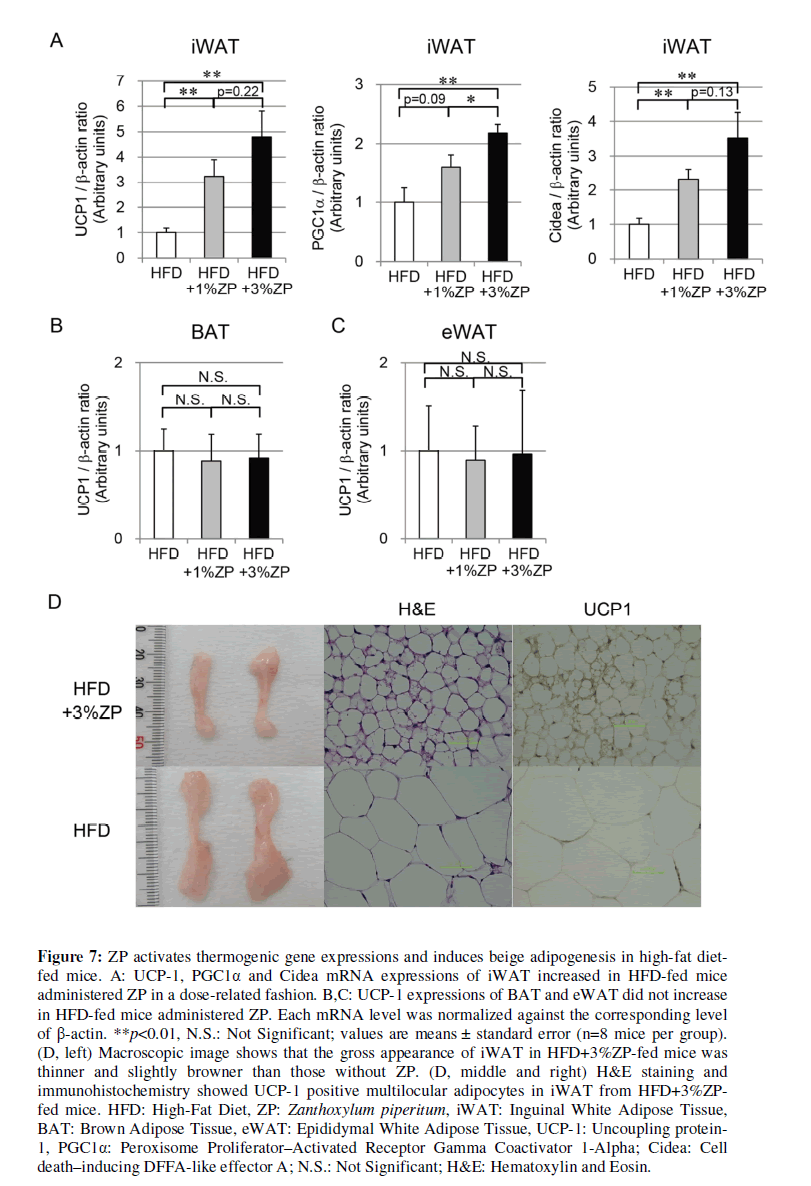
ZP activates thermogenic gene expressions and induces beige adipogenesis in HFD-fed mice
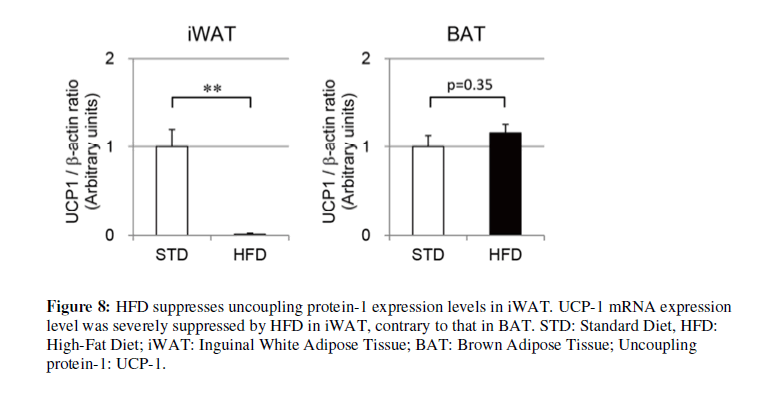
Based on our examination of STD-fed mice, we also evaluated thermogenic gene expression in iWAT from HFD-fed mice. UCP-1 expression in iWAT was significantly higher in HFD-fed mice with ZP than those without ZP in a dose-related fashion (Figure 7A). Other thermogenic genes, including PGC1α and Cidea, were also significantly elevated in HFD-fed mice with ZP in comparison with those without ZP (Figure 7A). They had a tendency to increase in a ZP concentration–dependent manner. UCP-1 expression in BAT and eWAT, however, did not differ significantly between the groups. UCP- 1 expression levels of iWAT in HFD-fed mice were lower than those in STD-fed mice (Figure 8), but this suppression was not confirmed in those of BAT in the HFD group.
Figure 7: ZP activates thermogenic gene expressions and induces beige adipogenesis in high-fat diet-fed mice. A: UCP-1, PGC1α and Cidea mRNA expressions of iWAT increased in HFD-fed mice administered ZP in a dose-related fashion. B,C: UCP-1 expressions of BAT and eWAT did not increase in HFD-fed mice administered ZP. Each mRNA level was normalized against the corresponding level of β-actin. **p<0.01, N.S.: Not Significant; values are means ± standard error (n=8 mice per group). (D, left) Macroscopic image shows that the gross appearance of iWAT in HFD+3%ZP-fed mice was thinner and slightly browner than those without ZP. (D, middle and right) H&E staining and immunohistochemistry showed UCP-1 positive multilocular adipocytes in iWAT from HFD+3%ZP-fed mice. HFD: High-Fat Diet, ZP: Zanthoxylum piperitum, iWAT: Inguinal White Adipose Tissue, BAT: Brown Adipose Tissue, eWAT: Epididymal White Adipose Tissue, UCP-1: Uncoupling protein-1, PGC1α: Peroxisome Proliferator–Activated Receptor Gamma Coactivator 1-Alpha; Cidea: Cell death–inducing DFFA-like effector A; N.S.: Not Significant; H&E: Hematoxylin and Eosin.
The gross appearance of iWAT in HFD+3%ZP-fed mice was slightly browner and thinner than that of HFD-fed mice without ZP (Figure 7D, left). H&E staining of iWAT from HFD-fed mice revealed unilocular adipocytes containing large lipid droplets typical of WAT (Figure 7D, center). By contrast, iWAT from HFD+3%ZP-fed mice exhibited some multilocular adipocytes containing smaller lipid droplets, but the number of multilocular adipocytes was not as high as those of STD+3%ZP-fed mice. Immunohistochemistry revealed that iWAT from HFD+3%ZP-fed mice was mildly UCP-1–positive (Figure 7F, right), consistent with the elevated mRNA expression levels of UCP-1. These findings suggest that ZP also activates thermogenic gene expression and induced beige adipogenesis in iWAT of HFD-fed mice, but the effects were milder than those in STD-fed mice.
Discussion and Conclusion
The present study revealed two major effects of Zanthoxylum piperitum; induction of beige adipogenesis and inhibition of hedonic eating.
In STD-fed mice, addition of 3% ZP significantly reduced body weight and fat mass without a corresponding decrease in food intake. We therefore focused on the potential fat-burning effects of ZP. Notably, thermogenic gene expression was elevated in STD+3%ZP-fed mice compared with those without ZP. Western blotting analysis also showed elevated UCP-1 protein expression. UCP-1 immunostaining revealed beige adipogenesis in iWAT, corresponding with the gene and protein expression analysis. Accordingly, we proposed that ZP is associated with beige adipogenesis followed by increase in energy expenditure. Similarities between mouse beige adipocyte and human brown adipocyte have been reported, so we expect these effects of ZP could be also beneficial in humans [3,4,6].
The speculated mechanism of thermogenic gene activation by ZP is as follows: i) ZP acts as a PPARγ agonist; ii) ZP activates beta-adrenergic receptor (βAR); and iii) ZP induces intrinsic beige adipocyte–promoting substances.
PPARγ is considered to be a master regulator of white and brown adipocyte biogenesis [10]. It also plays an important role in the induction of beige adipogenesis [11]. To determine whether ZP acts as a PPARγ agonist, UCP-1 expression in 3T3-L1 cells using ethanol and methanol extract of ZP was assessed (data not shown). As a result, ZP neither increased UCP-1 expression nor promoted beige adipogenesis; it is therefore unlikely that ZP acts as a PPARγ agonist.
As for βAR activation, capsaicin, a spice with similar tingling sensation to ZP, is reported to activate UCP-1 expression in BAT through transient receptor potential vanilloid 1 (TRPV1) in sensory neurons and promote thermogenesis [12,13]. It has also been reported that capsaicin and cold exposure synergistically promotes beige adipogenesis in iWAT through βAR signaling pathway [14]. We did not observe a significant increase in UCP-1 expression in BAT from STDfed and HFD-fed mice administered ZP in our study; it is therefore possible that ZP induces beige adipogenesis in iWAT through βAR activation via TRPs.
Another possible mechanism underlying beige adipogenesis is recruitment of intrinsic beige adipocyte–promoting substances. Bile acids are one of the candidates synthesized in the liver and associated with thermogenesis in BAT [15]. Recently, Gpbar1 ligand was shown to promote beige adipogenesis in WAT with overexpression of UCP-1 and PGC1α [16]. Another candidate is FGF21, also produced in the liver, it plays a physiological role in thermogenic activation of BAT and beige adipogenesis [17,18]. Future studies should seek to determine the association between ZP-induced beige adipogenesis and intrinsic substances.
In this study, we used a crude extract of ZP which included nonvolatile and volatile components. Non-volatile components have been reported to include α-sanshool, β-sanshool, γ-sanshool, hydroxy-α- sanshool, hydroxy-γ-sanshool, and hydroxy-α-sanshool [19,20]. Sanshool, especially hydroxy-α-sanshool, creates strong tingling and paresthetic sensations on the tongue [21]. According to the HPLC analysis of ethanol extract from ZP used in our study, the highest peak is from hydroxy-α-sanshool. Hydroxy-α-sanshool reportedly activates TRPs, such as TRPV1 and TRPA1, in sensory neurons [22]. These non-volatile components are therefore potentially the molecules that promote beige adipogenesis through βAR activation via TRPs.
In the present study, ZP markedly inhibited overeating and reduced body weight and fat mass in HFD-fed mice. HFD causes malfunction of the food-reward system, which results in overeating and obesity [23,24]. ZP is implied to possibly have the ability to modulate the hedonic state and suppress overeating. Volatile components of ZP reportedly include limonene, α-terpineol, linalool, citral, citronellal, cineol dipentene, and geraniol [25]. Epple et al. previously reported that these volatile components of ZP inhibited food intake of rats, so we speculated that in our study the components could be associated with the reduced food intake of HFD-fed mice. We also showed that an effect of ZP in activating UCP-1 expression in iWAT was also confirmed in HFDfed mice. In the present study, however, the expression levels were severely suppressed by HFD. A previous report also suggested UCP-1 suppression by HFD [26], but with an unclear mechanism.
A further limitation of this study was that when it was performed, we lacked metabolic cages and testing facilities for fecal analysis. We could not therefore directly assess the effects of ZP on intestinal absorption and basal energy expenditure. We used a crude extract of ZP in this study, so studies of single components will be future focus of study of the functional components of ZP.
In conclusion, ZP activates thermogenic gene expressions and induces beige adipogenesis in iWAT of mice. It also has the effect of inhibiting hedonic eating in mice. Both effects of ZP could be potentially utilized in a complementary agent for bodyweight control in obese patients.
Acknowledgments
We thank Ayumi Nakatani, Atsuko Ono, and Saeko Higashi for help with animal experiments. We also thank Asako Doi for technical advice. We also thank Benjamin Phillis for proofreading and editing the paper.
Funding
This work was supported by JSPS KAKENHI Grant Number JP25504010 (to H.I.), JP16K12728 (to H.I.), Grants-in-Aid from the Consortium of Higher Education Institution in Wakayama (2012 & 2016 to H.I., 2014 & 2015 to K.T.), Research and Development Support Project for Pioneering Industrial Technology by Wakayama Prefectural Government (to N.G.), and a Grant-in-Aid from the Regional Innovation Strategy Support Program 2011-2016 (to K.T.).
References
- World Health Organization (1999) Definition, diagnosis, and classification of diabetes mellitus and its complications: Report of a WHO Consultation.
- Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA (2010) Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol 6: 255-269.
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150: 366-376.
- Kajimura S, Spiegelman BM, Seale P (2015) Brown and beige fat: Physiological roles beyond heat generation. Cell Metab 22: 546-559.
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, et al. (2013) Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 19: 635-639.
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, et al. (2012) Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7: e49452.
- Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, et al. (2011) Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 19: 1755-1760.
- Epple G, Bryant BP, Mezine I, Lewis S (2004) Zanthoxylum piperitum (DC), a potential feeding deterrent for mammals: studies with Microtus ochrogaster (Wagner). Pest Manag Sci 60: 624-630.
- Gwon SY, Ahn JY, Kim TS, Ha TY (2012) Zanthoxylum piperitum DC ethanol extract suppresses fat accumulation in adipocytes and high fat diet-induced obese mice by regulating adipogenesis. J Nutr Sci Vitaminol (Tokyo) 58: 393-401.
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM et al. (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 270: 12953-12956.
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S (2012) PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15: 395-404.
- Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K (1986) Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med 183:250-256.
- Saito M, Yoneshiro T (2013) Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr Opin Lipidol 24: 71-77.
- Ohyama K, Nogusa Y, Shinoda K, Suzuki K, Bannai M, et al. (2016) A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte. Biogenesis Diabetes 65: 1410-1423.
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, et al. (2006) Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484-489.
- Carino A, Cipriani S, Marchianò S, Biagioli M, Scarpelli P, et al. (2017) Gpbar1 agonism promotes a Pgc-1α-dependent browning of white adipose tissue and energy expenditure and reverses diet-induced steatohepatitis in mice. Sci Rep 7: 13689.
- Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R (2010) Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab 11: 206-212.
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, et al. (2012) FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271-281.
- Yasuda I, Takesya K, Itokawa H (1982) Distribution of unsaturated aliphatic acid amides in Japanese Zanthoxylum species. Phytochemistry 21: 1295-1298.
- Kashiwada Y, Ito C, Katagiri H, Mase I, Komatsu K, et al. (1997) Amides of the fruit of Zanthoxylum spp. Phytochemistry 44: 1125-1127.
- Bryant BP, Mezine I (1999) Alkylamides that produce tingling paresthesia activate tactile and thermal trigeminal neurons. Brain Res 842: 452-460.
- Koo JY, Jang Y, Cho H, Lee CH, Jang KH et al. (2007) Hydroxy-alpha-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur J Neurosci 26: 1139-1147.
- Begg DP, Woods SC (2013) Hedonic and homeostatic overlap following fat ingestion. Cell Metab 18: 459-460.
- Murray S, Tulloch A, Gold MS, Avena NM (2014) Hormonal and neural mechanisms of food reward, eating behaviour and obesity. Nat Rev Endocrinol 10: 540-552.
- Pfänder HJ, Frohne D (1987) Szechuan pepper. The fruits of Zanthoxylum piperitum DC (Rutaceae). Dtsch Apoth Ztg 127: 2381–2384.
- Fromme T, Klingenspor M (2011) Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 300: R1-R8.
Citation: Takeshima K, Kadowaki A, Gato N, Fujita S, Kishida K, et al. (2020) Zanthoxylum piperitum Activates Thermogenic Gene Expression and Induces Beige Adipogenesis in White Adipose Tissues of Mice. J Obes Weight Loss Ther 11: 421. DOI: 10.4172/2165-7904.1000421
Copyright: © 2020 Takeshima K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2970
- [From(publication date): 0-2021 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2182
- PDF downloads: 788