Wild Jack Tree: An Underutilized Endemic Fruit Tree of Southern Western Ghats for Evaluation of Nutritional Security in Developing World
Received: 13-Apr-2019 / Accepted Date: 06-Jun-2019 / Published Date: 13-Jun-2019
Abstract
Artocarpus hirsutus (wild jack tree) is an important endemic tree species of the Southern Western Ghats of peninsular India belongs to moraceae family. It is well known for food commodity which has had enormous attention as the ‘natural nutrition of the people of Kerala state from time immemorial. It is popularized for its medicinal uses due to high nutraceutical and pharmaceutical properties for curing diseases or disorders in Ayurvedic since ancient times. Currently it is a cultivated crop in most parts of the Kerala with major focus on the isolation of its important secondary metabolites rather than its use as edible plant. Considering the fact that there is no literature regarding the nutritional analysis of fruits and seeds of the Artocarpus hirsutus. The aim of the present work was to analyses the physical, nutritional, anti-nutritional parameters, antioxidant activity and GCMS analysis of A. hirsutus. The nutritional value like total carbohydrate, protein, lipid, reducing sugar, vitamins (thiamine, riboflavin, niacin, ascorbic acid and carotene) of the fruit parts were determined. The result showed the significance of this wild jack fruit as important source of nutrients. The low levels of anti-nutrients were analyzed. Analysis of DPPH radical scavenging assay showed IC50 values of methanolic extract of fruit pulp, seed and seed coat were 580 ± 0.83 μg/ml, 336 ± 0.74 μg/ml, 158 ± 0.72 μg/ml respectively. In the light of these outputs, wild jack fruits should be considered for promoting the conventional utilization, and also as sources of bio active compounds for the addition to other food supplements, so that all the nutrients present are fully used, instead of being discarded.
Keywords: Artocarpus hirsutus; Underutilized tree; Wild jack fruit; Nutritional and antinutritional; Antioxidant
Introduction
The genus Artocarpus of the family Moraceae almost comprises about sixty species of evergreen and deciduous trees [1]. Economically, the genus is appreciable importance as a source of edible fruit, yield fairly good timber and is widely used in folk medicines [2]. A. hirsutus is an endemic tree species of the Southern Western Ghats of peninsular India and popularly known as the wild jack tree called as Anjali chakka is commonly used as Indian traditional medicine. The tree is reported in the famous Hortus malabaricus. There are 18 species of Artocarpus reported from India and A. hirsutus is the only endemic species of Southern India [3,4] and is considered a keystone species of Western Ghats [5]. It is an important food commodity which has had enormous attention as the natural nutrition of the people of Kerala state from time immemorial. In addition to the uses of wood, fruits and roasted seeds of this tree are used as a highly nutritive food in India, particularly in Kerala. Presently, the scarcity of trees, changing social status, advanced health care, development of technology and several other changes have altered these and other traditional uses such that they are no longer part of routine life of Keralites [3]. Arung et al. [6] reported that extracts of Artocarpus species have good cosmetic application. Melanocytes produced the melanin pigments that prevent the skin damage apart from improving the skin colour [7,8].
This deciduous tree is used in folk medicines and also reported to have antipyretic, antibacterial, antiviral, antifungal, antiarthritic activity [9]. Recent few reports emphasize the importance of this species and thus it needs to be explored more precisely. Pharmacological studies on bark, root, leaf and fruit extracts have demonstrated their importance as antioxidant agents, as well as their efficacies on diuretic, antibacterial, anti-fungal and antiulcer activity [10]. Despite its medicinal values, there are no other studies on anti-acne potential of its crude extracts, its isolated chemical constituents or their synthetic derivatives [11]. The crude extracts of leaf, stem bark and stem wood of A. hirsutus have anti-acne potentiality [12]. The main property and uses of unripe fruits are sour, astringent, sweet and thermogenic anaphrodisiac, constipating and cause flatulence [13]. Major disease preventative mechanisms of plants which have been identified in human dietary studies due to the action of phytochemicals [14]. Carcinogens and harmful chemicals, stimulation of the immune system, altered hormone metabolism, blood pressure reduction, antibacterial and antiviral properties, maintain normal DNA repair, inhibit tumor growth, decreases processes which promote cardiovascular disease. Agents inhibit or neutralize potentially harmful compounds known as free radicals. Free radicals are produced during metabolic activity [15].
Artocarpus hirsutus easily distinguished from the common jack fruit (Artocarpus heterophyllus) by the smaller size of spherical fruits. Nutritional facts of fruits and seeds are yet to be validated but several potential phytochemicals such as steroids, tannins, terpenoids, flavonoids, anthocyanins and saponins were evaluated. Active antioxidant compound such as isoflavones, flavones, anthocyanins, lignans, coumarins, catechins have been identified from fruit pulp [13]. A. hirsutus produce large number of edible fruit but the composition of the fruit is very low. In general, there is a lack of information on the levels of physical, nutritional in the fruits and seed parts, and this could be an important contributor to the antioxidant activity of wild jack fruit. In this context, the utilization of neglected and underutilized trees offers huge promise in satisfying the nutritional requirement of the needed peoples in developing countries, as the wild or local varieties have much climate tolerance and adaption potential than the hybrid varieties [16]. Moreover, wild varieties are rich in essential nutrients and can be considered as promising plants for dietary diversification. Prominently, as intended by the FAO (Food and Agricultural Organization of the United Nations), the food production depend on locally and seasonally cultivated plants can reduce the production and transport cost, thereby reducing the ecological footprint extensively. So, the present research is aimed to benefit from fruit parts which are a by-product by studying its nutritional value by the determination of its nutritional and anti-nutritional values for supplementing the food and nutritional requirements of rapidly growing human population in resource-poor developing countries. In addition GC-MS analysis of methanolic extracts of different parts and its antioxidant activity was also studied the determination of DPPH radical scavenging activity.
Materials and Methods
The fruits of A. hirsutus were collected in the month of April to May 2018, from naturally growing tree in kariavattom campus, University of Kerala, Trivandrum, India. Tree was properly identified with the help of authentic literature and documented with characteristic features.
Analysis of physical properties
Various physical parameters such as fruit size, weight, length of individual fruit as well as fruit core, fruit stalk were analysed using digital Vernier caliper and mean value were calculated. The weight of pulp, peel, seed and core in each fruit were evaluated by weighing each component separately.
Moisture content
The moisture content of the samples (fruit pulp seed and seed coat) was determined by using the method of ISTA, 1976.
Nutritional analysis
Total carbohydrate was estimated using Anthrone’s reagent method by Hedge et al. [17], Protein contents in the samples were done by the method of Bradford [18]. Total lipid was estimated following the method of Bligh and Dyer [19], Reducing sugar were obtained by adopting the method of Bailey et al. [20], Thiamine was estimated by method adopted to Lichtenthaler, [21], while Riboflavin in the samples was obtained determined by Arya, [22], Niacin content in the samples were estimated by following the method of Adeolu and Enesi, [23], Carotene content was estimated by following the method of Yoshida et al. [24], Estimation of ascorbic acid was done by following the method of Bessey [25].
Anti-nutritional analysis
Anti-nutrients such as phenol, saponin and oxalate were estimated by following the method of Soni and Sosa, [26], AOAC (1984) respectively.
Antioxidant activity
DPPH radical scavenging activity of methanol extract of samples was estimated following the method of Al-Saikhan et al. [27]. Different concentration of methanol extract ranging from 0.2 mg/ml, 0.4 mg/ ml, 0.6 mg/ml, 0.8 mg/ml and 1 mg/ml was prepared. 1 ml extract of different concentration was added to 2 ml of DPPH solution and the reaction mixture was incubated at 37ºC for 20 min. The absorbance was read at 517 nm against positive control which does not contain the extract.
GC-MS analysis
The methanolic extract of samples such as fruit, seed and seed coat were subjected to gas chromatography and mass spectroscopic analysis. The fresh samples were extracted by percolation with 95% methanol and evaporated at room temperature. The test was carried out in a Shimadzu Gas chromatograph equipped with mass detector. The column used 30 × 0.25 × 0.25 μM capillary column. The GC oven temperature was 80 and programmed to 280 with ratio of 50.0, the peak identification were carried out using NIST 11 & WILEY 8 library version.
Results and Discussion
The research findings obtained from the analysis of physical parameters, moisture content, nutrients and anti-nutrients were presented in Tables 1-4 respectively.
| Physical properties | Measurements |
|---|---|
| Fruit weight (g) | 189.268 ± 11.667 |
| Fruit length (cm) | 12.967 ± 1.167 |
| pulp weight (g) | 25.055 ± 3.412 |
| Peel weight (g) | 170.857 ± 12.884 |
| Seed weight (g) | 26.333 ± 4.030 |
| Number of seeds per fruit | 43.000 ± 6.683 |
| Core weight (g) | 24.061 ± 2.477 |
| Core length (cm) | 6.176 ± 0.654 |
| Stalk length (cm) | 2.800 ± 0.535 |
| Stalk weight (g) | 1.200 ± 0.390 |
| Bulb weight (g) | 66.252 ± 6.048 |
Table 1: Physical characteristic of A. hirsutus fruit.
| Samples | Moisture content (%) |
|---|---|
| Fruit pulp | 73.630 ± 0.915 |
| seed | 64.666 ± 0.714 |
| Seed coat | 48.133 ± 0.096 |
Table 2: The mean values of moisture content of fruit pulp, seed and seed coat of A. hirsutus.
| Sample | Thiamine (mg/g) | Riboflavin (mg/g) | Niacin (mg/g) | Vitamin C (mg/g) | Carotene (mg/g) |
|---|---|---|---|---|---|
| Fruit pulp | 0.048 ± 0.016 | 0.841 ± 0.082 | 0.984 ± 0.061 | 5.882 ± 0.001 | 0.00888 ± 0.008 |
| Seed | 0.100 ± 0.016 | 3.224 ± 0.008 | 0.738 ± 0.162 | 8.235 ± 0.026 | 0.06392 ± 0.008 |
| Seed coat | 0.0518 ± 0.024 | 4.115 ± 0.012 | 1.722 ± 0.008 | 18.823 ± 0.816 | 0.04692 ± 0.215 |
Table 3: Vitamin analysis of different parts of A. hirsutus.
| Sample | Phenol (mg/g) | Saponin (mg/g) | Oxalate (mg/g) | Flavonoid (mg/g) |
|---|---|---|---|---|
| Fruit pulp | 0.139 ± 0.081 | 1.470 ± 0.326 | 4.600 ± 0.326 | 0.134 ± 0.025 |
| seed | 0.159 ± 0.008 | 4.970 ± 0.024 | 2.300 ± 0.244 | 0.123 ± 0.049 |
| Seed coat | 0.762 ± 0.016 | 4.060 ± 0.048 | 2.300 ± 0.408 | 0.060 ± 0.016 |
Table 4: Anti-nutritional composition of different parts of A. hirsutus.
| Chemical constituents | R. Time | Parts | ||
|---|---|---|---|---|
| Fruit pulp | Seed | Seed coat | ||
| 2-furancarboxaldehyde 5-hydroxymethyl (C6H6O3) | 12.486 | |||
| threo-3, and 4-epoxy-2-octanol (C8H16O2) | 43.242 | |||
| cyclohexane methyl cyclohexane (C7H14) | 44.320 | |||
| 2-furancarboxaldehyde 5-hydroxymethyl (C6H6O3) | 28.492 | |||
| threo-3, and 4-epoxy-2-octanol (C8H16O2) | 31.692 | |||
| nonanoic acid methyl ester (C10H20O2) | 28.512 | |||
| 1-pentanone, 4-hydroxy-4-methyl-1-phenyl- (C12H16O2) | 32.433 | |||
| butanoic acid, 4-(ethoxyhydroxyphosphinyl) | 34.576 | |||
| +)-.alpha.-tocopherol, (C29H50O2) | 40.241 | |||
| furan, 3-(chloromethyl)-,(C5H5ClO) | 46.000 | |||
| oxirane, (bromomethyl) (C3H5BrO) | 47.881 | |||
Table 5: Chemical constituents in different parts of A. hirsutus by GC-MS analysis.
The fruit of A. hirsutus (189.26 g) easily distinguished from the common jack fruit (A. heterophyllus) by the smaller almost spherical fruits. Whereas the fruit of A. heterophyllus and A. odoratissimus are heavier than fruits of A. hirsutus. Compared to A. heterophyllus, the flesh edible portion (perianth) of this fruit (25.055 g) is smaller, thinner and ripening yellow in colour. Physical characteristics of fruits of A. hirsutus such as fruit weight, peel weight, bulb weight, pulp weight and seed weight were fruit collected for the present study shows comparatively higher than fruit collected from mid land and low land areas of Kerala studied earlier [13].
The present study an average of 43 numbers of seeds were found in each fruit. Whereas previous studies reported that fruit found in mid land area contain 14.14 and in low land contain 35.76 seeds. The present study showed the presents of more number of seeds in each fruit, core weight, stalk length and weight were also found to be more compare to the previous report [13].
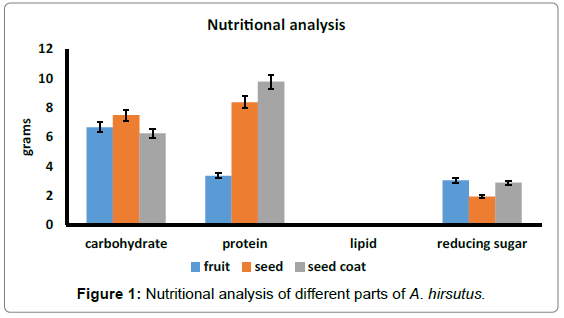
The fruit pulp of A. hirsutus were found to be high amount of moisture content 73.63%, whereas seeds and seed coat contain low amount of moisture content respectively 64.666 and 48.133% (Table 2). Graph 1 shows the nutritional analysis of different parts of fruits. Ripened fruits of A. hirsutus contain higher amount of carbohydrates than seed and seed coat whereas A. heterophyllus reported low amount carbohydrates. The protein content was ranged from 3.353 g (fruit) to 9.78 g (seed coat) in A. hirsutus. In the case of A. heterophyllus 1.2 to 1.9 g and 6.6 to 7.04 g of protein were reported in ripened fruit and seed respectively [28]. So compared to both seed and pulp of A. heterophyllus, A. hirsutus fruit pulp, seed and seed coat contain more amount of protein than jackfruits. Total lipid values were ranged from 0.02 g (seed coat) to 0.08 g (fruit pulp). And reducing sugar was found to be high in fruit pulp (3.046 g) and low in seed (1.926 g) (Figure 1).
The vitamin content of the analyzed parts was as shown in Table 3. Thiamine content of fruit pulp, seed and seed coat of A. hirsutus in the present study were found to be 0.048, 0.1 and 0.0518 mg respectively. These results signify that there are significant differences among the samples in thiamine levels and it agreed with the result obtained in the nutrient composition in ripened fruit and seeds of A. heterophyllus were 0.03 mg and 0.25 mg respectively in [28]. Whereas riboflavin content of pulp and seed were found to be 0.05 to 0.4 mg and 0.11 to 0.3 mg respectively. Niacin content was found to be ranged from 0.738 mg (seed) to 1.722 mg (seed coat) whereas carotene was found to be high in seed (0.063 mg). Vitamin C value was ranged from 7 to 10 and 11 mg in pulp and seed respectively.
Vitamin analysis of all the three samples viz fruit pulp, seed and seed coat shows that seed coat contain comparatively high amount of riboflavin (4.115 mg), niacin (1.722 mg) and vitamin C (18.823 mg) than fruit pulp and seed. Seed contain comparatively high amount of carotene (0.06392 mg) and thiamine (0.100035 mg) than others. Whereas fruit pulp showed comparatively low level of all the analyzed vitamins (Table 3).
Table 4 shows the level of anti-nutrients in various samples (fruit pulp, seed and seed coat), which hamper the utilizable nutrient in them. Anti-nutritional analysis of the samples shows that, seed shows comparatively high amount of saponin content (4.970 mg). Whereas seed coat contain comparatively high amount of phenol than others (0.762 mg). Fruit contain almost twice the amount of oxalate than seed and seed coat. Seed coat showed low amount of flavonoid (0.060 mg) compared to fruit and seed. The levels of the entire anti-nutrient fraction in the fruits of A. hirsutus were lower than the value that can cause malabsorption of other nutrients. The nutritional analysis revealed that the fruit of this valuable plant are safe for edible, all the nutritional analysis will helpful for the utilization and consumption of underutilized plant.
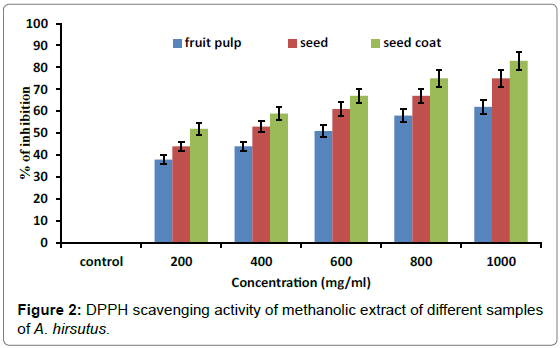
The DPPH method creates a rapid and cost effective method, which has been used frequently for the estimation of the anti-oxidative potential of natural products [29]. DPPH radical-scavenging abilities of the fruit pulp, seed and seed coat extracts are shown in Figure 2. Analysis of antioxidant activity showed that DPPH radical scavenging activity of the extract increases with increased concentration, 27.6% DPPH radical scavenging was present for 250 μl. Although this fruit extract showed lower scavenging activity in comparison to BHA reference standard BHA and ascorbic acid [30]. Fruit extract exhibited antioxidative potential and increased concentration of fruit extract has shown increased anti oxidative potential. These results indicate that fruit pulp has a noticeable effect on scavenging free radicals. This could be attributed to its high content of riboflavin, niacin and antinutritional value.
In the present study the change in absorbance produced by reduced DPPH was used to evaluate the ability of test compounds to act as free radical scavengers. In this test, DPPH was used as substrate to evaluate the antioxidant activity. The IC50 value of ascorbic acid was 209.8 ± 0.07 μg/ml. The IC50 values of methanolic extract of fruit pulp, seed and seed coat were 580 ± 0.83 μg/ml, 336 ± 0.74 μg/ml, 158 ± 0.72 μg/ ml respectively.
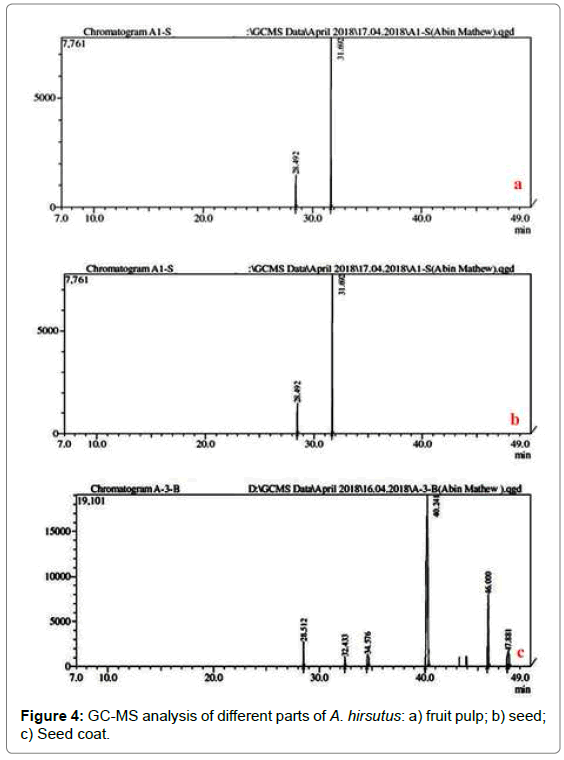
The bioactive compounds present in methanolic extract of fruit pulp, seed and seed coat extract of A. hirsutus are shown in Table 5. Their identification and characterization were based on their elution order in a MS column. The elution time and molecular formula of these bioactive compounds were also shown. Based on abundance and percentage of area present, the major compounds present in the methanolic extract of fruit pulp, seed and seed coat were 2-furancarboxaldehyde 5-hydroxymethyl, threo-3 (79.51%), 5-octen- 1-OL (82.52%) and alpha.(+)-tocopherol (71.89%) respectively. The GC chromatograms of the methanolic extracts of fruit pulp, seed and seed coat revealed in Figure 3. Figure 4a-c show the retention time in the column and the detected peaks which correspond to the bioactive compounds present in the extract of selected plant parts.
Conclusion
The present study concludes that A. hirsutus was highly nutritious and can be easily cultivated in tropical, sub-tropical and temperate regions of the world. From these results it can be concluded that fruit pulp has a good nutritional value because of its content of essential amino acids and lipids besides its high content of antioxidants such as, thiamine, ascorbic acid, riboflavin and niacin. It is therefore suggested that the fruits and fruit parts be subjected to further treatments or processing to reduce the toxic level before processing for consumption. The study revealed major bioactive compounds present in methanolic extracts of fruit pulp, seed and seed coat. Identification of these compounds in the plant serves as the basis in determining the possible health benefits of the plant leading to further biologic and pharmacological industry as antioxidant supplements. Moreover, the cultivation practice of such locally available wild species will create a sense of sustainable utility feeling in farmers and will pave an example for conserving other neglected and lesser-utilized plants in various climatic regions of the world. However, extensive field surveys in various agro-climatological regions are important for analyzing the genetic diversity of the species and also for rising suitable agronomic and breeding platforms for improving the nutritional quality and environmental adaptability.
Acknowledgement
The authors are thankful to Kerala State Council for Science Technology and Environment (KSCSTE), Government of Kerala for financial support and Head, Department of Botany for encouragement and providing the facilities for the present investigation. One of the authors (S. Muthukrishnan) thankful to the DSTSERB New Delhi for providing financial support through National-Post Doctoral Fellowship (N-PDF) (Ref: File No. PDF/2016/002503 dt. 28/03/2017).
References
- Zerega NJ, Ragone D, Motley TJ (2004) Complex origins of breadfruit (Artocarpus altilis, Moraceae): implications for human migrations in Oceania. Am J Bot 91: 760-766.
- Jagtap UB, Bapat VA (2010) Artocarpus: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 129: 142-166.
- Matthew SP, Mohandas A, Shareef S, Nair GM (2006) Biocultural diversity of the endemic ‘wild jack tree’on the Malabar coast of South India. Ethnobotany Res and App 4: 025-040.
- Ahmedullah M, Nayar MP (1986) Endemic plants of the Indian region, Botanical survey of India.
- Jayson EA (2006) Status distribution, food and feeding of Malabar Spiny Dormouse (Platacanthomys lasiurus Blyth) in the Western Ghats of Kerala. KFRI Research Report No 293.
- Arung ET, Shimizu K, Kondo R (2011) Artocarpus plants as a potential source of skin whitening agents. Nat Prod Commun 6: 1397-1402.
- Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, et al. (2002) Melasma: histopathological characteristics in 56 Korean patients. Br J Dermatol 146: 228-237.
- Im S, Kim J, On W, Kang W (2002) Increased expression of alpha-melanocyte-stimulating hormone in the lesional skin of melasma. British J Dermatol 146: 165-167.
- Dibinlal D, Sathish S, Senthil KL (2010) Pharmacognostical studies on the bark of Artocarpus hirsutus Lam. Hygeia JD Med 2: 22-27.
- Vinay Suvarna MN, Venkatachalapathy R, Makari Hanumanthappa K, Ramesh BS (2014) Phytochemical analysis and antimicrobial activity of Artocarpus hirsutus: An in vitro study.
- Nayak M, Nagarajan A, Majeed M, Mundkur LA (2017) Evaluation of in vitro antioxidant potential, anti-inflammatory activity and melanogenesis inhibition of Artocarpus hirsutus Lam. extracts. Inter J Sci and Tech Res 6: 196-203.
- Nayak M, Nagarajan A, Majeed M (2017) Pharmacognostic Evaluation of Leaf and Stem Wood Extracts of Artocarpus hirsutus Lam. Pharmacogn J.
- Thakur S, Vidyasagaran K (2014) Influence of zonal variation on physical characteristics of artocarpus hirsutus fruit and sizewise variation in biochemical composition of the fruit. J Recent Advances in Agriculture 2: 263-270.
- Bubici C, Papa S, Dean K, Franzoso G (2006) Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene 25: 6731-6748.
- Amarowicz R, Pegg R, Rahimi-Moghaddam P, Barl B, Weil JA (2004) Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chemistry 84: 551-562.
- Singh, Abhilash PC (2017) Agricultural biodiversity for sustainable food production. J Cleaner Production 172: 1368-1369.
- Hedge J, Hofreiter B, Whistler R (1962) Carbohydrate chemistry. Academic Press New York.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 7: 248-254.
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem and Physiol 37: 911-917.
- Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnology 23: 257-270.
- Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Portland Press Limited.
- Arya SP, Mahajan M, Jain P (1998) Photometric methods for the determination of vitamin C. Analytical Sciences 14: 889-895.
- Adeolu AT, Enesi DO (2013) Assessment of proximate mineral vitamin and phytochemical compositions of plantain (Musa paradisiaca) bract–an agricultural waste. Inter Res J Plant Sci 4: 192-197.
- Yoshida H, Kotani Y, Ochiai K, Araki K (1998) Production of ubiquinone-10 using bacteria. J Gen Appl Microbiol 44: 19-26.
- Bessey OA (1938) A method for the determination of small quantities of ascorbic acid and dehydroascorbic acid in turbid and colored solutions in the presence of other reducing substances. J Biol Chem 126: 771.
- Soni A, Sosa S (2013) Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J Pharmacognosy and Phytochemistry 2: 22-29.
- Al Saikhan M, Howard LR, Miller JC (1995) Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum L.) J Food Sci 60: 341-343.
- Baliga MS, Shivashankara AR, Haniadka R, Dsouza J (2011) Bhat HP Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam (jackfruit): A review. Food Research Inter 44: 1800-1811.
- Soler Rivas C, EspÃn JC, Wichers HJ (2000) An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochemical Analysis 11: 330-338.
- Kumar V, Lemos M, Sharma M, Shriram V (2013) Antioxidant and DNA damage protecting activities of Eulophia nuda Lindl. Free Radicals and Antioxidants 3: 55-60.
Citation: Gangaprasad A, Mathew AP, Muthukrishnan S (2019) Wild Jack Tree: An Underutilized Endemic Fruit Tree of Southern Western Ghats for Evaluation of Nutritional Security in Developing World. J Nutr Sci Res 4: 137.
Copyright: © 2019 Gangaprasad A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 5606
- [From(publication date): 0-2019 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 4765
- PDF downloads: 841