Will Probiotics Provide the Answer for Therapy of Non-alcoholic Fatty Liver Disease (NAFLD)? - A Systematic Review
Received: 04-Feb-2020 / Accepted Date: 20-Feb-2020 / Published Date: 27-Feb-2020 DOI: 10.4172/2168-9652.1000257
Abstract
As per current definition recognized by Food and Agricultural Organization of the United Nations (FAO) and world health organization (WHO) working group experts is that probiotics are live strains of strictly selected microorganisms, which once administered in adequate amounts, give a health benefit to the host. Globally non-alcoholic fatty liver disease (NAFLD) represents the liver problems of metabolic syndrome (Met S) that is also mostly related to obesity type2 diabetes mellitus (T2DM), as well as dyslipidemia. The correlation between gut microbiota and NAFLD has been emphasized recently. Lot of evidence is there that gut microbiota influences hepatic lipid metabolism as well as affects the correlation between pro and anti-inflammatory effectors within the liver. Though experimental studies have shown a correlation between gut microbiota dysbiosis and NAFLD exactly getting the answers of mode by which gut dysbiosis causes NAFLD is a problem. The probable pathophysiology which intertwines gut microbiota dysbiosis to NAFLD might be summed up as interrupting the energy balance among harvest as well as energy expenditure (EE), aiding in hepatic inflammation (interfering with intestinal integrity, stimulate endotoxaemia and start inflammatory cascades associated with cytokines liberation) and changes in biochemistry, metabolism and gut microbiota associated metabolites (like bile acids, short chain fatty acids (SCFA’s), Aromatic amino acid (AAA) derivatives, branched chain amino acid (BCAA), choline, ethanol). With the deposit that probiotics or synbiotics might reverse the gut microbiota associated dysbiosis in cases of NAFLD, work has escalated to evaluate the therapeutic efficacy of probiotics or synbiotics in NAFLD patients. Randomised controlled trials (RCT’S) done in recent times point that probiotics or synbiotics might better the tranmsaminases, steatohepatitis and decrease inflammation. Inspite of promising outcomes more further studies are warranted to get an insight in the full part that gut microbiota play in the formation of NAFLD and its future continuation. Moreover more results are required to know the effectiveness, safety profile and maintenance in the form of an innovative pharmacologic method for curing NAFLD.
Keywords: NAFLD; Gut microbiota; Dysbiosis; SCFA’s; Bile acids; AAA; Choline ; Ethanol; Probiotics; Synbiotics
Introduction
Globally non-alcoholic fatty liver disease (NAFLD) represents the commonest cause of chronic liver disorders. It involves a broad area of liver problems varying from simple steatosis to hepatocellular carcinoma (HCC) [1,2]. NAFLD involves 20-40% of adult population in western countries probably secondary to epidemics of obesity and type 2 Diabetes Mellitus (T2DM) [3]. The etiopathogenesis is interlinked with enhanced adiposity, insulin resistance (IR) as well as dyslipidemia [4]. Increased calorie intake, fructose and physical inactivity or sedentary lives are other causative factors of this problem [5]. Variable NAFLD phenotype might partly be secondary to genetics. Single nucleotide polymorphisms (SNPs) in protein controlling intracellular management of lipids within the liver which are constituted by palatin like phospholipase domain- containing 3(PNPLA3) transmembrane 6 superfamily member 2 (TM6SF2) and membrane bound O’acetyl transferase Domain-containing 7(MBOAT7), correlate with NASH development as well as fibrosis [6]. Lot of phenotypic alterations might be secondary to interactions among gene and environment known commonly as epigenetics that denotes a heritable although a process that can revert and has an influence on gene expression with no modification in the DNA sequence, like changes in the DNA nucleotides like methylation, histone modifications along with control of transcription by changing stability of mRNA via small RNA molecule like mi RNAs [7,8]. The word probiotic comes from the Greek word which means ‘for life’. Despite lot of change in definitions, currently the definition recognized by Food and Agricultural Organization of the United Nations (FAO) and world health organization (WHO) working group experts is that probiotics are live strains of strictly selected microorganisms which once administered in adequate amounts, give a health benefit to the host [9]. With mainly lipid metabolism being altered in NAFLD briefly we discuss about role of Lipoproteins in relation to lipid metabolism. Cholesterol, triglycerides and phospholipids get transported in the form of Lipoprotein complexes. These complexes markedly increase the solubility of lipids. There are 6 families of Lipoproteins that get graded as per size and lipid content. The density of these lipoproteins and thus the speed at which they sediment in the ultracentrifuge is inversely proportionate to their Chylomicrons, Chylomicron remnants, very low density lipoprotein (VLDL), intermediate density lipoprotein (IDL), density lipoprotein (LDL), high density lipoprotein (HDL). In general the lipoproteins consist of a hydrophobic core of triglycerides and cholesteryl esters surrounded by phospholipids and protein. These lipoproteins are arranged in an exogenous pathway that transports lipids from intestine to the liver and the endogenous pathways that transport lipids to and from the tissues. The protein components of lipoproteins are called apoproteins. The major apoproteins are called APO E, APO C, and APO B. There are 2 forms of APO B, a low molecular weight called APO B48 that is a characteristic of the exogenous pathway which transports ingested lipids and a high molecular weight form called APO B 100 [10,11]. Chylomicrons (CM) get formed in the intestinal mucosa during absorption of the products of fat digestion. CM is v large, lipoproteins complexes which enter circulation through the lymphatic ducts. CM get cleared from circulation by the action of Lipoprotein lipase that is located on the surface of endothelium of capillaries and breaks down triglycerides (TG) in CM to FFA and glycerol that then enters the adipose cells and get re esterified. Alternatively the FFA remains in circulation bound to albumin. Lipoprotein lipase that needs heparin as a cofactor also removes triglycerides from circulating VLDL. The Chylomicrons and Chylomicron remnants constitute the transport system for ingested exogenous lipids. There is also an endogenous system that is made up of VLDL, IDL, LDL and HDL that transports triglycerides and cholesterol throughout the body. VLDL are formed in the liver and transport triglycerides formed from fatty acids (FA’s) and carbohydrates in liver rich in triglycerides are secreted by the liver and converted to IDL and then to LDL rich in cholesteryl esters. Some of the LDL enter the sub-endothelial space of arteries and are oxidized, then taken up by macrophages which become foam cells. HDL receptor is primarily found in endocrine glands which make steroid hormones and in the liver. The HDL system transfers cholesterol to the liver which is then excreted in the bile and thus it lowers plasma cholesterol. Thus being a complicated disease NAFLD pathophysiology has not been clarified with it getting further affected by umpteen parallel hits like insulin resistance (IR), oxidative stress, inflammation, epigenetic modifiers with many other. Multiple risk factors recent suggestions indicate the importance of gut microbiota (GM) with their metabolites in the pathophysiology of alcoholic fatty liver disease (ALD) and NAFLD [12-14]. Thus, probably helping the manipulation of intestinal flora as a diagnostic method and ultimate therapy getting utilized in personalized therapy of NAFLD might be important in early diagnosis along with therapy.
Aim of review
In view of no proven treatment approved by FDA work is going on worldwide on how we try to restrict it to NAFLD and prevent the progression to more severe aspect like NASH and cirrhosis along with HCC, we chose this review on emphasizing on the role of gut microbiome impairment in the generation as well as progression of NAFLD with a key role as non-invasive biomarkers and therapeutic development. Earlier in our attempt to find more medical therapies for obesity, metabolic syndrome (Met S) and related disorders, we had reviewed the role of probiotics in the management of these conditions besides role of Vit D and its receptor as well as allyl isothiocyanate (AITC) and use of levo carnitine and nicotinamide riboside and volixbat along with role of rosmarinic acid in liver disorders that include HCC, here we further detail on probiotics role in NAFLD [15-22]. Here our aim is to further correlate the role of GM and liver axis in development of NAFLD and how utilization of probiotics might aid in prevention of development and progression of NAFLD to NASH, cirrhosis and HCC.
Methods
We carried out a pubMed search using the MeSH terms like NAFLD, NASH, obesity, gut microbiota, probiotics cirrhosis, HCC with relation to pathophysiology and management of NAFLD, HCC, Cirrhosis from 1970’s to 2019.
Results and Discussion
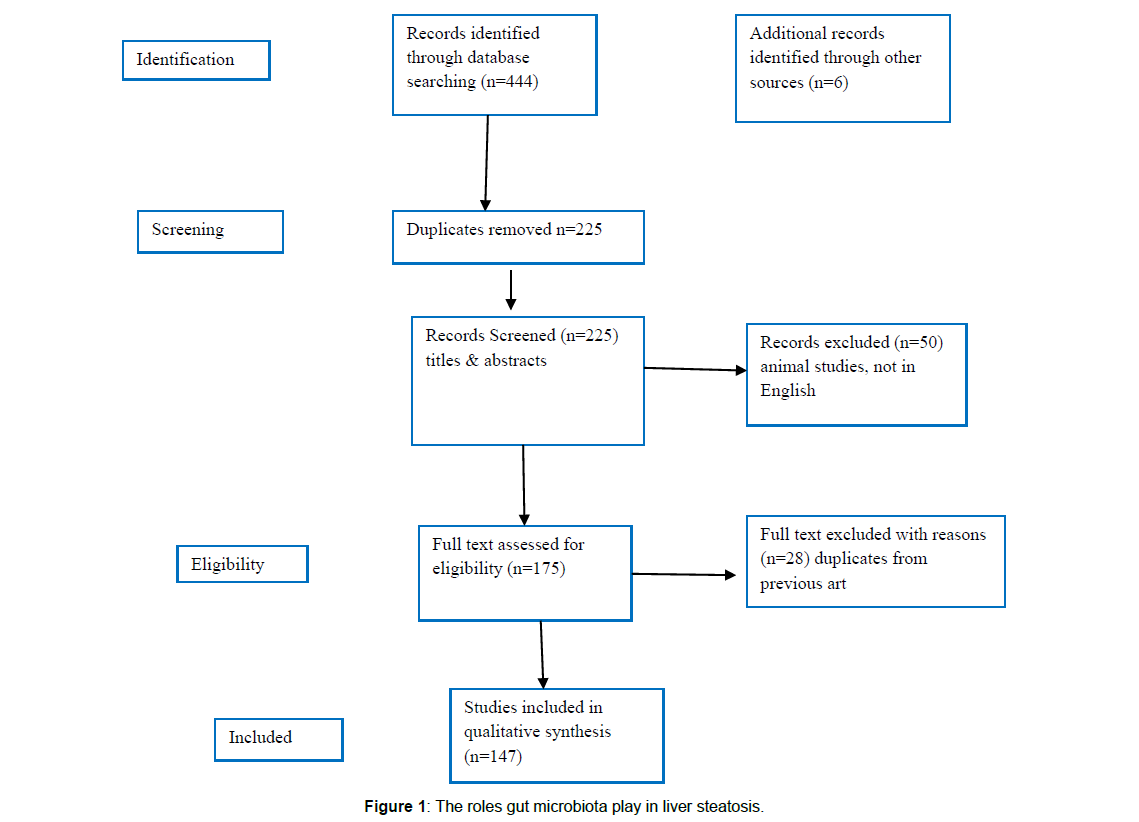
We found a total of 450 articles relevant to this field of which we selected 147 articles for this review. Further references were obtained from cross references obtained from the original articles. No metaanalysis was done. See Figure 1 for criteria of selection.
Gut- microbiota in NAFLD
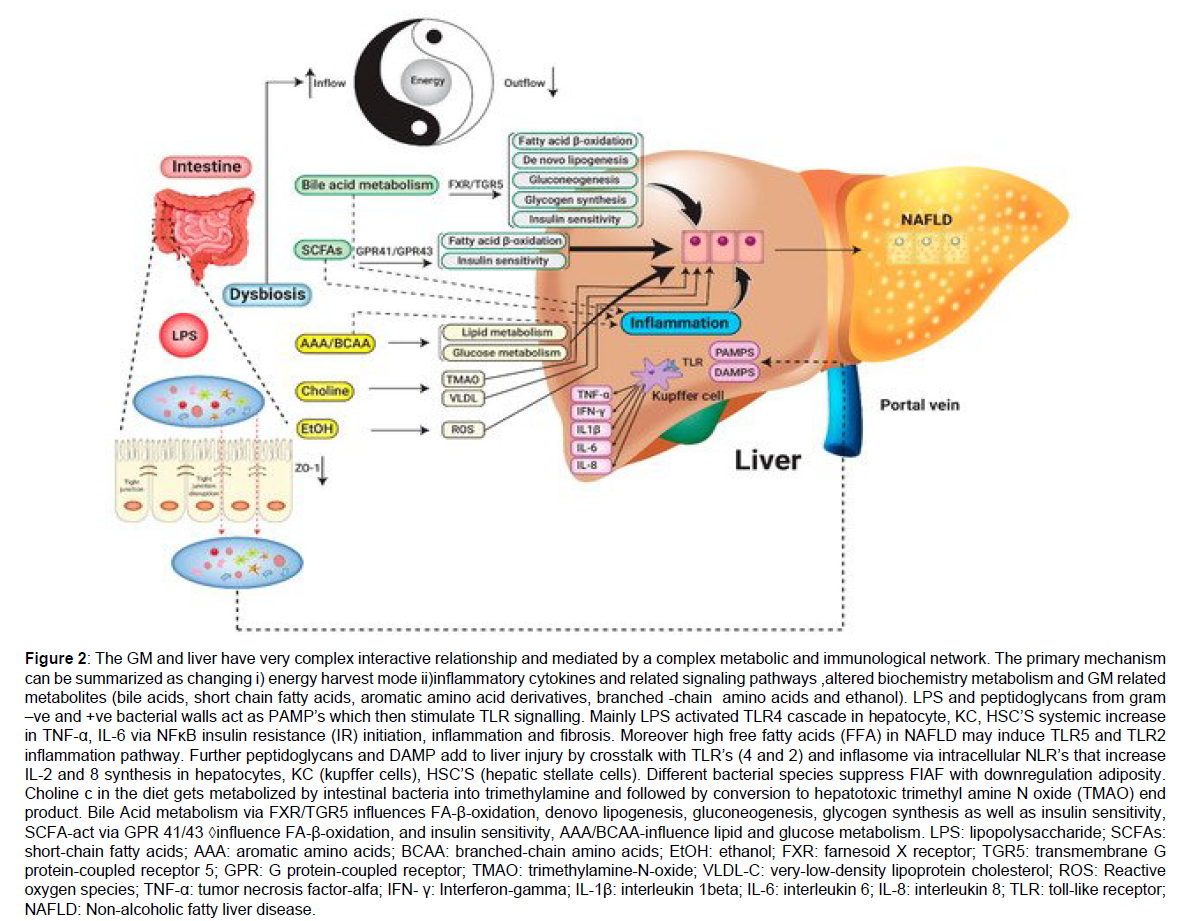
It is well known that both small and large intestine house a trillion microorganisms that belong to over 100 species. Changes in intestinal bacteria has a role in development of obesity and glucose tolerance has been proven [9,23,24]. This belief started from Buckhead’s early findings regarding germ free mice had decreased body fat and did not develop both obesity and insulin resistance (IR) when put on a high fat diet (HFD) [25,26]. But following reconstitution with the gut microbiota of conventionally raised mice these very germ free mice gained the adiposity and developed IR and glucose intolerance within 2 weeks [25]. This occurred despite decreasing diet that added more data on role of gut bacteria as being regulators of energy metabolism. There is change in gut microbiota which has been associated with both obesity and metabolic syndrome (Met S) that is referred to as dysbiosis [27]. Metagenomic analysis showed that most bacteria in the distal gut and faeces are from 2 main bacterial phyla both in mice as well as humans namely Bacteroides and Firmicutes [28]. A balance is maintained between these 2 phyla in lean mice, while in models of obese mice, a greater ratio of Firmicutes: Bacteroides has been observed commonly [27,29]. Yet some studies have reported opposite results [30,31] which points that this issue is still controversial. Large scale metagenomics studies in humans correlated microbial gene signatures with Met S [32-34]. Low bacterial prevalence, pointed to low microbial gene count in faecal DNA that correlated with dyslipidemia, IR and inflammation as shown by Le –Chatellier [33]. Those who had a high gene count had prevalence of potentially anti-inflammatory species like Faecalibacterium prausnitzii, which are associated with increased production of short chain fatty acids (SCFAs), which included butyrate [35]. Once bacterial numbers were increased in subjects with a low gene count by diet induced weight loss an improved metabolic outcome was seen in a study by Coutilajo et al. [35] that pointed that changes in the microbiota could be brought via diet. In toto bacterial richness might be an indicator of inflammation and metabolic diseases. In accordance with this, another small study in humans revealed that intestinal transfer of faecal microbiota from lean donors could improve insulin sensitivity along with richness of butyrate producing bacteria in recipients having Met S [36]. Transferring gut flora from obese to germ free mice increased obesity than use of gut flora from lean mice showing that obesity signals the collection of pathogenic bacteria which promote its occurrence [27]. Hence antibiotic therapy of obese mice can decrease adiposity and adipose inflammation and improve glucose metabolism [37,38]. Following weight loss and decreased adiposity and improvement of metabolic parameters by gastric bypass surgery, changes in microbial composition is also observed that further adds to the interlinking of obesity, dysbiosis and metabolic diseases [39,40]. How changes in bacteria cause obesity, various mechanisms have been given namely gut bacteria suppress the lipoprotein lipase suppressor also referred to as fasting induced adipocyte factor (FIAF) or angiopoietin like protein -4 (ANGPTL4) in intestinal cells an increased lipoprotein lipase activity along with increased triglyceride storage in adipocytes and the liver [25,26]. Further gut microbiota, control metabolism and regulation of bile acid profiles in the bowel which bind to the farsenoid X receptor (FXR) and G protein coupled bile acid receptors TGR5. It’s seen that a gut restricted FXR agonist decreases diet induced weight gain, systemic inflammation along with hepatic glucose production [41]. Obesity associated microbiota might be more efficient in harvesting energy from the diet by producing enzymes which break down nutrients in a more efficient manner [27]. Changed microbial composition in obesity also has other big consequences in the form of greater intestinal permeability that causes leakage of bacteria, bacterial products like lipo-polysaccharide (LPS) across intestinal barriers [42-44]. These bacterial products stimulate the innate immune system chronic inflammation. Giving continuous LPS infusion for 4 weeks recalls most metabolic changes that occur post HFD consumption, like increased fasting glucose and insulin, raised liver, adipose tissue (AT) and body weight and AT inflammation [43]. This bacteria-related leaking into blood and tissues like AT can be found as fast as 1 week following HFD initiation that is dependent on the microbial pattern receptors NOD1 or CD14 [44,45]. These LPS might enter the systemic circulation and AT via uptake by chylomicrons [46]. Increased energy intake, especially saturated fat is associated with endotoxemia in humans [47] along with concentration of bacterial 16S rRNA in the blood and associated with abdominal obesity along with risk of DM [48]. If host microflora is deleted with sterilization of the gut, it might repress tumor generation, decreasing remarkably size as well as number of nodules in diethylnitrosamine (DEN)- stimulated hepatocellular carcinoma(HCC) [49]. Similarly Dapito et al. documented that mice that were brought up in germ free situations formed little and lesser HCC and therapy with low dose of endotoxins reversed the situation [50]. These studies showed that gut microbiota and toll like receptor (TLR4) are needed for aiding in tumorigenesis progress which modulate proliferation as well as prevent apoptosis Figure 2 [49,50].
Figure 2: The GM and liver have very complex interactive relationship and mediated by a complex metabolic and immunological network. The primary mechanism can be summarized as changing i) energy harvest mode ii)inflammatory cytokines and related signaling pathways ,altered biochemistry metabolism and GM related metabolites (bile acids, short chain fatty acids, aromatic amino acid derivatives, branched -chain amino acids and ethanol). LPS and peptidoglycans from gram –ve and +ve bacterial walls act as PAMP’s which then stimulate TLR signalling. Mainly LPS activated TLR4 cascade in hepatocyte, KC, HSC’S systemic increase in TNF-α, IL-6 via NFκB insulin resistance (IR) initiation, inflammation and fibrosis. Moreover high free fatty acids (FFA) in NAFLD may induce TLR5 and TLR2 inflammation pathway. Further peptidoglycans and DAMP add to liver injury by crosstalk with TLR’s (4 and 2) and inflasome via intracellular NLR’s that increase IL-2 and 8 synthesis in hepatocytes, KC (kupffer cells), HSC’S (hepatic stellate cells). Different bacterial species suppress FIAF with downregulation adiposity. Choline c in the diet gets metabolized by intestinal bacteria into trimethylamine and followed by conversion to hepatotoxic trimethyl amine N oxide (TMAO) end product. Bile Acid metabolism via FXR/TGR5 influences FA-β-oxidation, denovo lipogenesis, gluconeogenesis, glycogen synthesis as well as insulin sensitivity, SCFA-act via GPR 41/43 influence FA-β-oxidation, and insulin sensitivity, AAA/BCAA-influence lipid and glucose metabolism. LPS: lipopolysaccharide; SCFAs: short-chain fatty acids; AAA: aromatic amino acids; BCAA: branched-chain amino acids; EtOH: ethanol; FXR: farnesoid X receptor; TGR5: transmembrane G protein-coupled receptor 5; GPR: G protein-coupled receptor; TMAO: trimethylamine-N-oxide; VLDL-C: very-low-density lipoprotein cholesterol; ROS: Reactive oxygen species; TNF-α: tumor necrosis factor-alfa; IFN- γ: Interferon-gamma; IL-1β: interleukin 1beta; IL-6: interleukin 6; IL-8: interleukin 8; TLR: toll-like receptor; NAFLD: Non-alcoholic fatty liver disease.
NAFLD and human gut microbiota
Lot of studies have shown that bacterial excessive growth might badly affect metabolic functions as well as immune responses that result in obesity as well as obesity associated comorbidities which are NAFLD and IR [51]. But the exact role of dysbiosis in the full variety of NAFLD lesions has not been worked out. In 35 patients attending consecutively having biopsy corroborated NAFLD it was shown that NAFLD patients had a significantly higher gut permeability as compared to healthy people and the incidence of small intestinal bacterial overgrowth was associated strictly with the degree of steato hepatitis although not with the lobular inflammation, ballooning and fibrosis was shown by Miele et al. [52]. Further number of patients afflicted by NASH showed intestinal bacterial overgrowth as checked with (14) C-D-xylose-lactulose breath test enhanced endotoxins along with inflammatory cytokines in to the circulation [53]. Hence the severity of NAFLD is associated with dysbiosis as well as the manipulation of metabolic qualities of intestinal flora [54]. Main bacterial changes seen in NAFLD patients are enhancement of Protobacteria, Enterobacteriacea, Lachnospiraceae, Escherichia, Bacteriodes but various differences in the amounts of the latter among different studies with heterogeneity of results in part in view of obesity and MetS acting as confounders [55-57]. An imbalance in the ratio of Bacteroides and Firmicutes was documented by Zhou et al. in faecal samples of obese as well as children [58]. They checked the composition of gut bacterial communities especially in 22 biopsy tested NASH children of 25 obese individuals and 16 healthy controls by 16S ribosomal RNA (rRNA) pyrosequencing and they displayed an escalated amounts of Bacteroides with a reduction in the amount of Firmicutes in the fecal samples obese and NASH children. Further the amounts of Actinobacter were decreased in NASH patients and on the reverse the numbers of Protobacteria enhanced progressively from healthy to obese to NASH patients [57]. Most important part of this work was the presence of escalated blood levels of alcohol with the maximum action of alcohol dehydrogenases (ADH’S) only in NASH children in view of escalated quantities of ethanol synthesizing bacteria from carbohydrate catabolism like E. coli. Physiologically, endogenous alcohol is synthesized continuously by intestinal bacteria and immediately cleared from portal blood by hepatic ADH’S, catalases and microsomal ethanol-oxidizing processes [59,60]. In case of NASH-induced dysbiosis on the other hand excessive representation of ethanol synthesizing bacteria saw to it that there is an excessive ethanol liberated into the blood circulation proving further that liver inflammation, reactive oxygen species (ROS), synthesis of cytochrome P4502E (CYP2E1) as well as intestinal permeability [58-61]. Finding of faecal dysbiosis as well as reduced amount of Firmicutes was seen by Wong et al. in 16 NASH patients as compared to controls [56]. They demonstrated that the reduced amount of Faecalibacterium and Anaerorosporobacter in these patients but levels of Para Bacteroides and Allisonella. Further Sobhonslidsuk et al. emphasized on the increase in the Bacteroides: Firmicutes ratio in 16 adult NASH subjects independent of age, body mass index (BMI), DM and any other medicines [62]. Especially in Bacteroides phylum the Bacteroides and Prevotellae genera are present in maximum numbers in NASH patients. On the other hand the group of Mouzaki [55] showed a decrease in Bacteroides as well as amounts of Clostridium coccoides in 22 NASH patients as compared to 17 healthy controls and 11 simple steatosis thus aiding in the growth of separate bacterial species and hence the excessive energy sapping from dietary fat [63-65]. The differences in the degree of Bacteroides: Firmicutes seen by Zhu et al. and group of Mouzaki might point to variations in age, BMI, environmental as well as dietary factors of the 2 study groups. Thus to find the effect of obesity on gut microbiota, Wang et al. tried to find the differences in fecal microbiota in non-obese adult subjects with and without NAFLD (43 NAFLD a visual healthy controls) [66]. As per their findings non-obese NAFLD patients had 20% higher Bacteroides phylum as well as 24 & lower phylum pointing to a significant association of metabolic markers with the impaired microbiota in NAFLD [65]. Thus the Firmicutes higher levels depict the fingerprint as far as NAFLD is associated with obesity is concerned while Bacteroides excess points to a thinner NAFLD. Increasing proof has been passed on by Loomba et al. regarding the gut microbiota induced signature that forecasts the presence of exaggerated fibrosis in NAFLD patients [67]. Utilizing a whole genome shotgun sequencing technique on stool samples they evaluated the bacterial composition in 86 biopsy corroborated NAFLD in which 72 had mild fibrosis while 14 with exaggerated fibrosis (stage ¾). 37 separate bacterial species were found that helped them to differentiate mild and exaggerated fibrosis in NAFLD patients and they demonstrated that exaggerated fibrosis in NAFLD patients is represented by an enhancement of Proteobacteria and E. coli as well as reduction in Firmicutes [67]. Recently same workers found that the particular intestinal microbiota profile of cirrhosis patients secondary to NAFLD found about 27 bacteria in a panel of faecal bacteria which might differentiate cirrhosis due to NAFLD utilizing a random forest classifier model [68]. Also Boursier et al. found that enhancing amount of Rhinococcus in NASH patients afflicted with increased fibrosis and Prevotella excess was reduced with amounts of E. coli and Staphylococcus have been detected in stool of subjects having mild encephalopathy as well as cirrhosis [69]. 75, 245 genes were detected with quantitative metagenomic evaluation which could distinguish cirrhotic patients from healthy subjects displaying Bacteroides but amounts of Proteobacteria and Fuso bacteria [70]. Trying to find the Circulating microbiome from the portal vein Schierwagen et al. of 7 patients having decompensated cirrhosis after implantation of a transjugular intrahepatic porto systemic shunt they showed that 65 genera from the 4 phyla, mainly Proteobacteria, were strictly associated with cytokine secretion [71]. Lastly checking for fecal microbiota differences in HCC patients Ren et al. demonstrated excessive phylum Actinobacteria and in 13 genera, which included Gemmiger and Para Bacteroides in fecal samples of 75 HCC patients as compared to 40 cirrhotic patients. Especially butyrate forming genera were reduced while lipopolysaccharide (LPS) synthesizing genera were enhanced in early stage of HCC [72].
Gut- liver axis-importance in Non-alcoholic Fatty Liver Disease (NAFLD) aetiopathogenesis
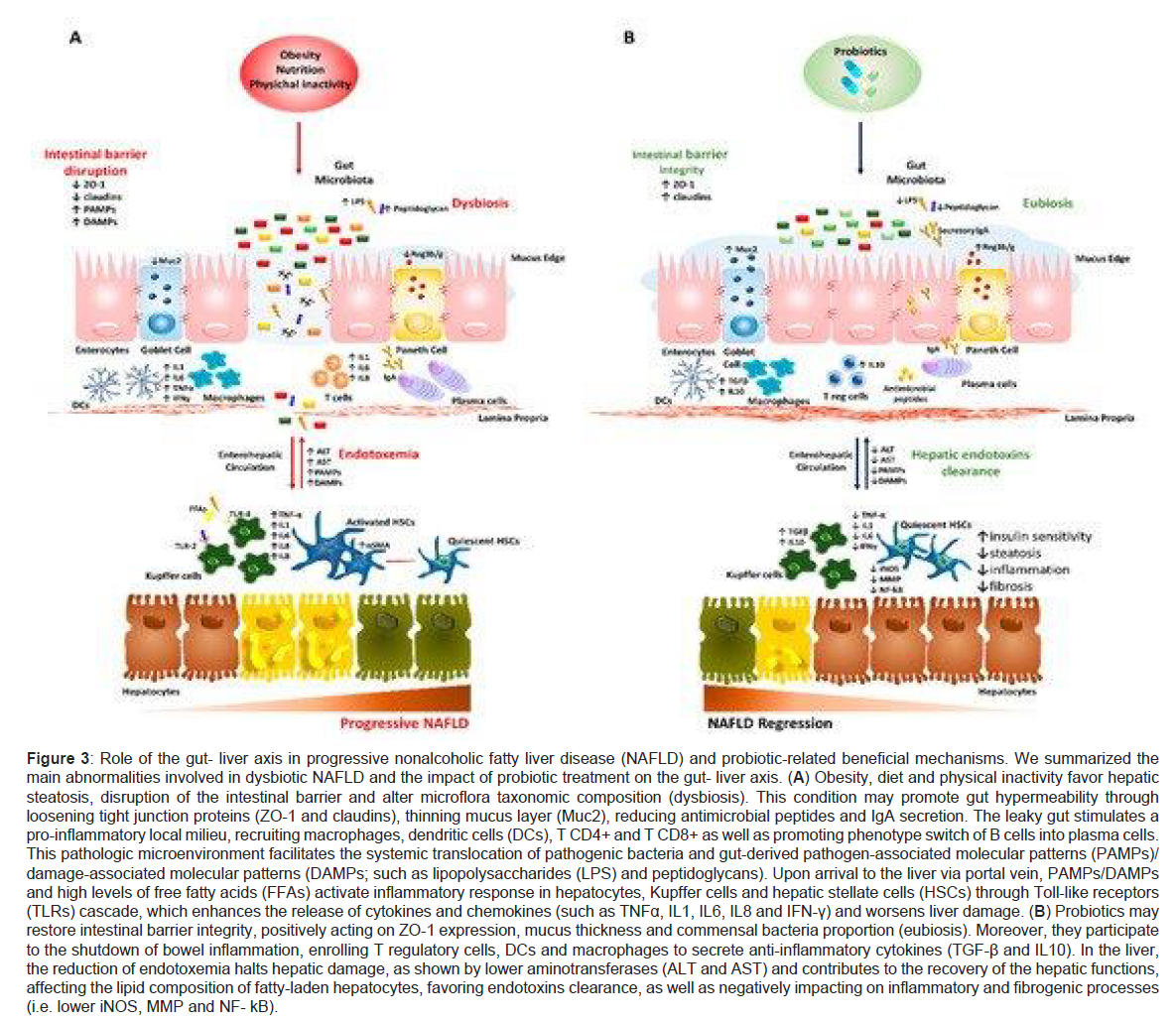
Importance of Gut-Liver Axis in dysbiosis of the intestine lies in view of the close anatomic along with functional cross talk among the 2 organs. Liver gets constantly exposed to gastrointestinal tract (GIT) microbiota end products along with nutrients through the portal vein (constituting 70% of blood supply) and hence takes part in the composition of bacteria via cycling of bile acids that get liberated within the duodenum lumen through the utilization of enterohepatic circulation [10]. This total gut microbe’s component is a key for manipulating innate as well as adaptive immune response at local as well as systemic level promoting host defence in fighting pathogenic organisms. The wall of the intestines takes a key part by acting as a barrier that is selective in controlling flux in both sides among the gut and liver as it is made up of tight junctions that are adherent occludins, claudins and Zona Occludens 1(ZO1) along with desmosomes that see to it that epithelial cells are kept together. Moreover, it causes multiple immunological roles in view of it possessing many layers along with particular cells having special functions like Goblet, Paneth along with plasma cells that liberate mucins, antimicrobial peptides (like defensins, lysozyme and c-lectin Reg 3b/g) and immunoglobulin A (IgA), respectively. Collectively it is to protect host from invading pathogens and prevention of excessive bacterial growth and their systemic movement. Enhanced erosion of this protective mucus lining along with decrease in antimicrobial mediators correlates with transmission of pathogenic organisms as observed in both preclinical along with human experiments [73]. Once the integrity of this intestinal barrier gets interfered with called leaky gut and simultaneous change in the metabolic action of gut microbes are often observed in subjects with NAFLD associated dysbiosis [52,74] and is proportional to the severity of NAFLD. Relative enhancement of Bacteroides and Ruminococcus has been found to correlate independently with NASH and fibrosis [75]. Due to increased permeability of the GIT much bacteria move into the blood along with by products that might be damaging and from there to the liver and hence adding to the escalation of the circulating toxin levels generated from the GIT (endotoxaemia) and getting chronic low grade inflammatory state typical of obesity formed along with NAFLD [76]. Variety of endogenous molecules like ethanol, ammonia, acetaldehyde whose circulating escalated amounts are secondary to dysbiotic microbes (like numerous E. coli) can induce pro inflammatory cytokine generation from hepatic Kupffer cells with the same modes acting in ALD. Similarly Lipopolysaccharides (LPS) and peptidoglycans obtained from both gram negative as well as positive bacterial walls act as maximum depicting pathogen associated molecular patterns (PAMPs) that then stimulate toll like receptor (TLR) signalling. Especially LPS-Stimulated TLR4 cascade within the hepatocytes, Kupffer cells as well as hepatic stellate cells (HSCs) resulting in systemic amounts of tumor necrosis factor alpha (TNFα) and interleukin (IL-6) through nuclear factor kappa B (NFκB) hence initiating insulin resistance(IR), inflammation and fibrosis [9,77]. Separate from that circulating free fatty acids (FFA) having amounts in NAFLD might induce TLR4 and TLR2 inflammatory pathways [78,79]. Moreover peptidoglycans along with damage associated molecular patterns (DAMP) add to liver injury via crosstalk among TLRs (TLR5 and TLR2) as well as inflammasome through intracellular nucleotide binding and oligomerization (NOD)- like receptors (NLRs) that enhance IL1 and IL-8 synthesis within hepatocytes, Kupffer cells and HSCs [61]. Further, changes in gut microflora communities add to liver pathology and interference of intestinal barrier integrity. Like dysbiosis can influence lipid metabolism along with trafficking within liver as well as Adipose tissue (AT) via upregulation of lipogenic enzymes orlipoprotein lipase (LPL) and hence contributing to both obesity as well as steatosis formation. Notably various intestinal species of bacteria depress the Fasting Induced Adipocyte factor (FIAF) with the downregulation correlating with enhanced adiposity along with hepatic de novo lipogenesis [80]. Excessive collection in Cytophagia, Flavobacter, Bacteroides phyla stimulates the formation of fatty liver along with hepatic inflammation inducing (IL)- 17 liberation from T-helper cells (Th17) [81]. Further choline in the diet gets metabolized by intestinal bacteria into trimethylamine followed by conversion to hepatotoxic trimethyl amine N oxide (TMAO) end product. Actually shortage of choline or escalated TMAO synthesis are correlated with amounts of gram negative Gamma proteobacteria and Erysipelotrichi along with steatosis as its amounts are key to enhance very low density lipoprotein (VLDL) assembly along with their liberation. SCFA from microbes might influence intestinal barrier integrity as well as mucosal immune tolerance that escalate the amounts of intestinal short chain fatty acids (SCFA) generating species that increase the strength of barrier integrity helping tight junctions and generation of mucins and working as source of energy for the intestinal mucosal cells [82]. Like the decrease in butyric acid synthesis that is generated by Faecalibacterium prausnitzii causes weakening of some connections among intestinal epithelial cells by reducing the expression of tight junction proteins and mucins. Reinstallment of physiological excess of microorganisms that synthesize butyrate might abrogate the high gut permeability as well as systemic inflammation [83]. These molecular changes in gut- liver axis depicted Figure 3.
Figure 3: Role of the gut- liver axis in progressive nonalcoholic fatty liver disease (NAFLD) and probiotic-related beneficial mechanisms. We summarized the main abnormalities involved in dysbiotic NAFLD and the impact of probiotic treatment on the gut- liver axis. (A) Obesity, diet and physical inactivity favor hepatic steatosis, disruption of the intestinal barrier and alter microflora taxonomic composition (dysbiosis). This condition may promote gut hypermeability through loosening tight junction proteins (ZO-1 and claudins), thinning mucus layer (Muc2), reducing antimicrobial peptides and IgA secretion. The leaky gut stimulates a pro-inflammatory local milieu, recruiting macrophages, dendritic cells (DCs), T CD4+ and T CD8+ as well as promoting phenotype switch of B cells into plasma cells. This pathologic microenvironment facilitates the systemic translocation of pathogenic bacteria and gut-derived pathogen-associated molecular patterns (PAMPs)/ damage-associated molecular patterns (DAMPs; such as lipopolysaccharides (LPS) and peptidoglycans). Upon arrival to the liver via portal vein, PAMPs/DAMPs and high levels of free fatty acids (FFAs) activate inflammatory response in hepatocytes, Kupffer cells and hepatic stellate cells (HSCs) through Toll-like receptors (TLRs) cascade, which enhances the release of cytokines and chemokines (such as TNFα, IL1, IL6, IL8 and IFN-γ) and worsens liver damage. (B) Probiotics may restore intestinal barrier integrity, positively acting on ZO-1 expression, mucus thickness and commensal bacteria proportion (eubiosis). Moreover, they participate to the shutdown of bowel inflammation, enrolling T regulatory cells, DCs and macrophages to secrete anti-inflammatory cytokines (TGF-β and IL10). In the liver, the reduction of endotoxemia halts hepatic damage, as shown by lower aminotransferases (ALT and AST) and contributes to the recovery of the hepatic functions, affecting the lipid composition of fatty-laden hepatocytes, favoring endotoxins clearance, as well as negatively impacting on inflammatory and fibrogenic processes (i.e. lower iNOS, MMP and NF- kB).
Bile acid pool: controller of intestinal barrier integrity
Increasing proof points that change in bile Acid metabolism correlates with chronic liver diseases and its comorbidities like cholestasis formation. Normally liver produces primary Bile Acids that initially collect within the gall bladder and then get liberated in the lumen of the duodenum, where their conversion to secondary Bile Acids occurs by the intestinal microbes and stimulate lipid solubilisation, emulsification and absorption. Additionally, Bile Acids work as signalling molecules like deoxycholic acid (DCA) and dihydroxy cheno deoxycholic acid (CDCA) since they stimulate the intestinal farsenoid X receptor (FXR) causing the liberation of fibroblast growth factor 19 (FGF19) that can manipulate GIT barrier integrity and β-Klotho within the blood stream [10]. Both fibroblast growth factor 19(FGF19) and β Klotho might depress bile acids generation within the liver by inhibition of cholesterol 7-α-hydroxylase 1(Cyp7A1) [84]. The Increased synthesis of Bile Acids pool can further activate Takeda G protein coupled receptor 5 (TGR5) to stimulate the pro inflammatory cascade on surface of Kupffer cells [85]. Actually NASH subjects demonstrate increased amounts of cytotoxic Bile Acids in the liver, serum, stool and urine that might further aggravate liver injury to cirrhosis [86,87]. Many FXR agonists having hepatoprotective actions (i.e. obeticholic acids) have been hypothesized to be having the capacity to decrease hepatic steatosis formation as well as necroinflammation [87,88]. Hence alternate methods like ω-3 long chain polyunsaturated fatty acid intake and/or combined for manipulation of gut microbes that might benefit in adjusting bile acid Pool is still under evaluation to treat nafld [89].
Aromatic amino acids derivatives and branch chain amino acids
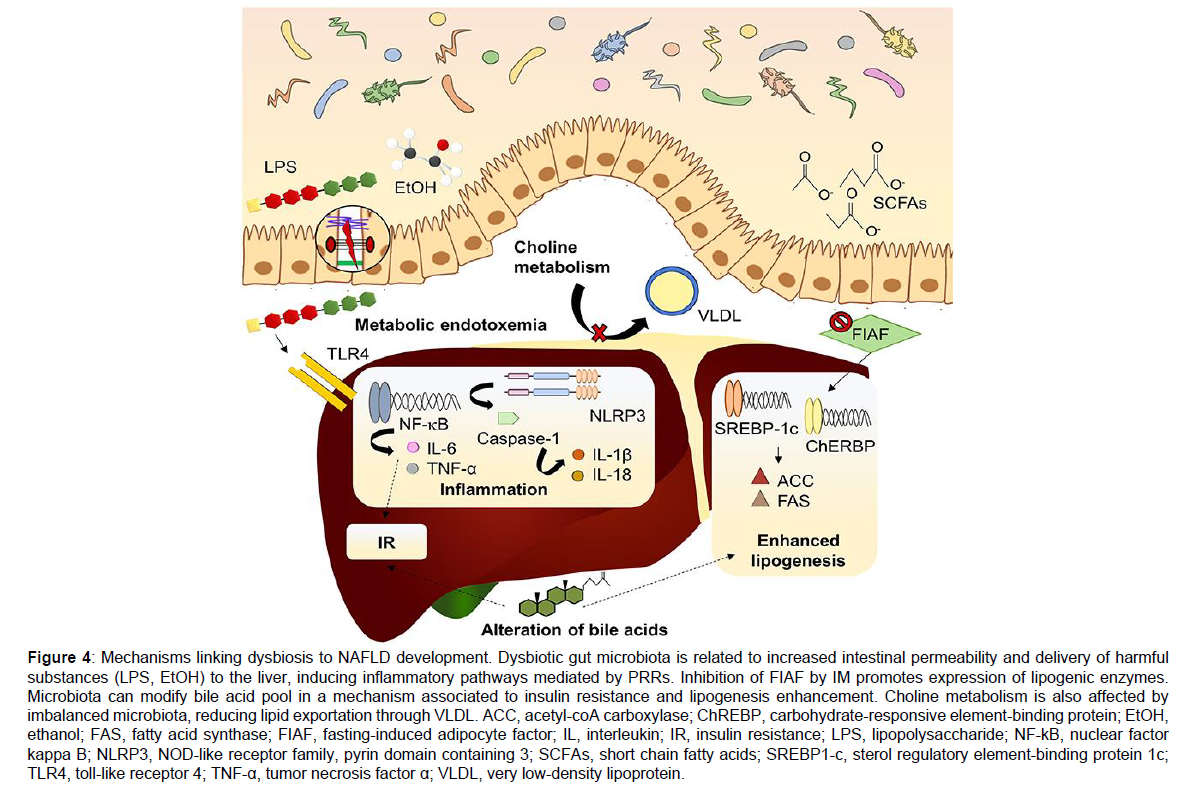
Some novel bacterial metabolites that are produced from Aromatic Amino Acids (AAA) are tryptophan, phenylalanine and tyrosine have got highlighted recently and thought of as possible mechanisms needed in NAFLD formation [90,91]. Bacterial metabolites that are produced via tryptophan are made up of indole, indole-3 propionic acid, indole-3 –acetic acid, indole-3-acetaldehyde, tryptamine and 3 methindole. Among these main part is indole [92]. These compounds sustain intestinal integrity, decrease Bacterial endotoxaemia translocation that avoids the liberation of microbiota produced components and control inflammatory cascades [90,91]. Oral indole supplementation can decrease the expression of crucial genes as well as proteins in LPS stimulated pro inflammatory signaling pathway, which has also been demonstrated to avoid LPS stimulated changes of the cholesterol metabolism in mouse models [93]. Indole-3 acetate also possesses similar protection conferring actions on liver which ameliorates inflammatory actions and decreases lipid collection in liver cells through the aryl hydrocarbon receptor (AhR) pathway [94]. Bacterial metabolites that are produced from phenylalanine are phenyl acetic acid, phenylpropionic acid and benzoic acid [95]. Plasma phenyl acetic acid (PAA) amounts were observed to be positively associated with the degree of steatosis severity in a cohort of women who possessed morbid obesity (n=56) as well as mice having therapy with PAA for 2 weeks had markedly enhanced triglyceride (TG) collection. Further, PAA was correlated with changed gene expression that was responsible for both glucose as well as lipid metabolism [96]. Branched Chain Amino Acids (BCAA) are valine, leucine and isoleucine belong to other group of metabolites that was highlighted in a recent hepatic steatosis research. Presence of crosstalk regarding the association among microbiome host gene expression as well as BCAA hepatic metabolism [96]. Biosynthesis of BCAA is upregulated regarding obesity and IR [97,98]. Further a positive association between hepatic steatosis and plasma and urine BCAA amount exists. PAA can also significantly escalate hepatic BCAA usage that can in synergy aid in hepatic lipid collection Figure 4 [96].
Figure 4: Mechanisms linking dysbiosis to NAFLD development. Dysbiotic gut microbiota is related to increased intestinal permeability and delivery of harmful substances (LPS, EtOH) to the liver, inducing inflammatory pathways mediated by PRRs. Inhibition of FIAF by IM promotes expression of lipogenic enzymes. Microbiota can modify bile acid pool in a mechanism associated to insulin resistance and lipogenesis enhancement. Choline metabolism is also affected by imbalanced microbiota, reducing lipid exportation through VLDL. ACC, acetyl-coA carboxylase; ChREBP, carbohydrate-responsive element-binding protein; EtOH, ethanol; FAS, fatty acid synthase; FIAF, fasting-induced adipocyte factor; IL, interleukin; IR, insulin resistance; LPS, lipopolysaccharide; NF-kB, nuclear factor kappa B; NLRP3, NOD-like receptor family, pyrin domain containing 3; SCFAs, short chain fatty acids; SREBP1-c, sterol regulatory element-binding protein 1c; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor α; VLDL, very low-density lipoprotein.
Changes in gut-liver axis in NAFLD and NASH
Newer understanding in NAFLD pathogenesis have been thought to be secondary to the gut permeable barrier since it adds to a microenvironment that suits the extra growth of bacteria, favouring endotoxaemia and thus leading to chronic liver injury secondary to endogenous or exogenous components like dietary factors and lifestyle changes. Actually fatty liver is a common component of obese subjects with 20-30% of patients that are pathologically obese display histological evidence of necroinflammation and fibrosis [99], probably in diet stimulated abnormality of barrier integrity. High fat diet (HFD) and intestinal bacteria favour inflammation of the intestines via TNFα generation as well as NFκB stimulation that is a mode that happens just prior to IR generation in mice [100]. Feeding high sucrose high fat (HSHF) diet in NAFLD rats it was demonstrated that animals showed injured intestinal epithelium villi along with low grade inflammatory state secondary to enhanced gut generated endotoxins as well as inflammatory cytokines which get translocated to the circulation as shown by Zhou et al. [101]. Brun et al. [102] showed that leptin deficient (Lepob/ob) and hyperleptinemic (Lepdb/db)mice that were obese exhibited a dysmorphic mucosal barrier as shown by an amazing ZO1 and tight junctions redistribution with a marked enhancement of liberation of IL-1, IL-6, TNFα and interferon gamma (IFN-ƴ) in the portal vein Importantly sodium butyrate administration to HFD fed mice led to a better gut mucosa with intestinal ZO1 restoration and helping in marked enhancement of bacteria that are useful like Christen senallaceae, Blautia as well as Lactobacillus [103]. Moreover butyrate has more advantages like markedly decreasing fat collection within the liver along with inflammation and fibrosis markers [103]. HSCs that were obtained from Lep0b/ob and Lepdb/dblivers that had been chronically treated with LPS displayed a pro inflammatory as well as profibrotic phenotype in comparison to HSCs obtained from lean mice that corroborated that enhanced intestinal permeability might be responsible for the formation and further progress in obesity associated NASH [102]. Intake of fructose chronically correlates with absence of tight junctions protein as well as compounds that decrease SCFA formation that influence movement of PAMP’s enhance amount of macrophages in the liver and stimulate TLR1-9 along with myeloid differentiation factor 88 (Myd88) based pro inflammatory pathways in case of mice [104]. In the same way both acute as well as chronic fructose supplementation accelerated endotoxinaemia in case of paediatric NAFLD as well as associates with IR and liver inflammation markers [105]. Importantly GPC chemokine receptor CXC3CR1 safeguards mice from steatohepatitis stimulated HFD or MCD diet since it sustains intestinal homeostasis along with barrier integrity [106]. Ultimately in an initial model of NASH stimulated with high glucose/fructose diet (HFHGD), it was seen that rats had portal hypertension formation which indicates an extreme side effect of liver cirrhosis. Thus they evaluated if intestinal dysbiosis had the ability of manipulating endothelial as well as hepatic functions. Interestingly HFHGD fed mice had abundance of Firmicutes instead of Bacteroides species with selected stimulation of intestinal FXR which pointed that alterations in intestinal microbes communities interferes with Bile Acid metabolism that further promotes NASH associated side effects [107].
Changes in gut-liver axis in cirrhosis and HCC
The above detailed modes have an effect moreover on liver injury promotion probably ending in marked fibrosis and cirrhosis which signifies the end stage of chronic liver disease which is the main reason of liver failure as well as HCC. Stimulated HSCs display great LPS-stimulated TLR4 responsivity and hence continuous escalating profibrotic features which reduces immune defence as well as toxins clearing through liver tissue and thus promoting cirrhosis formation [108]. Further LPS/TLR4 signaling has a key part in stimulating hepatocarcinogenesis by support of senescence correlated secretory phenotype (SASP) in HSCs and activating chemoattractant cytokines synthesis from HSCs as well as monocytes. Translocation of bacteria also plays a role in haemodynamic side effects related to cirrhosis like hepatic encephalopathy, bleeds from the oesophageal varies as well as portal hypertension. Soribas et al. demonstrated that cirrhotic mice decreased mucus thickness and had Goblet cells deletion along with mucin expression, ending in bacterial overgrowth with the pathological shift in E. coli [109]. Importance of a leaky gut in human cirrhosis was first shown by Assimakopoulous et al. emphasizing on the role of occludin as well as claudin 1 downregulation in cirrhotic patients and further more in the ones having decompensated cirrhosis [110]. Additionally amounts of IL- 6, nitric oxide (NO) and reduced transepithelial resistance correlate with the activated intestinal macrophages present in cirrhotic patients [111]. Alterations in obesity stimulated gut microbes might enhance the quantity of DCA which has been correlated with phenotype SASP phenotype in HSCs that escalates the pro-inflammatory cytokines and tumor forming components along with DNA injury and reactive oxygen species (ROS) formation in the liver [112]. DEN stimulated HCC experimental model, changes in intestinal permeability appears to be the first hit causing enhanced tumorigenic liver response to LPS. Actually antibiotics regimen or TLR4 amelioration aborted the tumor growth along with multiplicity in mice [50]. C3H/HeJ and C3H/HeOuJ mice were exposed to a combination of DEN and the hepatotoxin carbon tetrachloride (CCL4), by Dapito et al. that is a model simulating human microenvironment for HCC increase and showed that intestinal microbes and TLRs help in liver cancer as a long time effect of chronic liver damage. Recently, Ponzani et al. Evaluated the gut microbiome profile as well as intestinal features of 21 NAFLD subjects with both cirrhosis and HCC as compared to 20 cirrhotic patients without HCC and healthy controls. Though they observed a similar amount of intestinal barrier abnormality among HCC and cirrhotic patients systemic amounts of IL-18, IL13, Chemokine C-C motif ligand 3 (CCL3), CCL4 and CCL5 markedly associated with the circulating activated monocytes in the presence of HCC [113].
Probiotics
Over the last 10 years great work has been tried for formulating newer methods that use Gut-Liver Axis as a target since it seems to be important area of convergence required to prevent NAFLD initiation and/or its progress. Various methods that have been tried to manipulate dysbiosis are untargeted methods (diet, probiotics, prebiotics, antibiotics and faecal transplant (FMT) microbiota targeted therapy (MTT) that targets microbial as well as host metabolites [114]. The mode through which imbalancing gut microbes influence liver pathology is still not clear though favouring outcomes of manipulation of GIT flora have been documented in variety of preclinical as well as human work. Great work has been put in to utilize the capacity of probiotics to convert the gut dysbiosis to normal and only now they have been suggested in treating NAFLD. The definition was accepted by the International Scientific Association of Probiotics and Prebiotics (ISAPP) in 2013 [23]. Though there are dead bacteria and their components can also show probiotic properties. Most commonly used bacterial strains are Bifidobacteria and Lactobacillus that exhibit probiotic properties and get included in many functional foods and dietary supplements [24].
Preclinical studies of NAFLD probiotics
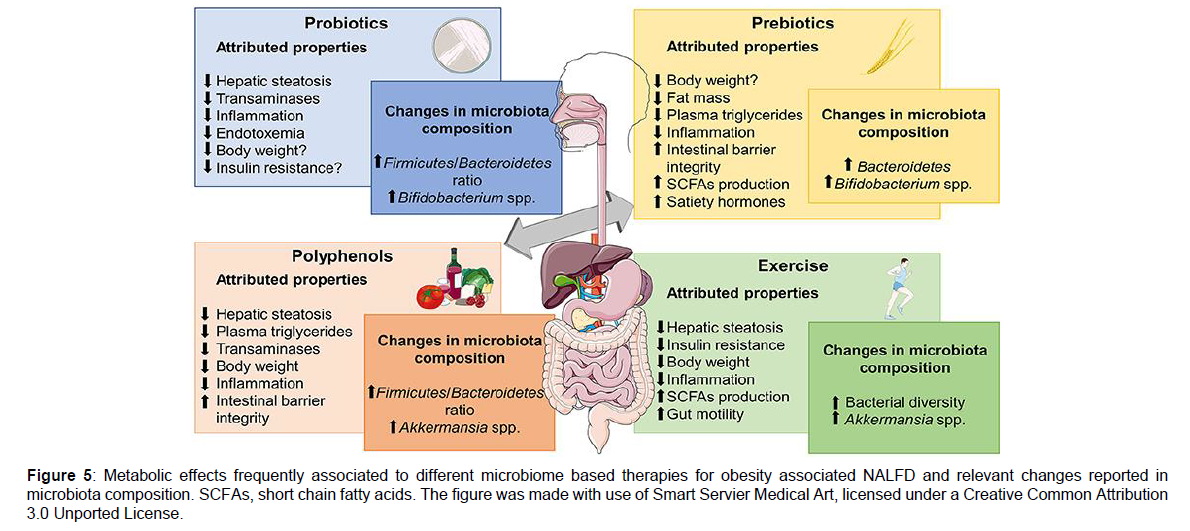
Various studies displayed that Probiotics intake might ameliorate NAFLD components in animal models [115-119]. Supplementing VSL#3, i.e. a combination of 3 genera of bacteria (multistrain formulation made by Streptococcus, Thermophilus and variety of species of Bifidobacterium and Lactobacillus) for 4 weeks to Lep0b/ob mice enhanced insulin sensitivity, total FA amount and serum alanine aminotransferase (ALT) amounts and the histological changes in liver injury. Main cause of these actions were secondary to decreases in c-Jun N terminal kinase (JNK) and NFκB activation in HFD fed mice as well as reduced expression of UCP- 2 in Lepob/obmice that had been exposed to VSL#3 corroborating the posit that intestinal bacteria might control the stimulation of signalling in the host that hamper the hepatic insulin response as well as lipid metabolism [115]. But still VSL#3 administration to HFD fed young rats reduced the synthesis of TNFα inducible nitric oxide synthadse (iNOS), metalloproteinases (MMP) and cyclooxygenase- 2 (COX-2) along with better lipid peroxidation markers that halts oxidative and inflammatory injury within liver [116-119]. Similar outcomes were noted by Ma et al. Where VSL#3, probiotics exposure attenuated IR, steatosis, proinflammatory cytokine liberation interfering with NFκB activation in HFD fed mice. Moreover Bifidobacterium longum administration to HFD –fed rodents for 12 weeks decreased hepatic fat collection than that with Lactobacillus acidophilus, despite the intestinal permeability getting reverted back to normal [120]. Additionally Lactobacillus johnsonii BS15 had a protective effect on HFD mice from hepatic steatosis as well as hepatocytic apoptosis displaying positive action on lipid peroxidation maintaining the antioxidant defence mechanism and better efficacy on mitochondrial impairments [121]. The conversion to Bifidobacterium or Akkermansia muciniphilia as well as oligofructose addition decreased endotoxinaemia, hepatic fat depots and metabolic syndrome (Met S) Signs in HFD- fed mice [122]. Further DM rats supplementation with Akkermansia muciniphilia benefitted in better liver function decrease gluco or lipotoxicity, ameliorate oxidative stress, repress inflammation and restore intestinal microbes to normal and thus attenuating type 2 diabetes mellitus (T2DM) [123]. In the same way nano selenium added Bifidobacterium longum postpones the initiation of streptozocin stimulated DM [124]. Intestinal microbes manipulation via VSL#3 mixture in an inherited dyslipidaemia (ApoE-/-) mice improved hepatic and AT IR as well as overcame atherosclerosis along with NAFLD initiation. Particularly VSL#3, reverted IR abrogated the formation of histologic changes of mesenteric AT inflammation NASH and decreased the degree of aortic plaques, via manipulation of PPAR ƴ, FXR and Vitamin D receptor [125]. VSL#3 might even effect fibrosis formation in mice given MCD stimulating the decrease of collagens MMPs and α-smooth muscle actin (α-SMA) without amelioration of hepatic steatosis and inflammation as shown by Velayudham et al. [119]. Corroborating Velayudham, Noradone et al. also displayed that Lactobacillus paracasei F19 (LP-F19) protected in an experimental ischemia-reperfusion models in rats given MCD [117]. Similarly, heat killed Lactobacillus reuteri GMLN-263 reverted hepatic steatosis along with heart fibrosis setting the expression of profibrotic markers again, like transforming growth factor beta (TGF-β) in HFD- fed hamsters [126]. Besides LP-F19 and GMLN 26, many different strains of Lactobacillus i.e. Lactobacillus reuteri GMLN-13 have been shown to abrogate the bad effects of impaired gut microbes in relation to NAFLD and NAFLD associated comorbidities like hypertension, obesity, glucose intolerance AT inflammation, dyslipidaemia as well as oxidative stress [127]. Oral VSL#3 supplementaion/day in rodents resurrected the enterocyte structure as well as intestinal barrier integrity by stimulating mucus liberation Muc2 colonic expression and ideal occluding amounts, preventing viable microorganisms along with bacterial products movement into circulation [128]. Tight junction proteins expression i.e. occludins, claudins and Zona Occludens 1(ZO1) was increased with Lactobacillus rhamnosus, Lactobacillus paracasei and Bifidobacterium adolescentis [114]. Interestingly Clostridium butyricum strain MIYAIRI 588 i.e. a butyrate synthesizing probiotic avoids the full pathologic spectrum of NAFLD right from steatosis to HCC in a rodent choline deficiency/L-amino acid (CDAA) diet model by establishing the intestinal barrier integrity again [129]. Other than that MIYAIRI 588, has a positive influence on the IR formation as well as increased triglyceride accumulation and ameliorates serum, endotoxin amounts, hepatic inflammation as well as oxidative stress [127]. In the end Lactobacillus johnsonii along with anti-oxidants avoid bacterial movement as well as endotoxemia in a rat model that is cirrhosis stimulated by CCL4, stimulating the thought that utilization of probiotics and antioxidants as an alternate method to antibiotics in avoiding bacterial infections in cirrhotic patients Figure 5 [130].
Figure 5: Metabolic effects frequently associated to different microbiome based therapies for obesity associated NALFD and relevant changes reported in microbiota composition. SCFAs, short chain fatty acids. The figure was made with use of Smart Servier Medical Art, licensed under a Creative Common Attribution 3.0 Unported License.
Human utilization of probiotics in NAFLD, cirrhosis and HCC
A double blind study was carried out in 20 obese children in whom USG confirmed steatosis were randomized for Lactobacillus rhamnosus, GG/placebo for 8 weeks by Vajro et al. inspite of no effect on adiposity as well as fatty liver it has been proposed as therapy for hyper transaminasemia in obese kids that were not following the lifestyle interventions [131]. Same kind of decreased ALT and aspartate aminotransferases (AST) was seen in 30 adults afflicted with NASH who got exposed acidophilus in contrast to those getting placebo [132]. With the combination of multi strains compound the probiotics influence has provided improved results in randomized controlled trial (RCT’s). A triple blind trial was conducted on 64 children having obesity displaying sonographic NAFLD was done by Famouri et al. Adolescents who obtained a probiotic capsule containing Lactobacillus acidophilus ATCC B3208 with Bifidobacterium lactis DSMZ 32, 269, Bifidobacterium Bifidum ATCCSD576 and Lactobacillus rhamnosus DSMZ21, 690 for 12 weeks displayed marked decreases in ALT, lipid changes in profile as well as intrahepatic fat amount analysed by USG as compared to placebo group. In another randomized, placebo controlled trial the effectiveness of ‘Symbiter’ that was constituted of 14 alive probiotic strains of Lactobacillus + Lactobacillus, Bifidobacterium, Propionobacterium and Acetobacter was checked by Kobyliak et al. in NAFLD patients. Attenuation of hepatic steatosis, aminotransferase action, TNFα, and IL-6 was observed with the use of this cocktail made of multiple probiotics in these NAFLD subjects [133]. In the same way in subjects having histology confirmed NASH administered Lepicol probiotic formula over 6 month’s ameliorated intrahepatic triglycerides and serum AST amounts [134]. Ma et al. carried out a meta-analysis that comprised of NAFLD/NASH through 4 RCT’s they emphasized that giving probiotic therapy utilizing Lactobacillus, Bifidobacterium and Streptococcus improved hepatic fat amount total cholesterol (TC), amounts of aminotransferase as well as HOMA-IR [135]. Gao et al. examined the effectiveness of probiotic therapy in pediatric as well as adult NAFLD that consisted of 9 RCT’s having 535 NAFLD subjects [136]. The maximum examined multi strains probiotics, VSL#3 has been shown to guard barrier integrity of the intestine reduces endotoxaemia as well as oxidative stress and nitrosative stress and hence helping in reversing of liver pathology in subjects presenting with variety of chronic liver diseases of which 20 presenting with ALD and 22 with NAFLD [137]. 48 pediatric NAFLD got included in a double blind RCT’s (NCT 01650025) administration of 4 month of VSL#3 reduced severity of NAFLD secondary to VSL#3 stimulated eubiosis [138]. Supplementation with Bifidobacterium longum as well as prebiotic fructo-oligosaccharides (FOS) markedly corrected the circulating metabolic as well as inflammatory markers including fibrosis scores in cases having biopsy confirmed NASH [139]. Funnily, Bifidobacterium longun in the VSL#3 compound manipulates gut microbes to synthesize conjugated linoleic acid (CLA) that further changes FA constitution in the liver that proves further that crosstalk among liver and GIT in NAFLD takes an essential role in the formatting of various treatment interventions. Intake of Lactobacillus GG over 8 weeks in a phase I study stimulated alterations in gut microbiome of cirrhotic patients having least hepatic encephalopathy (n=30) that decreased the excess of Enterobacteriaceae, endotoxaemia along with TNFα and helped in the growth of Clostridiales incertae sedis XIV and Lachnospiraceae [140]. An analysis of the combination of 8 strains for 12 weeks was conducted in 36 subjects with cirrhosis by Roman et al. and observed that this multi strains probiotics enhanced cognitive function, decreased the incidence of falls as well as reduced inflammatory responses [141]. On the other hand in a randomized double blind RCT that comprised of 44 cirrhotic patients long term probiotics administration made up of Bifidobacterium Bifidum W23, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Lactobacillus brevis W63, Lactobacillus casei W56, Lactobacillus salivarius W24, Lactobacillus lactis W58 had a significant effect on neutrophil resting burst but no effect on circulating endotoxins gut permeability or inflammatory markers [142]. Though most of the present day work on efficacy of probiotics is not very exciting initial work has pointed that they get well tolerated and are safe in cirrhotic patients. Very little work on the use of probiotics regarding HCC treatment has been documented in humans. It has been seen that if probiotics administration is done preoperatively and post operatively in HCC patients that had hepatic resection aided in liver working returning and displayed decreased frequency of intra and post-operative side effects [143].
Clinical Trials -in Relation To NAFLD And Probiotics
The action of probiotics on NAFLD formation and on Met s is recently getting evaluated in larger trials designed for long duration. Actually the effect of diet administration for 6 months of Lactobacillus acidophilus ATCCSD5221 and Bifidobacterium lactis HN019 on hepatic alterations in NASH cases is being defined in a single centre double blind, placebo controlled, parallel group study NCT027647 [134,137]. Final results of this experiment are the non-invasive examination of fibrosis, AST, ALT, reinstallment of gut microbes with the abrogation of lipid profile through measuring circulating TC, HDL, LDL and triglycerides. Efficacy of probiotics in getting better liver working is the target of another randomized, double blind, placebo controlled, trial NCT0404889 of 6 months period. In this study the supplementation of a combination of Lactobacillus and Bifidobacterium present in the probiotics Lactobacillus acidophilus, Lactobacillus casei subsp, Lactobacillus lactis, Bifidobacterium Bifidum, Bifidobacterium infantis, Bifidobacterium longum). Dietary administration in NAFLD patients who were obese who had to go through BS was the topic of a randomized, interventional study NCT01922830, where a combination of a variety of bacterial species (present in formula of BIO-25 (Supherb) constituted by 11 separate species that were of patented probiotic bacteria). Similarly in NCT03585413 examined the action of modulation of gut microbes and the mini gastric bypass surgery in the context of obesity. Furthermore phase II of NCT03511365 the Supplementation of VSL#3 in NAFLD patients. Lastly, NCT03511365 and NCT 02972567 are at present enrolling patients with NASH with the hope of attenuating hepatic inflammation and fibrosis and to better the prognosis of such subjects and their CVS risk following intestinal microbe recovery. Further in a systematic review and meta-analysis on probiotics effects in NAFLD Tang et al. found that weight reduced by 2.31 kg and BMI by 1.08 kg/m2 with probiotic therapy, ASP level by 7.22 U/L, Alkaline phosphatase by 25, 87 U/L and the glutamyl preptidases levels by -5.76 U/L, TG, LDLC and TG were reduced significantly and plasma glucose got reduced as did cytokine TNFα by 0.62 and leptin by 1.14. Relative risk of restoring degree of liver fat infiltration (DFI) for probiotics was 2.47 and thus concluding that administration of probiotics is a promising way of treatment of NAFLD [144]. The efficiency of probiotics in metabolic disease is debatable requiring more evaluation for safety dosages short/long term exposure effects and is it effective alone or in combination of today’s therapy of NAFLD.
Conclusion
The rapid escalation of both incidence as well as prevalence of NAFLD with lack of any efficacious pharmacological therapies other than the ones being tried like LC, NR trying vit D supplementation and agonists against Vitamin D receptor have being tried. Further this interaction among gut microbes and NAFLD have received emphasis with increasing amount of studies showing that host gut microbiome action as well as pathology of dysbiosis might aid in the formation of NAFLD. Metabolites obtained from bacterial products, immune associated cells as well as energy removal/intake balance impairment may aid in NAFLD progress. Hence reversion of dysbiosis might be a probable method for NAFLD that is supported by lot of recent probiotics and symbiotic clinical trials in relation to NAFLD and NASH. Till date we have publications in support regarding both probiotics and symbiotic that might help in correcting aminotransferase levels, hepatic steatosis as well as action score. To some degree probiotics and symbiotic might also easily decrease pro- inflammatory cytokines like TNF alpha as well as interleukin family (IL-1, IL-6, IL-8).
In total probiotics and symbiotic are safe, get well tolerated although safety examinations are required in the coming future. In this time of increasing NAFLD epidemic more work needs to be carried out to clearly find the effectiveness, safety and continuation of probiotics and symbiotic in the treatment of NAFLD.
References
- WaiSun W, Wong G, Yip G (2006) Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gastroenteriol 130: 207-10.
- Wang RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, et al. (2015) Non-alcoholic steato hepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenteriol 148: 547-55
- Younossi ZM (2019) Nonalcoholic fatty liver disease a global public health perspective. J Hepatol 70: 531-44.
- Byrne CD, Targher G (2015) NAFLD: A multisystem disease. J Hepatol 62: S47-S64.
- Dongiovanni P, Valenti L (2017) Nutrigenomic approach to Non-alcoholic fatty liver disease. Int J Mol Sci 18: p: 534
- Dongiovanni P, Romeo S, Valenti L (2015) Genetic factors in the pathogenesis of Non-alcoholic fatty liver and steato hepatitis. Biomed Res Int p: 460190.
- Dongiovanni P, Meroni M, Longo M, Fargion S, Franczani AL (2018) mi RNA Signature in NAFLD: A turning point for a non-invasive diagnosis. Int J Mol Sci 19: p: 3966.
- Meroni M, Longo M, Rametta R, Dongiovanni P (2018) Genetic and epigenetic modifiers of alcoholic liver disease. Int J Mol Sci 19: p: 3857
- Food and Agricultural Organization (FAO). Report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food FAO: London 2002
- Kaur KK, Allahbadia GN, Singh M (2015) An update on micro RNA's and metabolic regulation with future therapeutic potentials regarding diagnosis and treatment of obesity, metabolic syndrome and other related disorders. J Health Med Informat 6: 2.
- Kaur KK, Allahbadia GN, Singh M (2019) Chronically elevated triglycerides as a result of high fat palatable diet resulting in a vicious cycle on reinforcing reward and dopamine signaling a possible cause for the obesity epidemic worldwide in the food environment available a comprehensive review. J Endocrinol 3: 000143.
- Buzzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple hit pathogenesis of Nonalcoholic fatty liver disease (NAFLD). Metab Clin Exp 65: 451-64.
- Meroni M, Longo M (2019) Alcohol or gut microbiota: Who is the Guilty? Int J Mol Sci 20: 4568.
- Borrelli A, Bonelli P, Tucillo FM, Goldfine ID, Evans JL, et al. (2018) Role of gut microbiota and oxidative stress in the progression of Nonalcoholic fatty liver disease to hepato carcinoma: Current and innovative therapeutic approaches. Redox Biol 15: 467-79.
- Kaur KK, Allahbadia GN, Singh M (2016) An Update on Aetiopathogenesis and Management of Obesity. Obes Control Ther Open Acc 3: 1-17.
- Kaur KK, Allahbadia GN, Singh M (2017) Hypothalamic inflammation and glioses as aetiopathogenetic factor inhigh fat diet induced obesity and various therapeutic options to resolve it. Obes Res Open J 4: 44-60.
- Kaur KK, Allahbadia GN, Singh M (2018) Current advances in pathogenesis in obesity: Role of Hypothaalamic glioses. J Obes Weight Loss 3: 1-11
- Kaur KK, Allahbadia GN, Singh M (2019) Have Probiotics and Synbiotics passed the test of time to be implemented in management of obesity and related metabolic disorders a comprehensive review. Adv Obes Weight Manag Control9: 21-28
- Kaur KK, Allahbadia GN, Singh M (2018) Weight loss Associated with high protein Intake in Obesity: Interactions of Gut Microbiota in Protein Sources influencing this positive effect.Acta Scientific Nutr Health 2: 80-89
- Kaur KK, Allahbadia GN, Singh M (2019) Rosmarinic Acid A New Hope for Liver Diseases Like Cirrhosis, Hepatocellular Carcinoma Needs Translation to Humans. EC Endocrinol Metabol Res4: 289-301.
- Kaur KK, Allahbadia GN, Singh M (2020) An update on further progression of NAFLD, NASH with prospective therapies like L- Carnitine (LC), Nicotinamide ribose (NR) combination as well as apical sodium dependent bile acids transporter (ASBT) or Volixibat and Silybin as alternatives. Int J Clin Med Cases 3: 238.
- Kaur KK, Allahbadia GN, Singh M (2019) Advances in Targeting the Gut Microbiota for Obesity Therapy. Acta Scientific Microbiol2: 78-79.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, et al. (2014) Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of term probiotic. Nat Rev Gastroenterol Hepatol 11: 506-514
- Plaza Diaz I, Gomez Llorente C, Abadia-Molina F, Saez LaraMJ, Campana Martin L, et al. (2014) Effects of Lactobacillus paracasei CNCM I 4034, Bifidobacterium breve CNCM I 4035 and Lactobacillusthamnosus CNCM I- 4036 on hepatic steatosis in Zucker rats. PLOS ONE 9: 401
- World Health Organization and Food and Agricultural Organization in the United Nations (2001) Health and Nutritional Properties in the Food including Powder milk with Live Lactic Acid Bacteria FAO Nutrition Paper FAO, Cordoba, Argentina. 85: 1-33.
- Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, et al. (2004) Maintaining remission of ulcerative colitis with the probiotic Escheria Coli Nissle 1917 is as effective as with standard mesalazine. Gut 53: 1617-23
- Bermudez Brilo M, Plaza-Diaz I, Munoz Quezada S, Gomez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Ann Nutr Metab 61: 160-74.
- Fontana I, Bermudez BM, Plaza DI, Munoz Quezada S, Gil A (2013) Sources isolation, chracterisation and evaluation of probiotics. Br J Nutr 2: S35-S50
- Pineiro M, Asp NG, Reid G, Macfarlane S, Morelli L, et al. (2008) FAO Technical meeting on probiotics. J Clin Gastroenterol 42: 156-59.
- Upadhyay N, Moudga LV (2012) Probiotics: A Review. J Clin Outcomes Manag 19: 76-81.
- Plaza DI, Fernandez JA, Chueca N, Garcia F, Gomez LC, et al. (2015) Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immuno-modulatory probiotic strains. Nutr 7: 3999- 4015.
- Kim SW, Suda W, Kim S, Oshima K, Fukuda S, et al. (2013) Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high throughput pyrosequencing. DNA Res 20: 241-53
- Ferraro C, Taverniti V, Milani C, Flore W, Laureati M, et al. (2014) Modulation of fecal Clostridial bacteria and butyrateby probiotic intervention with Lactobacillus paracasei DC, varies among healthy adults. J Nutr 144: 1787-96.
- Yoo JY, Kim SS (2016) Probiotics and Prebiotics. Present status and future perspectives on metabolic disorders. Nutr 8: 173
- Laursen N, Vogensen FK, Gobel RJ, Michaelson KF, Forssten SD, et al. (2013) Effect of Lactobacillus salivarius Ls33 on faecal microbiota inobese adolescents. Clin Nutr 32: 935-40.
- Gobel RJ, Laursen N, Jacobsen M, Molgaard C, Michaelson KF (2012) Probiotics to adolescents with obesity Effect on inflammation and metabolic syndrome. J Paediatr Gastroenterol Nutr 55: 673-78.
- Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, et al.(2010) Regulation of abdominal obesity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 64: 636-43.
- Kadooka Y, Sato M, Ogawa A, Miyoshi M, Uenishi H, et al. (2013) Effect of Lactobacillus gasseri SBT2055 in fermented milk on abdominal obesity in adults in a randomized controlled trial. Br J Nutr 110: 1696-03.
- Sharafedtinov KK, Plotnikova OA, Alexeeva RI, Sentsova TB, Songisepp E, et al. (2013) Hypocalorie diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertendive patients - A randomized double blind, placebo controlled pilot study. Nutr J 12: 138.
- Zarrati M, Salehi E, Nourijelyani K, Mofid V, Zadeh MJ, et al. (2014) Effect of probiotic yogurt on fat distribution and gene expression of pro-inflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight loss diet. J Am Coll Nutr 33: 417-25.
- Zarrati M, Shidfar F, Nourijelyani K, Mofid V, Hossein-sadeh Atar MJ, et al. (2013) Lactobacillus acidophilus La5, Bifidobacterium BB12 and Lactobacillus casei DN001 modulate gene expression of subset specific transcription factors and cytokines in in peripheral blood mononuclear cells of obese and overweight people. Biofac 39: 633-43.
- Zarrati M, Salehi E, Mofid V, Hossein-sadeh Atar MJ, Nourijelyani K, et al. (2013) Relationships between a probiotic consumption and IL10 and IL17 secreted by PBMC in overweight and obese people. Iran J Allergy Asthma Immunol 12: 404-06.
- Agerholm Larsen L, Raben A, Haulrik N, Hansen AS, Manders M, et al. (2000) Effects of 8 weeks intake of probiotic milk products on risks of cardiovascular diseases. Eur J Clin Nutr 54: 288-97.
- Rajkumar N, Mahmoud N, Kumar M, Varikuti SR, Challa HR, et al. (2014) Effects of probiotic (VSL#3) and 3 on lipid profile, insulin sensitivity, inflammatory markers and gut colonization in overweight adults. A randomized controlled trial. Mediat Inflamm p: 348959
- Brahe LK, Le Chatalier E, Prifti E, Pons N, Kennedy S, et al. (2015) Dietary modulation of the gut microbiota - A randomized controlled trial in obese postmenopausal women. Br J Nutr 114: 406-17.
- Ivey KL, Hodgson JM, Kerr DA, Lewis JR, Thompson PL (2014) The effect of probiotic bacteria on glycaemic control in overweight men and women: A randomized controlled trial. Eur J Clin Nutr 68: 447-52.
- Ivey KL, Hodgson JM, Kerr DA, Thompson PL, Stojcesi B, et al. (2015) The effect of yogurt and its probiotics on blood pressure and serum lipid profile A randomized controlled trial. Nutr Metab Cardiovasc Dis 25: 46-51.
- Sanchez M, Darimont C, Drapeau V, Emady-Asar S, Lepage M, et al. (2014) Effect of Lactobacillus rhamnosus CGMCCI.3724 supplementation on weight loss and maintainance in obese men and women. Br J Nutr 111: 1507-19.
- Yu LX, Yan HX, Liu Q, Yang W, Wu HP, et al. (2010) Endotoxin accumulation prevents carcinogen induced apoptosis and promotes liver tumorigenesis in rodents. Hepatol 52: 1322-33.
- Dapito DH, Mencin A, Gwak GY, Pradere GP, Jang MK, et al. (2012) Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21: 504-16.
- Machado MV, Cortez-Pinto H (2016) Diet, microbiota, obesity and NAFLD: A dangerous quartet. Int J Mol Sci 17: 481.
- Miele L, Valenza V, La Totte G, Montalto M, Cammarota G, et al. (2009) Increased intestinal permeability and tight junction alterations in Non-alcoholic fatty liver disease. Hepatol 49: 1877-87.
- Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, et al. (2001) The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia and tumor necrosis factor alpha in the pathogenesis of Non-alcoholic steato hepatitis. Gut 48: 206-11.
- Boursier J, Mueller O, Barrett M, Machado M, Fizanne L, et al. (2016) The severity of Non-alcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of gut microbiota. Hepatol 63: 764-75.
- Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, et al. (2013) Intestinal microbiota in patients with Nonalcoholic fatty liver disease. Hepatol 58: 120-27.
- Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, et al. (2013) Molecular characterization of the fecal microbiota in patients with Non-alcoholic steato hepatitis- a longitudinal study. PLoS ONE 8: p: E62885.
- Raman M, Ahmed I, Gillevet PM, Probet CS, Ratcliffe NM, et al. (2013) Fecal microbiome and volatile organic compounds metabolome in obese humans with Nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 11: 861-63.
- Zhou L, Baker SS, Gill C, Liu W, Alkhouri R (2013) Characterization of gut microbiome in Non-alcoholic steato hepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatol 57: 601-09
- Blomstrand R (1971) Observations of the formation of ethanol in the Intestinal tract in man. Life Sci Biochem Gener Mol Biol 10: 575-82.
- Sarkola T, Eriksson CJ (2001) The effect of 4-methyl pyrazole on endogenous plasma ethanol and methanol in humans. Alcohol Clin Exp Res 25: 513-16.
- Kolodziejczyk AA, Zheng D, Shibollet O, Elinav E (2019) The role of the microbiome in NAFLD and NASH. EMBO Mol Med 11.
- Sobhonslidsuk A, Chan prasertyothin S (2018) The Association of gut microbiota with Nonalcoholic steato hepatitis in Thais. Biomed Res Int p: 9340316
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: Human gut microbes associated with obesity. Nature 444: 1022-23.
- Wang B, Jiang x, Cao M, Ge J, Bao Q, et al. (2016) Altered Fecal microbiota correlates with liver biochemistry in nonobese patients with Nonalcoholic fatty liver disease. Sci Rep 6: p: 32002.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER (2006) An obesity associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027-31.Â
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, et al. (2009) A core gut microbiome in obese and lean twins. Nature 457: 480-84.
- Loomba R, Seguritan V, Li W, Long T, Klitgord N, et al. (2017) Gut microbiome based Metagenomic signature for cirrhosis due to Non-alcoholic fatty liver disease. Cell Metab 25: 1054-62.
- Caussey C, Tripathy A, Humphrey G, Bassirian S, Sigh S, et al. (2019) A Gut microbiome signature for cirrhosis due to Nonalcoholic fatty liver disease. Nat Commun 10: p: 1406.
- Liu X, Lazenby AJ, Clements RJ, Jhala N, Abrams GA (2007) Resolution of Non-alcoholic steato hepatitis after gastric bypass surgery. Obes Surg 17: 486-92.
- Qin N, Yang F, Li A, Prifti E, Chen Y, et al. (2014) Alterations of the human Gut microbiome in liver cirrhosis. Nature 513: 59-64.
- Schierwagen R, Alvarez-Silva C, Madsen MSA, Kolbe CC, Meyer C, et al. (2018.) Circulating microbiome in blood of different circulatory compartments. Gut 68: p: 3.
- Ren Z, Li A, Jiang J, Zhou L, Yu Z, et al. (2019) Gut microbiome analysis as a tool towards targeted noninvasive biomarkers for hepatocellular carcinoma. Gut 68: 1014-23.
- Wang L, Fouts DE, Starkel P, Hartmann P, Chen P, et al. (2016) Intestinal REG3 Lectins protect against Alcoholic steato hepatitis by Reducing Mucosa Associated microbiota and preventing bacterial translocation. Cell Host Microbe 19: 227-39.
- Giorgio V, Miele L, Principessa L, Ferretti F, Villa MP, et al. (2014) Intestinal permeability is increased in children with Nonalcoholic fatty liver disease and correlates with liver disease severity. Dig Off J Ital Soc Gastroenterol Ital Assoc Stud Liver 46: 556-60.
- Boursier J, Diehl AM (2015) Implication of gut microbiota in Nonalcoholic fatty liver disease. PLoS Pathogens 11: p: E1004559
- Cani PD, Osto M, Geurts L, Everard A (2012) Involvement of gut microbiota in the development of low-grade inflammation and type 2 Diabetes associated with obesity. Gut Microbes 3: 279-88.
- Schnabi B, Brenner DA (2014) Interactions between the Intestinal microbiome and liver diseases. Gastroenterol 146: 1513-24.
- Ferreira DF, Fiamoncini J, Prist LH, Ariga SK, DeSouza HP, et al. (2015) Novel role of TLR4 in NAFLD development: Modulation of metabolic enzymes expression. Biochym Biophys Acta 1851: 1353-59.
- Cai C, Zhu X, Li P, Li J, Gong J, et al. (2017) NLRP3 Deletion inhibits the Non-alcoholic steato hepatitis development and inflammation in Kupffer cells induced by palmitic acid. Inflamm 40: 1875-883.
- Leung C, Rivera L, Furness JB, Angus PW (2016) The role of gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol 13: 412-25.
- Ivanov I, Frutos Rde l, Manel N, Yoshinaga DB, Rifkin DB, et al. (2008) Specific microbiota direct the differentiation of IL-17 producing T- helper cells in the mucosa of small Intestine. Cell Host Microbe 4: 337-49.
- Barahe LK, Astrup A, Larsen LH (2013) Is butyrate the link between diet, Intestinal microbiota and obesity related metabolic diseases? Obes Rev Off J Int Assoc Stud Obes 14: 950-59.
- Riviera A, Selak M, Lantin D, Leroy F, De Vuyst L (2016) Bifidobacteria and butyrate-producing colon bacteria: Importance and Stragiers for their stimulation in human Gut. Front Microbiol 7: 979.
- Bajaj JS, Hylemon PB (2018) Gut-Liver axis alterations in Alcoholic liver disease: Are bile acids the answer? Hepatol 67: 2074-75.
- Pols TW, Noriega LG, Nomura M, Auwerx J, Schoojans K (2011) The bile acid membrane receptor TGR5: A valuable metabolic target. Dig Dis 29: 37-44.
- Ferslew BC, Xie G, Johnston CK, Su M, Stewart PW, et al. (2015) Altered bile acid metabolome in patients with nonalcoholic steato hepatitis. Dig Dis Sci 60: 3318-28.
- Chavez Talavera O, Tailleux A, Lefebvre P, Staele B (2017) Bile acid control of metabolism and inflammation in obesity, type 2 Diabetes, dyslipidaemia and Non-alcoholic fatty liver disease. Gastroenterology 152: 1679-94.
- Milosevic L, Vujovic A, Barac A, Djelic M, Korac M, et al. (2019) Gut-Liver Axis, Gut microbiota and its modulation in the management of liver diseases: A review of the Literature. Int J Mol Sci 20: p: 395
- Chen J, Thomsen M (2019) Interaction of Gut microbiota with dysregulation of bile acids in the pathogenesis of Non-alcoholic fatty liver disease. J Cell Biochem 120: 2713-720.
- Agus A, Planchais, J, Sokol H (2018) Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23: 716-24.
- Roager HM, Licht TR (2018) Microbial tryptophan catabolites in health and disease. Nat Commun 9: p: 3294.
- Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, et al. (2014) Microbiome derived tryptophan metabolites and their aryl hydrocarbon receptor dependent agonist and antagonist activities. Mol Pharm 85: 777-88.
- Beaumont M, Neyrinck AM, Olivares M, Rodriguez J, de Rocca Serra, et al. (2018) The gut microbiota metabolite indole alleviates liver inflammation in mice. FASEB J 32.
- Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, et al. (2018) Gut microbiota derived tryptophan metabolites modulate inflammatory responsein hepatocytes and macrophages. Cell Rep 23: 1099-11.
- Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, et al. (2013) Major phenylpropanoid derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res 57: 523-35.
- Hoyles L, Fernandez Real, Federici, Serino MA, bbott J, et al. (2018) Molecular phenomics and metagenomics of hepatic steatosis in non diabeticobese women. Nat Med 24: 1070-80.
- Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, et al. (2016) Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535: 376-81
- Shoaie S, Gha ari P, Kovatcheva Datchary P, Mardinoglu A, Sen P, et al. (2015) Quantifying diet-induced metabolic changes of the human gut micro biome. Cell Metab 22: 320-31.
- Reid AE (2001) Nonalcoholic steato hepatitis. Gastroenterol 121: 710-23.
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, et al. (2010) High fat diet: Bacteria Interaction promotes intestinal inflammation which precedes and correlates with obesity and insulin resistance In mouse. PLoS ONE 5: E12191.
- Zhou X, Han D, Xu R, Li S, Wu H, et al. (2014) A model of metabolic syndrome and related diseases with intestinal endotoxaemia in rats fed a high fat and high sucrose diet. PLoS ONE 9: E115148.
- Brun P, Castaglioulo I, Di Leo V, Buda A, Pinzani M, et al. (2007) permeability in obese mice: new evidence in the pathogenesis of Non-alcoholic steato hepatitis. Am J Physiol Gastroenterol Liver Physiol 292: 518-25
- Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, et al. (2017) Sodium butyrate attenuates high fat diet induced steato hepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol 23: 60-75.
- Wagnerberger S, Spruss A, Kanuri G, Volynets V, Stahl C, et al. (2012) Toll like receptors 1-9 are elevated in livers with fructose induced steato hepatitis. Br J Nutr 107: 1727-38.
- Jin R, Willment A, Patel SS, Sun X, Song M, et al. (2014) Fructose induced endotoxaemia in Paediatric Nonalcoholic fatty liver disease. Int J Hepatol 2014: 560620.
- Schneider KM, BieghtsV, Heymann F, Hu W, Deymueller D, et al. (2015) CX3CR1 is a gatekeeper for Intestinal barrier integrity in mice: Limiting steato hepatitis by maintaining Intestinal homeostasis. Hepatol 62: 1405-16.
- Garcia-Lezana T, Raurell I, Bravo M, Torres-Arauz M, Salcedo MT, et al. (2018) Restoration of a healthy Intestinal microbiota normalizes portal hypertension in a rat model of Non-alcoholic steato hepatitis. Hepatol 67: 1485-98.
- Tao X, Wang N, Qin W (2015) Gut microbiota and hepatocellular carcinoma. Gastrointest Tumors 2: 33-40.
- Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, et al. (2019) FXR modulates the Gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol 7: 1126-40.
- Assimakopoulous SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, et al. (2012) Altered intestinal tight junctions expression in patients with liver cirrhosis: A pathologic mechanism of intestinal hyperpermeability. Eur J Clin Investig 42: 439-46.
- Du Plessis- J, Vanheel H, Janssen CE, Roos L, Slavik T, et al (2013). Activated Intestinal macrophages in patients with liver cirrhosis release NO and IL-6 that may disrupt Intestinal barrier function. J Hepatol 58: 1125-32.
- Scwabe RF, Jobin C (2013) The microbiome and cancer. Nat Rev Cancer 13: 442.
- Ponzani FR, Bhoori S, Castelli C, Putignani L, Rivolgtini L, et al. (2019) Hepatocellular carcinoma is associated with Gut microbiota profile and inflammation in Non-alcoholic fatty liver disease. Hepatol 69: 107-20
- Bluemel S, Williams S, Knight R, Schnabi B (2015) Precision medicine in alcoholic and Non-alcoholic fatty liver disease via modulating the Gut microbiota. Am J Physiol Gastroenterol Liver Physiol 311: 1018-36.
- LiZ, Yang S, Lin H, Huang J, Watkins PA, et al. (2003) Probiotics and antibodies to TNFα anti inflammatory activity and improves Nonalcoholic fatty liver disease. Hepatol 37: 343-50.
- Ma X, Hua J, Li Z (2008) Probiotics improve high fat diet induced steato hepatitis and insulin resistance by increasing hepatic NKT cells. J Hepatol 49: 821-30.
- Nardone G, Compare D, Ligouri E, Di Maum V, Rocco A, et al. (2010) Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am J Physiol Gastroenterol Liver Physiol 299: 669-76.
- Espito E, Iacono a, Bianco G, Autore G, Cuzzocrea S, et al. (2009) Probiotics reduce the inflammatory response induced by a high fat diet in the liver of young rats. J Nutr 139: 905-11.
- Velayudham A, Dolga nieue A, Ellis M, Petrasek J, Kodya K, et al. (2009) VSL#3 Probiotic treatment attenuates fibrosis without changes in steato hepatitis in a diet induced nonalcoholic steato hepatitis modelsin mice. Hepatol 49: 989-97.
- Xu RY, Wan YP, Fang QY, Lu W, Cai W (2012) Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat Nonalcoholic fatty liver disease model. J Clin Biochem Nutr 50: 72-77.
- Xin J, Zeng D, Wang H, Ni X, Yi D (2014) Preventing Non-alcoholic fatty liver disease through Lactobacillus johnsonii B515 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl Microbiol Biotechnol 98: 6817-29.
- Kirpich LA, Marsano LS, McClain CJ (2015) Gut Liver Axis, nutrition and Non-alcoholic fatty liver disease. Clin Biochem 48: 923-30.
- Zhang L, Qin Q, Liu M, Zhang X, He F (2018) Akkermansia muciniphilia can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation and normalizes Intestine microbiota in streptozocin induced diabetic rats. Pathogens Dis p: 76.
- Lin Y, Ren Y, Zhang Y, Zhou J, Zhou F, et al. (2018) Protective effect of Nano selenium enriched Bifidobacterium longum in delaying the onset of streptozocin induced diabetes. R Soc Open Sci 5: p: 181156.
- Mencarelli A, Cipriani S, Renga B, Bruno A, D’Amore C (2012) VSL#3 Resets insulin signalling and protects against NASH and Atherosclerosis in a model of genetic dyslipidaemia and intestinal inflammation. PLOS ONE 7: E45425.
- Ting WJ, Kuo WW, Hsieh DJ, Yeh YL, Day CH, et al. (2015) Heat killed Lactobacillus reuteri GMLN-263 reduces fibrosis effects on the liver and heart in high fat diet hamsters via TGF-β Suppression. Int J Mol Sci 16: 25881-896.
- Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, et al. (2013) Oral administration of Lactobacillus reuteri GMLN-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose fed rats. Nutr Metabol 10: 35.
- Caballero FC, Keller K, De Simone C, Chadee K (2007) The VSL#3 Probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastroenterol Liver Physiol 292: 315-22.
- Endo H, Niloka M, Kobayashi N, Tanaka M, Watanabe T (2013) Butyrate producing probiotics reduces Non-alcoholic fatty liver disease progression in rats: New insights into the Probiotics for Gut liver axis. PLOS ONE 8: E63388
- Chiva M, Soriano G, Rochat I, Peralta C, Rochat F, et al. (2002) Effect of Lactobacillus johnsonii La1 and anti oxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatol 37: 456-62.
- Vajro R, Mandato C, Licenziati MR, Franzese A, Vitale DF, et al. (2011) Effect of Lactobacillus rhmnosus strain GG in paediatric obesity related liver disease. J Gastroenterol Nutr 52: 740-43.
- Abdel Monem SM (2017) Probiotics therapy in patients with Non-alcoholic steato hepatitis in Zagazag University Hospitals. Eur J Hepato Gastroenterol 7: 101-06.
- Kobyliak N, Abenovali L, Mykhal chyshyn G, Kononenko L, Boccuto L, et al. (2018) Multistrain probiotics Reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J Gastroenterol Liver Dis JGLD 27: 41-49.
- Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, et al. (2013) Treatment of Nonalcoholic steato hepatitis with Probiotics. A proof of concept study. Ann Hepatol 12: 256-62.
- Ma YY, Li L, Yu CH, Shen Z, Chen LH (2013) Effect of probiotics on Non-alcoholic fatty liver disease: A Meta-analysis. World J Gastroenterol 19: 6911-18.
- Gao X, Zhu T, Wen Y, Liu G, Wan C (2016) Efficacy of probiotics on Non-alcoholic fatty liver disease: A Meta-analysis of randomized controlled trials. Hepatol Res off J Japan Soc Hepatol 46: 1226-33.
- Loguercio C, Federico A, Tuccillo C, Terra ciano F, D’Auria MVDeSimone C, et al. (2005) Beneficial effect of probiotics VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 39: 540-43.
- Allai A, Bedogni G, Baviera G, Giorgio V, Porro E, et al. (2014) Randomized Clinical Trial: The beneficial effect of VSL#3 in obese children with Non-alcoholic steato hepatitis. Aliment Pharmacol Ther 39: 1276-85
- Malaguarnera M, Vacante M, Antic T, Gioordano M, Chisari G, et al. (2012) Bifidobacterium longum with fructo oligosaccharides in patients with Nonalcoholic steato hepatitis. Dig Dis Sci 57: 545-53.
- Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, et al. (2014) Randomized clinical trials: Lactobacillus GG modulates gut microbiome, metabolome and endotoxaemia in patients with cirrhosis. Aliment Pharmacol Ther 39: 113-25.
- Roman E, Nieto JC, Gely C, Vidal S, Pozuelo M, et al. (2019) Effect of a Multi-strain probiotic on cog native function and risk of falls in patients with cirrhosis: A Randomized Trial. Hepatol Commun 3: 632-45.
- Horvath A, Leber B, Schmerboeck B, Tawdrous M, Zettel G, et al. (2016) Randomized clinical trial: The Effect of a multispecies probiotic vs placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment Pharmacol Ther 44: 26-35.
- Rifatheg Mesic D, Ljucas F, Zildzic M, Avdagic M, Gribic K, et al. (2010) Effect of Probiotics on liver function after surgery resection in malignancy in the liver cirrhotic. Med Arch 64: 208-11.
- Tang Y, Huang G, Zhang WY, Qin S, Yang YX, et al. (2019) Effect of probiotics on Non-alcoholic fatty liver disease: A systematic review and meta-analysis. Therap Adv Gastroenterol 12.
Citation: Kaur KK, Allahbadia G, Singh M (2020) Will Probiotics Provide the Answer for Therapy of Non-alcoholic Fatty Liver Disease (NAFLD)? - A Systematic Review. Biochem Physiol 9: 257. DOI: 10.4172/2168-9652.1000257
Copyright: © 2020 Kaur KK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3877
- [From(publication date): 0-2020 - Oct 17, 2025]
- Breakdown by view type
- HTML page views: 3023
- PDF downloads: 854