Review Article Open Access
What is on the Horizon for Hyperthermic Cancer Therapy?
Szigeti GyP1, Lee DY2 and Hegyi G3*
1Institute of Human Physiology and Clinical Experimental Research, Semmelweis University, Hungary
2Department of Thoracic Cardiovascular Surgery, Bundang CHA Medical Center, CHA University, Kyunggido, Republic Korea
3Department of Complementary Medicine, Medical School, Pecs University, Hungary
- *Corresponding Author:
- Hegyi G
Department of Complementary Medicine
Medical School
Pecs University, Hungary
Tel: +36 30 922-5347, +36 72 535-994
E-mail: drhegyi@hu.inter.net
Received Date: April 19, 2017; Accepted Date: April 21, 2017; Published Date: April 26, 2017
Citation: Szigeti GyP, Lee DY, Hegyi G (2017) What is on the Horizon for Hyperthermic Cancer Therapy? J Tradit Med Clin Natur 6:217.
Copyright: © 2017 Szigeti GyP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Traditional Medicine & Clinical Naturopathy
Abstract
Cancer is a complex disease. Malignancy can often obscure the localization of the original tumor, which can mislead the patient and clinicians. Cancer means that the tumor is not benign; rather, it is malignant and thus systemic, even when dissemination or micro-metastases have not been discovered yet. Conventional hyperthermia induces tumor cell necrosis, but with isothermal heating and poor depth selection, it has multiple drawbacks. The new trend is mild hyperthermia, with support from complementary treatments. Furthermore, the trend for loco-regional treatment is developing toward immune modulation, especially toward tumor-specific immune reactions. Our objective is to present a review and discuss the trends regarding hyperthermia in oncology.
Keywords
Hyperthermia; Oncology; Trend; Immune effect; Bystander effect; Abscopal effect; Oncothermia
Introduction and Interpretation of a Relative “Old Method” in New Form
Hyperthermia is an old medical treatment. The long history of hyperthermia, from the status of the only cancer treatment in ancient medicine to the present situation, where hyperthermia is rarely used in oncotherapy, was never simple. The history of hyperthermia has had great peaks and disappointing troughs.
Modern electromagnetic heating techniques were able to renew this methodology 200 years ago. The new technology used electromagnetic effects, which were expected to not only heat but provide special, up to that time unknown affects, at the very beginning of modern hyperthermia [1]. The German Electric Belt Agency advertised directly that practitioners should discontinue the use of drugs and use only electricity; “Electricity is life” was their slogan. But the double expectations led to frustration with local hyperthermia in oncology. In the first half of the 19th century, two competing concepts were formed [2] emphasized the heating process and temperature increase of the absorbed energy, while d’Arsonval focused on the electromagnetic effects of the absorbed energy [3-5] (arsonvalization, fulguration, etc.) The competition was quickly ended when Nagelschmidt [6] promoted the heating mechanism, given it a new name: diathermia. Diathermia is centered on the concept of heating by radiofrequency currents. The use of the temperature approach became robust when Siemens, one of the largest producers of electromagnetic devices at that time, marketed the first diathermia devices devoted to curing cancer [7]. This shift in thinking was easy; the explanations were clear enough. Physiology, in general, is obviously temperature dependent. One of the strongest factors of its stability is thermal homeostasis. The efforts to make the feasibility of the hyperthermia method clear were enormous. To reduce the physiological control of thermal homeostasis, pulse application was introduced [8] and applied on lung tumors. RF-diathermy led to extensive degeneration of the tumor vasculature followed by massive infiltration of lymphocytes. Despite initial success, this type of heating approach did not continue, probably because the pulse-peak created conductive channels in the tissues (like a discharge path).
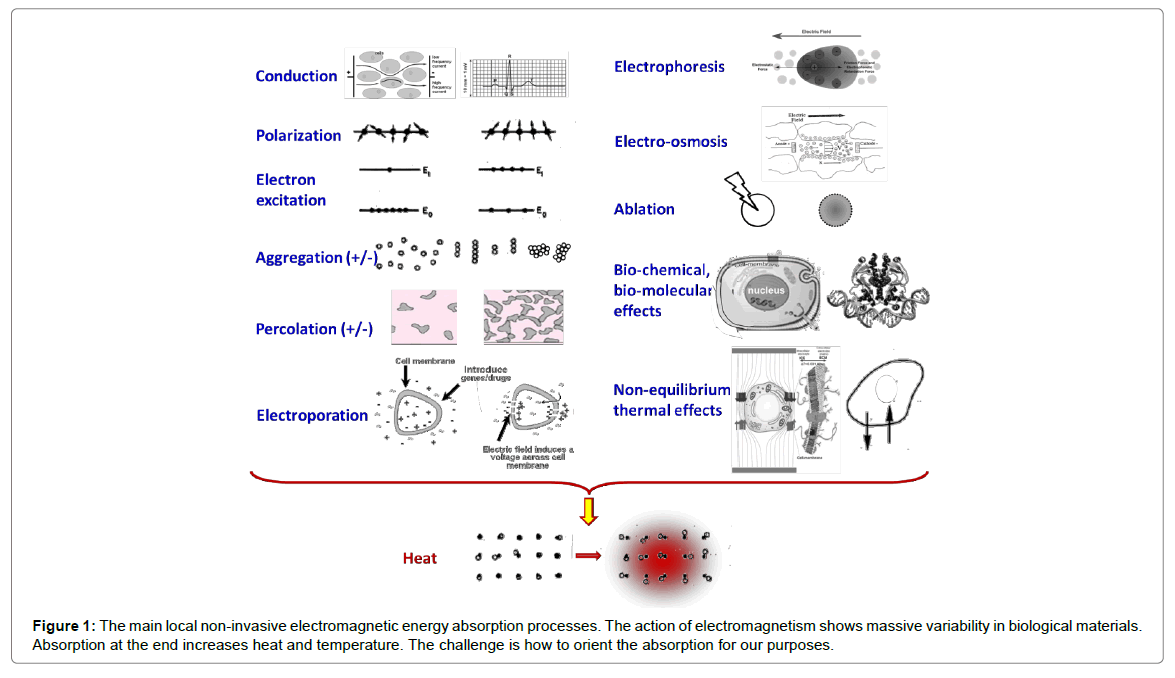
With the inevitable incursion of new techniques, a clear and simple explanation was requested. The main mode of energy delivery is performed by the electric component of the electromagnetic field. This interacts with biomatter, but with huge variations in energy loss in heterogeneous systems (Figure 1).
The study of bioelectromagnetics was almost impossible at the dawn of the 20th century. The missing physical and biological knowledge, which could describe at least part of the underlying interactions and the complex feedback controls of the phenomenon, were completely missing. This hindered the bioelectric concept of thermal solutions, and oriented attention toward the final results: heat and temperature. In this heating concept, the solution of the heating effect is the conversion of the absorbed energy into heat; this concept has become popular among medical professionals. Only relatively weak attempts [9-11] were made to re-establish the electromagnetic treatments in curative therapies, which were followed later by studies on spontaneous biological charge transfers [12,13]. The concept of “biologically closed electric circuits” (BCEC) was developed by Nordenstrom at the Karolinska Institute in Sweden [14,15]. Nordenstrom also introduced clinical Electrochemotherapy (ECT) [16,17], which became a popular method for cancer treatment [18,19]. The first commercial unit for galvanic applications in tumor treatment was released to the medical community in 1992. This became known as electro-cancer therapy (ECT), and was pioneering work for later developments in nanothermia. The results were amazing, and this method was accepted in Japan and China [20- 22], with results reported in several peer-reviewed journals [23-27]. These methods apply an electric field to generate currents without any remarkable increase in temperature (using less than 5 W of power), which has been found to be effective against cancer [28-30] when using galvanic (DC) current applications.
Double-faced behavior in physiology leads to frustration in developing treatment protocols. A vast amount of research and clinical papers, as well as many books, have summarized the research, efficacy and power of hyperthermia in oncology [31-33]. The method is a part of the universal and well-accepted oncology knowledge [34] 1 discussed in detail in large textbooks on radiology/radiotherapy [35] and general oncology [36]. The method has become a part of standard cancer care in a few countries, although limited to only a couple cancer types and to some types of heat delivery. There are numerous advantages of using hyperthermia in oncology [37,38]; however, the method is often questioned and is extensively criticized [39]. The success of this method is an important message, pointing to goals and visions, but the critics highlight the failures and keep us on a different path toward reaching the final goal. Dr. Storm provides one critique: “The mistakes made by the hyperthermia community may serve as lessons, not to be repeated by investigators in other novel fields of cancer treatment” [40]. The uncertainties are always there, regulating our thinking [41] “Once we accept our limits we go beyond them” [42].
Our aim is to provide an overview of the present directions of the development of oncological hyperthermia, and to summarize the challenges, the trends in development and the emerging solutions, while concentrating mainly on developments that have occurred over the last three years (since the last report [43], discussing where we are and where we must go from here with this promising methodology.
Simple, natural heating methods, such as saunas and hot-baths, are in the same spectrum as sophisticated mechanical (ultrasound) or electrical (non-ionizing radiation) techniques and their various combinations. One of the traditional therapies is the whole-body hyperthermia therapy [44]. The main technical solution here is to heat the blood in the periphery of the body, and allow the heated blood to heat the whole body via infrared application. This water-window effect in the infrared region (denoted as IR-A) is intensively used for wholebody treatments by multi-reflection filtering [45], water-filtering [46], and multilayer reflection filtering [47]. The method is used in local hyperthermia treatments [48] as well, which are also performed using an extracorporeal setup [49]. Direct contact heating for whole-body treatment (hot water, hot wax, wrapping the body in a heated blanket, etc.) is rarely applied, but water-heating at extremely high temperatures has had a renaissance recently [50].
In this review, we concentrate on local/regional hyperthermia methods, where the physiological feedback of thermal homeostasis is active. We do not discuss emerging techniques like ablation technologies. The reason is that these methods concentrate the energy in a relatively small spot, with short time of action, and so the physiological response (most of all changes to blood flow) is negligible.
Surprisingly, numerous methods of providing active hyperthermia therapy exist [51]. The classical technique is bulky heating (diathermia), by various technical solutions. These are widely applied in various physiotherapies, but most of them are generally contraindicated in oncology, due to their high metastatic risk. The more updated techniques use oriented electromagnetic waves of radiofrequency currents focused on the tumor.
The modern focused deep-heating local technologies are electromagnetic. Deep penetration is easier with a magnetic field. This penetration depth, however, has a drawback: the interaction of a magnetic field with the living tissues is poor due to the negligible magnetic permeability of living systems [52]. In natural cases, the energy absorption is induced by eddy currents [53-55] which are not focusable. A better solution is to inject magnetic materials into the targeted volume [56]. Various kinds of heating can be developed by these materials, including micro-particles2, ferrite rods3, nano-particle magnetic suspensions4 and other magnetic liquids. Development in the last few years has been oriented to nano-heating technologies [57-59].
A popular heat treatment is radiative antenna array coupling [60-64]. It uses a relatively high radio frequency (60–150 MHz). The antennas are arranged around the body in a belt-like shape, and their electromagnetic radiative properties (including their frequency) are tuned to focus on the body. Controlled clinical studies have validated the method [65-67]. Due to the sharp decrease in the penetration depth with increased frequency, microwave solutions are mostly applied to surface lesions [68].
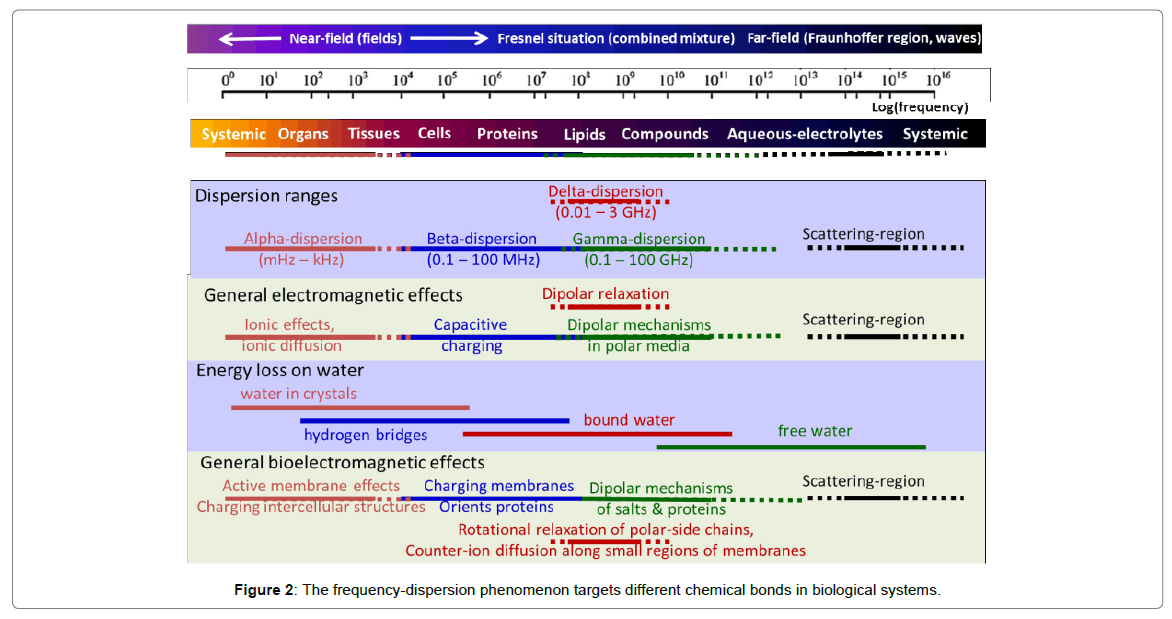
The dominant hyperthermia applications are a capacitive coupling, which has long history too [69-71]. It is mostly applied in the Radiofrequency (RF) range [72-74], via plane-wave radiation or a tightly matched impedance (RF current) solution [75,76]. The applied frequency could have important roles in targeting, because the frequency dispersion phenomenon targets different chemical bonds in biological systems (Figure 2).
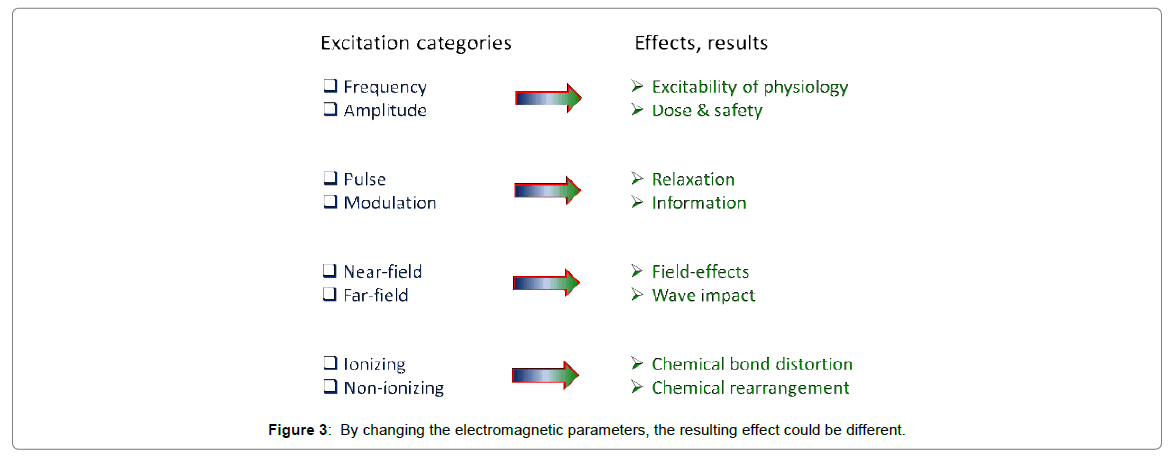
Bio electromagnetism depends on many different factors (Figure 3).
Nowadays, many capacitive coupling techniques apply the standard medical frequency of 13.56 MHz, which is approved for hospital use (Industrial, Scientific, Medical (ISM) band of frequencies) [77]. This frequency (when it is applied well, considering the parameters in Figure 3) could be optimized for lipid-protein interactions, especially for transmembrane proteins.
The advantages of oncological hyperthermia were valuable enough keep the method alive; it is an ideal combination therapy. Hyperthermia is mostly applied as a complementary therapy with “gold standards” such as chemo- or radio-therapy. Hyperthermia provides synergies with most conventional treatment modalities, boosts their efficacy and helps to resensitize to previously non-effective treatments. Moreover, as an advantage of the treatment, increased blood flow is a positive effect, delivering more of the drug, thus increasing chemo-efficacy and delivering more oxygen for radiotherapy success. Additionally, hyperthermia could effectively reduce pain [78]. Consequently, feedback control has two faces and we have to learn how to balance the two sides.
The possible vasodilatation caused by hyperthermia enhances synergy by increasing the overall blood perfusion (oxygenation) [79], creating considerable sensitization to ionizing radiation. The most active regions of tumor growth and microvolumes far from the arteriolar blood supply are usually severely hypoxic; therefore, radiation has reduced efficacy in these regions. However, hyperthermia has selectively high activity in these regions due to the relatively low cooling effect of blood flow. The heated areas furthermore develop higher blood flow as feedback due to thermal homeostasis, making these areas sensitive to ionizing radiation.
This approach was one of the first to use the modern hyperthermic effect. Its characterization was introduced by the Thermal Enhancement Ratio (TER) [80], which measures the efficacy of the treatment. Hyperthermia, however, speeds up cellular metabolism, while possibly accentuating hypoxia, which works in an opposite manner; it reduces the efficacy of radiotherapy. Here, both approaches could be used: a reduced radiotherapy dose, promoted by hyperthermia using the complex TER factor [81-83]. Different review articles have summarized the sensitizing effect on classical ionizing radiation by hyperthermia [80,84-86]. Considerable success has been presented for hyperthermia in combination with radiotherapy for deep-seated tumors [87,88- 141], and success has also been observed in shallow tumors [89-140]. The multinational group of radiotherapies for oncology (RTOG) also evaluated the method as being feasible [90].
The efficacy of chemotherapy is determined by blood perfusion into the tumor. Around the arterioles, a gradient forms regarding the concentration of the drug and also of reactive oxygen species. A higher temperature promotes blood perfusion and enhances the penetration of the drug into the tissue. This effect could also be achieved by a temperature sensing drug [91]. The thermo-chemotherapy results in a more efficient therapeutic effect, increases the target specificity and reduces the systemic side effects [92]. Synergy has been observed with a combination of hyperthermia and chemotherapy for deep seated tumors [93-95].
In some cases, low dose chemotherapy can be used [96,97] and enhanced by hyperthermia, on the same way like applied in low-dose metronomic chemo-regulation [98]. A robust synergy occurs with the combination of chemotherapy and heat, as thermally increased metabolism (enhanced chemo-metabolism) increases the absorption of cytotoxins [99,100]. Cellular chemo-penetration is strongly promoted by non-equilibrium heat-flows (electro-osmosis) [101] This optimized chemo-intake helps to overcome the failings of chemotherapy due to patient intolerance (the patient cannot take large doses of drugs, for example, due to renal or liver insufficiency, insufficient blood composition, etc.). In these cases, the same results may be achieved by combining a decreased chemo-dose and heat therapy [102].
Combined therapies are (radio-chemo-thermo-complementary applications) also frequently applied [103]. Hyperthermia inhibits angiogenesis, the contrast of the tumor becomes clearer, and turning previously inoperable cases to successfully operated ones [104,105]. The postoperative application of hyperthermia is also applied [106], and the minimally invasive radiofrequency ablation [107] and local hyperthermia [108] have been used to improve surgical results. Postoperative application to the brain has also been published [10], using an interstitial method (minimally invasive treatment using needles as electrodes in the tumor itself). Interstitial hyperthermia has shown surprisingly good efficacy in a randomized, controlled double-armed (with and without hyperthermia) clinical study [110]. The median survival increased from 76 to 85 weeks, and the two-year survival increased from 15% to 31%. As a consequence, the FDA certified brain hyperthermia in its interstitial form. The combination of interstitial hyperthermia with external radiation has also been tried for gliomas [111]. In one study, radiofrequency hyperthermia was also applied extra-cranially [112].
The combination of hyperthermia with gene therapies also looks promising [113-115].
Promising application of hyperthermia induces enhancement of the local rate of drug-release from thermosensitive liposomes [116].
The combination of hyperthermia with hormone therapies; enzyme therapy, photodynamic therapy, gene therapy, immune therapy and other supportive therapies are also frequent clinical applications [117- 123]. The methods are compared according temperature used, assuming that the same temperature provides the same results irrespective of which technology is applied to make it. This reference approach has led to some controversies and cannot explain the controversial definite differences in uterine cervix results [124-126].
As a consequence of increased temperature, multiple factors are modified. Changes to the cellular membrane, by softening or melting the lipid bilayer [127,128], can change lipid-protein interactions [129]. Heat treatment causes structural alterations in transmembrane proteins, inducing changes in active membrane transport and membrane capacity [130], thus leading to a substantial changes in potassium, calcium, and sodium ion gradients, changes in membrane potential and alterations to cellular functions [131-135]. Hyperthermia increases biochemical reaction rates and, therefore, the metabolic rate as well. Accelerated metabolism quickly impoverishes mitochondrial ATP sources and drives glycolysis, thereby producing lactate [136] and causing hypoxia. ATP depletion creates a severe ionic imbalance in cells5 [137] as well. These changes could easily destroy cancer cells, if the triggering action is selective enough.
Despite successful applications of hyperthermia, some questions remain. The central problem is the correlation between local control (remission rate, shrinking the tumor) and survival time. Most clinical studies concentrate on local control, and few compare this with survival time. Some early clinical studies indicated problems with survival time, but in fact, this was ignored. It was shown that radiotherapy and complementary hyperthermia have positive outcomes regarding local control compared to solely applied radiotherapy [138]; however, the measured survival was lower in the Complete Remission (CR) group of patients in the hyperthermia + radiotherapy arm. On the other hand, for patients having not CR, the survival rate was higher in the combined arm than in the arm where radiotherapy was solely applied. A problem with toxicity was shown in the same year [139], when combined therapy was found to be more toxic in both the acute and late stages; this was ignored again. However, this problem with the contradiction between local control and overall survival was recently measured on surface tumors in a rigorously performed clinical study [140]. This characteristic story shows that the survival time problem was ignited by a milestone publication about uterine cervix carcinoma [141]. The four-year survival time in the hyperthermia + radiotherapy treatment arm compared to radiotherapy alone was doubled, which was a breakthrough. A subsequent study [142] showed similar results, but the measured distant metastases were more than three times higher (as a percentage) in the combined arm, which had been observed earlier [143]. A repetition of the study, which was sponsored by a non-medical institution, i.e., the International Atomic Energy Agency, was not successful [144], showing the opposite trend, with better four-year survival in the radiotherapy alone arm. However, the remission rate was better when hyperthermia was applied as well, but again significantly more distant metastases were observed in the hyperthermia arm. The explanation of the challenge was the way the missing reference point [145]. In the framework of sponsorship by the International Atomic Energy Agency, a prospective, randomized, controlled, multicenter phase III trial was performed [146] for Non-Small Cell Lung Cancer (NSCLC). The result was again problematic: the difference in overall survival in the two arms was statistically insignificant.
To develop this method, we first have to consider its shortcoming, which will have to be addressed in the future.
The thermal effects of electromagnetic energy absorption are much easier to study, explain, and understand than the complex bioelectromagnetic interactions dominantly characterized by an Electric Field (EF). The heating concept has become common in oncological hyperthermia, while the electromagnetic effects are considered only regarding changes in temperature. The energy is absorbed from the field has a measurable Specific Absorption Rate (SAR) in units of W/kg. The absorbed energy is transformed into heat and increases the temperature of the target. However, this concept has multiple challenges.
Due to physiological thermoregulation maintaining the temperature difference, increasingly higher amounts of energy are necessary. This has initiated a race for more and more energy delivery, which is presently over 1 kW of power in some popular devices. The huge medical effort and market-oriented fight for higher temperatures in the target has focused on increasing power, instead of using temperature as a tool to destroy the tumor; this has become a goal of technical solutions and the aim of medical applications. Substituting the goal with a tool risks losing the reasons for the therapy.
The consequences of high-power constraints are accompanied by serious challenges:
1. A high power over 1 kW of course cannot be used on any living object without inflicting a serious burn injury. The limitation of skin burning was studied in detail [147,148], and showed an increasing risk of burning with a longer heating time. This is avoided by intensive cooling, which protects the skin from burns. However, the price is huge, as surface cooling complicates the energy-dose definition, because cooling energy is incalculable. Thus, it is unknown as to how much energy is absorbed by the target, and which part is cooled down. In this case, only one method remains to determine the amount of energy absorbed: measuring the temperature, which is expected to be proportional to the absorbed energy.
2. Together with the obvious loss of the energy-absorption dose, a positive physiological feedback loop develops with surface cooling. When the skin is cooled, it suppresses cutaneous blood flow, and the capillary bed impoverished from blood, forming more heat-isolating layer, maintaining thermal homeostasis in the local area. The heat-isolating layer (mainly adipose tissue) is an electric conductor as well. Pumping the same power through the isolating layer requires a higher voltage. A consequence of a higher voltage potential is electric burns, so further cooling is necessary to prevent the burn. But this additional cooling creates an additional isolator, which requires a higher voltage and so on. This positive feedback can be accelerated with cooling to 10°C [149,150].
3. One of the obvious challenges is focusing. It is not easy, but possible, to focus the energy on certain volumes at a specific depth, characterized by the SAR. However, the absorbed heat in the case of any precise focusing is not localized, as it naturally spreads over time throughout the areas that are definitely out of focus. The focusing of temperature is illusory in longer time frames than the physiological feedback reaction time, when active cooling feedback adds a heat distribution force to natural heat conduction. By increasing the power, the whole body temperature increases, but the real selective heating of the tumor becomes more difficult. The temperature gain with a higher power is soon equalized by thermal homeostasis in real clinical use [151]; clinically, the same provided energy produces very different temperatures in a patient cohort [152].
4. There are remarkable differences between the macro and micro (meso) environments of the tumor and its vicinity. When hyperthermia is applied, the gradients of the parameters in the meso-environment increase, due to local differences in blood flow (according to body temperature) and the heated tissues in the target. The macro-environment behaves in the opposite manner; a higher temperature directly spreads throughout the environment, decreasing the differences between the parts. To keep the macro selection functional, we have to avoid massive heating, when leading to widespread temperature changes and a loss of selectivity.
5. In the conventional hyperthermia concept, the isothermal idea requires the best homogeneous temperature in the tumor [153], and also assumes that heating is ideally selective, so the healthy neighborhood is not directly heated. This ideal situation is not realistic, even in a homogeneous model system [154] and is far away from the reality in robustly heterogeneous tumors and neighboring tissues. Not only are the structures of tissues rather inhomogeneous, but the micro- and macro blood flow which regulate thermal homeostasis make cooling occur heterogeneously, so the SAR distribution and temperature mapping are not correlated due to variations in blood flow in the micro- and meso-scopic environments [155]. Homogeneous heating of the tumor is not realistic, except when the temperature is high (over 43°C), or when thermal homeostasis is compromised.
6. The leading concept of tumor control by hyperthermia is based on necrosis. It harmonizes well with the presently applied dose concept of Cumulative Equivalent Minutes (CEM) [156]. This conventionally functional dose is based on the thermal characterization measured by the Arrhenius function [157- 159]. These changes have been experimentally studied by in vitro measurements of necrosis, blistering and burns to skin, or by assessing burn injuries to the skin of humans [160-164]. A dose of hyperthermia certainly induces necrosis, with 43°C as the reference (CEM 43°C). This dose concept requires isothermal heating of the target, which is formally solved by introducing the percentage of the target which is isothermal during the treatment [165]; this is denoted by the complete CEM, i.e., CEM 43°C T70, where T70 denotes the isothermal portion of the target as a percentage (70%). CEM efficacy is measured in correlation with tumor size (local control) [166,167], which do not take into account longer outcomes such as survival time. It the case of hyperthermia, this could be a crucial mistake, because local control and overall survival time are independent [168], and sometimes even contradictory [169]. A further problem with CEM-based protocols is that the dose only correlates slightly with the clinical outcome [170- 172]. Hyperthermia protocols using forced immediate necrotic cell death cannot use CEM, as it fails at higher temperatures [173] However, solving the dosing problem is mandatory for hyperthermia in oncology [174].
7. We have to consider the effect of physiological conditions, mainly blood flow, which has a double-edged sword effect. Blood flow is responsible for thermal adjustment (feedback mechanisms), and drug (chemotherapy) and oxygen delivery (radiotherapy), which are essential for the complementary applications of hyperthermia. Higher temperature leads to higher blood flow, thus maintaining the thermal equilibrium. The extra blood flow could support cell killing by delivering more drugs to the tumor tissue in complementary therapy, or tumor growth could be supported by blood-delivered glucose and other nutrients. In addition, high blood flow could aid in the dissemination of malignant cells, forming micro, and later macro, metastases.
8. The central fact is that changes in blood flow are temperature dependent. Blood flow undergoes a turning point with increasing temperature, i.e., blood flow begins to decrease at high temperatures. When blood flow is strong, the temperature increase needs a high SAR; when it is low, the temperature could increase rapidly. It has been shown that an increase in temperature can cause vasoconstriction in certain tumors, leading to decreased blood perfusion and heat conduction [175-177], while causing vasodilatation in healthy tissues, leading to increased relative blood perfusion and heat conduction in this region [178,179]. However, the blood perfusion of the tumor relative to the surrounding healthy tissue is always lower, providing an effective heat trap [180]. Detailed reviews have been published showing state-of-the-art results on tumor blood flow and the effect of applied temperature.
9. A strong indication from clinical practice is that patient tolerance (safety) governs therapy. A high number of treated patients do not obtain the prescribe dose due to tolerance. The protocol of the actual treatment is based on patient tolerance, such that the increase in power stops when the patient experiences remarkable discomfort. Some authors have excluded low-tolerance patients (not-heatable) from the study [181-186].
Bioelectromagnetics could induce a vast number of variants of these effects on biological systems. Heat delivery, which causes the temperature to rise, is only the final consequence of a variety of EF interactions with cells and tissues. Increased temperature under physiological conditions depends on many factors that are triggered by the primary heating of the EF: Increased metabolic rate, changes in blood flow and other transport systems like lymph, heat conduction and impedance changes to the surrounding volumes, etc. Careful study and the use of the complex assessments may change the paradigm of hyperthermia in oncology and provide solutions to the challenges listed above.
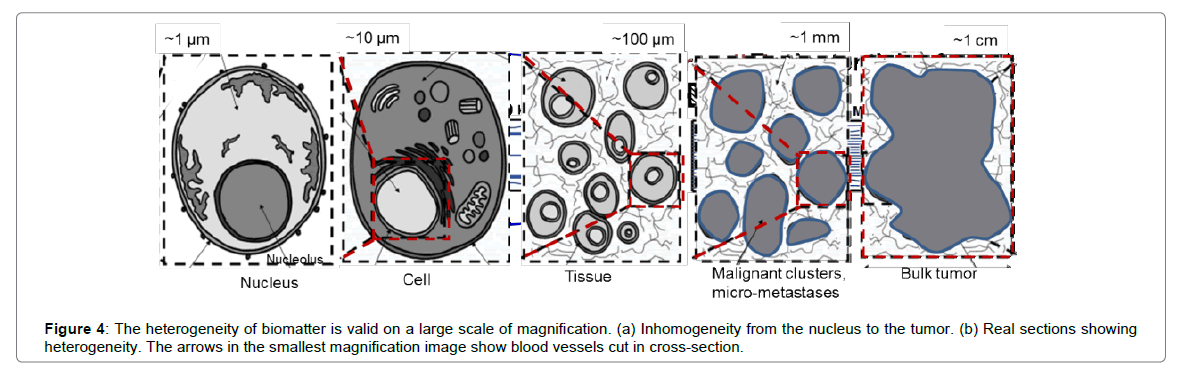
Most challenges regarding local hyperthermia are connected to the necessary delivery of proper and controlled energy to the active area. The desired temperature develops via energy absorption, which is not delivered precisely enough to provide the expected outcome. Biological material is very heterogeneous (Figure 4), so to heat it up homogeneously requires a large amount of absorbed energy, more than the largest considered interaction energy, which in hyperthermia is protein denaturation at 43°C. The new paradigm must avoid the complication of homogeneous (isothermal) heating. Heating must be selective microscopically, i.e. overheating malignant cells, but not directly heating the remaining mass of the tumor. In this approach, the main mass of the tumor (together with the healthy tissue) is not heated directly, and only heat conduction from selected malignant cells will cause mild hyperthermia in the tumor mass.
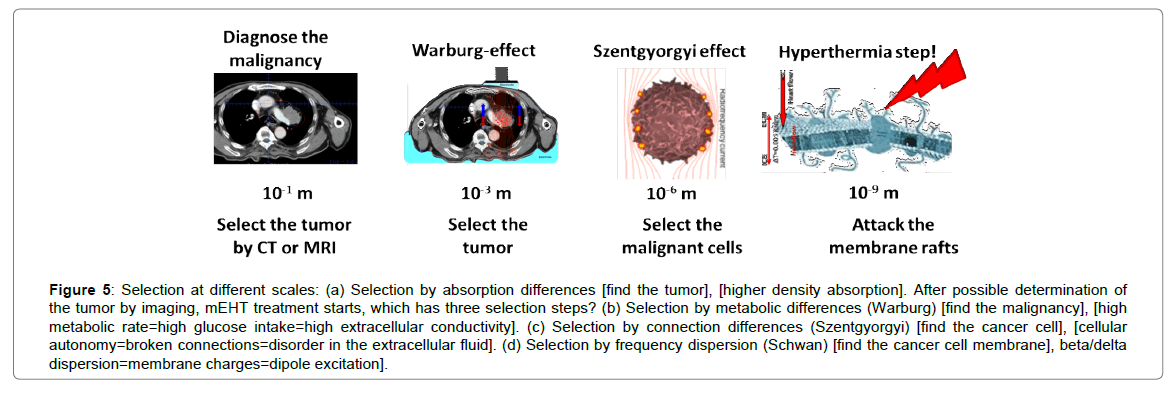
Modulated-electro-hyperthermia (mEHT; trade name, oncothermia) uses the precise delivery of energy to the membrane of malignant cells [187,188]. It is a radical change compared to conventional heating methods, and has drastically different consequences than those of isothermal hyperthermia. mEHT uses certain bio-electromagnetic differences between malignant and healthy cells to select them properly. The first selection step is made considering the conductivity of the tumor mass, which is much higher than the than that of healthy tissue. The conduction difference is caused differences in the concentration of ionic metabolites, a result of intensive glycolytic activity (Warburg effect [189]). The second selection step is more microscopic: identifying the dielectric constant (cellular connections) in the immediate vicinity of the malignant and healthy cells (Szentgyorgyi effect [190]). Malignant cells have drastically different intercellular connections (they are usually autonomic) which changes the Extracellular Matrix (ECM) around them, making selection possible due to the electrical permittivity of the ECM. The final and real EF absorption, i.e., the real hyperthermia step occurs via selective heating of transmembrane protein domains (rafts), which have very different electromagnetic properties than any other materials in their vicinity. The frequency dispersion specialty of cellular membranes (Schwan effect [191]). allows us to optimize the selection. The RF currents in the range of delta dispersion weakly penetrate into the cytoplasm, and their energy is robustly absorbed into the membrane and its immediate vicinity in the extracellular fluid. This could create hot spots in the nano-range at membrane rafts, which can be heated quickly. These spots heat the whole cell, which heats the tumor to the median temperature. Finally, the tumor structure and its consequent reaction kinetics (dynamism) differ robustly from those of healthy counterparts, which can be measured by noise analysis [192]. Recognizing these structural/dynamic differences (pathological pattern recognition) in malignant and healthy tissues could be discussed in the framework of fractal physiology [193]. This selection factor is promoted in mEHT by the time-fractal modulation of the carrier frequency [194,195]. The technical realization of the method is discussed elsewhere [196-198]. The approach for the selection to the nano-scopic hyperthermia step in the mEHT process is shown in Figure 5.
Figure 5: Selection at different scales: (a) Selection by absorption differences [find the tumor], [higher density absorption]. After possible determination of the tumor by imaging, mEHT treatment starts, which has three selection steps? (b) Selection by metabolic differences (Warburg) [find the malignancy], [high metabolic rate=high glucose intake=high extracellular conductivity]. (c) Selection by connection differences (Szentgyorgyi) [find the cancer cell], [cellular autonomy=broken connections=disorder in the extracellular fluid]. (d) Selection by frequency dispersion (Schwan) [find the cancer cell membrane], beta/delta dispersion=membrane charges=dipole excitation].
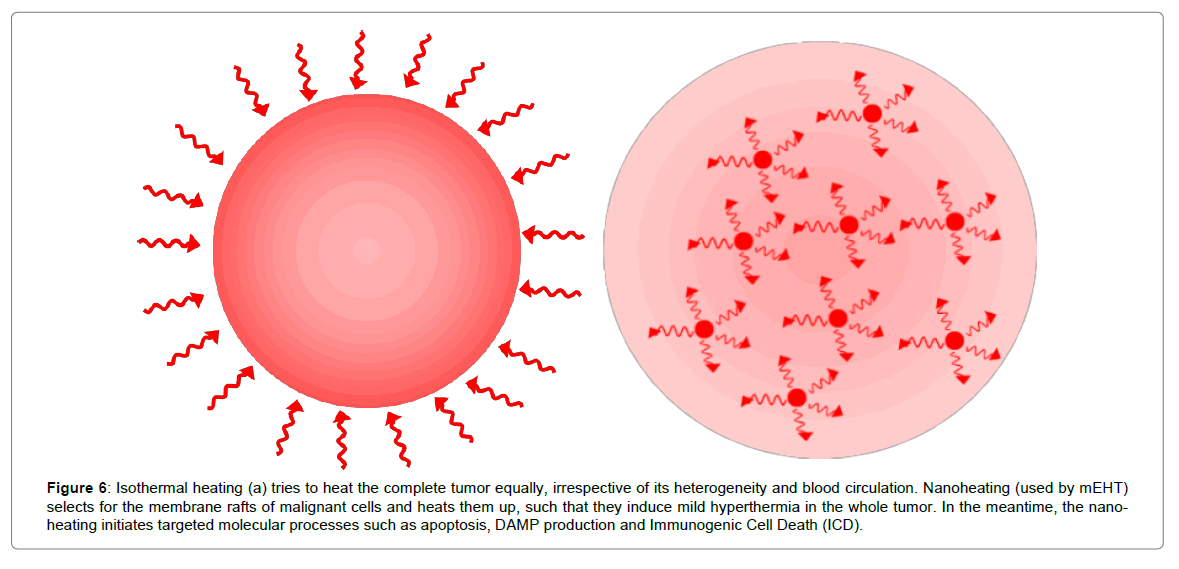
Nano-selection and nano-heating provides a different concept of heating than the isothermal approach (Figure 6).
Figure 6: Isothermal heating (a) tries to heat the complete tumor equally, irrespective of its heterogeneity and blood circulation. Nanoheating (used by mEHT) selects for the membrane rafts of malignant cells and heats them up, such that they induce mild hyperthermia in the whole tumor. In the meantime, the nano-heating initiates targeted molecular processes such as apoptosis, DAMP production and Immunogenic Cell Death (ICD).
Studies on mEHT application complementary to chemotherapy have shown excellent results in advanced brain gliomas, advanced NSCLC, and Small-Cell Lung Carcinoma (SCLC). Advanced sarcomas (n=13) (n=24), advanced liver tumors, and advanced inoperable pancreas tumors have also been treated without mentionable side effects. mEHT can be applied as monotherapy, when all other possibilities fail [199-213].
The success of mEHT is connected to its mechanism. It is a new type of heating: nanothermia. Using the above considerations, it concentrates on the nano-heating mechanism and the nano-manipulation of biomolecular changes [214,215]. It is supported by various experiments showing how energy absorption in the nano-range works. One study targeted the question: is nanothermia really hyperthermia? Various in vitro and in vivo experiments showed the thermal origin of the nano-effects. The U937 human lymphoma cell line was compared in Water Bath Hyperthermia (WHT) and nanothermia. It was rigorously shown that the membrane rafts were heated at least 3°C higher than the medium, which was the reference (WHT). It should be noted that this result is in agreement with much earlier in vivo experiments [216]. The measured Arrhenius plot showed a definite lowering of the activation energy in the case of nanothermia compared to WHT [217]. It was theoretically shown that membrane rafts were the basic target of nano-heating and Transient Receptor Potential Vanilloid (TRPV) receptors played a definite role in temperature sensing [218]. Next, Ca2+ influx into the cell (compared to a reference) was measured, which can be connected to TRPV sensing. The results showed the start of Ca2+ influx at least 3°C earlier in nanothermia6; the direct staining temperature measurement showed the same result [219,220].
Nanothermia rebuilds E-cadherin/β-catenin complexes as the first step in the bystander effect, which has been independently shown [221,222]. In comparison to with WHT and conventional (plane-wave capacitive coupling) hyperthermia (CHT), cleaved caspase-3, caspase-8 and caspase-9 levels were significantly increased, together with Reactive Oxygen Species (ROS) and the release of extracellular HSP70. Increased calreticulin expression was also measured compared to WHT and CHT.
Immune-oncology is an emerging science [223,224]. It was named as the breakthrough of 2013 [225], and immune therapy combined with hyperthermia therapy is emerging. In this method, tumor-specific immune absorption (like vaccination) drives the therapy. The general immune reactions support the improvement of the quality of life. The trend is moving toward immune effects and technologies that take systemic action against the malignancy. This may be readily targeted by nanothermia: an in situ, tumor-specific adaptive immune attack against cancer.
The apoptotic process in nanothermia provides a new type of local hyperthermia treatment. Recognizing the problem that malignancy is not a local disease and should not only be treated locally, nanothermia concentrates on systemic effects. Nanothermia kills cells by apoptosis [226]. As apoptotic bodies release damage-associated molecular pattern molecules, calreticulin and HMGB1 are released, and the membrane expresses HSP70, HSP90 and the DR5 death receptor. This pattern leads to immunogenic cell death [227], which could lead to the bystander and abscopal effect [228]. With this approach, local nanothermia could be a new kind of tumor vaccination [229]. This concept has been demonstrated by extended in vivo model experiments, where the abscopal effect of mEHT was shown on treated tumors in the femoral region as well as non-treated chest tumors in immune-competent mice. Recently, a new publication had shown the same effect, even with unsuccessful re-challenging of the animals by new tumor inoculations after the first NED (no evidence of disease) results. Promising results have also been shown with the non-tumor-specific immune-stimulant Marsdenia tenacissima, which promotes dendritic cell (DC) maturation in situ into antigen presenting cells (APC) for CD4+ and CD8+ T-cells, which act tumor-specifically and destroy cancer cells. This type of mechanism with an immunostimulatory “leukine” was shown in one human case of non-small-cell lung cancer.
Future Perspectives
We are convinced that electromagnetic technologies (a physical approach to change molecular structures and interactions in bio-systems) will rapidly emerge. Electromagnetic interactions (energy absorption) mean heat production at the nano-, micro-, meso- or macro-level. Like the chemical approach to targeting and modifying bio-interactions in the body, electromagnetism (and in general “hyperthermia”) will obtain a strong position in the weaponry of the war against cancer.
The main points which will be changed soon in hyperthermia at any scale:
1. The problem of overemphasizing the importance of temperature is a misleading aim: the goal has to be the changes needed to destroy tumor cells. The temperature is only one of the possible tools. Exchange the tool with the goal is false.
2. Technical challenges will be overcome, and better deep-heating and targeting technologies will be developed.
3. The dosing will be fixed, leaving the isothermal heating concept behind and taking into account the heterogeneity of biomatter.
4. Personalization will garner more attention. The treatment has to be close to homeostatic control, not have a contra-active effect on thermal homeostasis.
5. Cell-killing will be more natural. Heating will not force necrosis; rather it will occur in alliance with natural processes, mainly apoptotic cell destruction.
6. While isothermal heating breaks intercellular connections, the new approach will try to re-establish them, avoiding the invasion and dissemination of tumor cells.
7. Low acute and late toxicity is a characteristic of emerging hyperthermia methods, together with the suppression of side effects from complementary therapies.
8. Immune-related actions of bioelectromagnetic absorption will be more intensively studied and the locally applied treatment will have whole body action (abscopal effect), killing far-distant micro and macro metastases, similar to classical vaccination.
Conclusion
Conventional hyperthermia in oncology currently aims for isothermal heating of the tumor, irrespective of its heterogeneity and the physiological feedback of thermal homeostasis. mEHT represents a new paradigm, introducing selective heterogeneous heating, and using thermal homeostasis as a supporting factor and extending local effects to systemic by very local nano-manipulation in the primary tumor. Despite this, mEHT is a local treatment. It could be effective against systemic sequelae, and cooperate with natural systemic immune mechanisms. This is facilitated of by nanothermal heating, which fits with homeostatic control, and leads to immunogenic cell death. Nanothermia is an immune-supportive, immune modulation method. It fits the trend with earlier developed invasive techniques, which include such important treatments as ablation, electroporation and electro-chemo-therapy which exert immunomodulatory actions.
Author Disclosure Statement
Authors declare no conflict of interest.
References
- Lurito PW (1984) The message was electric. IEEE Spectrum 21: 84-96.
- Busch W (1886) On the influence of violent erysipelas occasionally on organized neoformations. Negotiations of the Natural History Association of the Prussian Rhineland and Westphalens 23: 28-30.
- d’Arsonval A (1893) Influences de la frequence sur les effects physiologique des courants alternatifs. Comptes Rendus de l’Academie des Sciences 116: 630-633.
- http://trade.ec.europa.eu/doclib/press/index.cfm?id=1519
- Kratzer GL, Onsanit T (2007) Fulguration of selected cancers of the rectum: report of 27 cases. Dis Colon Rectum 15: 431-435.
- Nagelschmidt F (1911) The thermal effects produced by high-frequency currents, and the therapeutical uses of diathermic treatment. Proc Royal Soc Med 4: 1-12.
- Seegenschmiedt MH, Vernon CC (1995) A historical perspective on hyperthermia in oncology. Thermoradiotherapy and Thermochemotherapy 1: 3-46
- Sugaar S, LeVeen HH (1979) A histopathologic study on the effects of radiofrequency thermotherapy on malignant tumors of the lung. Cancer 43: 767-783.
- Tesla N (1898) High frequency oscillators for electro-therapeutic and other purposes. Electrical Eng 26: 477.
- Rhees DJ (1999) Electricity-“The Greatest of All Doctors”: An Introduction to “High Frequency Oscillators for Electro-Therapeutic and Other Purposes”. Proc IEEE 87: 1277-1281.
- http://www.netowne.com/technology/medical/
- Becker RO, Selden G (1985) The Body Electric. Morrow, NY, USA.
- Becker RO (1990) Cross Currents. In: Jeremy P (ed.) Tarcher Inc., Los Angeles, USA.
- Nordenstrom BWE (1983) Biologically Closed Electric Circuits: Clinical Experimental and Theoretical Evidence for an Additional Circulatory System. Nordic Medical Publications, Stockholm,
- Nordenstrom BWE (1998) Exploring BCEC-Systems (Biologically Closed Electric Circuits). Nordic Medical Publications, Stockholm.
- Nordenström BEW (1978) Preliminary clinical trials of electrophoretic ionization in the treatment of malignant tumors. IRCS Med Sci 6: 537.
- Nordenström BEW (1985) Electrochemical treatment of cancer. Ann Radiol 28: 128-129.
- Mycielska ME, Djamgoz MBA (2004) Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci 117: 1631-1639.
- Sersa G, Miklavcic D, Cemazar M, Rudolf Z, Pucihar G, et al. (2008) Electrochemotherapy in treatment of tumors. Eur J Surg Oncol 34: 232-240.
- Xin YL (1994) Organization and spread electrochemical therapy (ECT) in China. Eur J Surg 574: 25-30.
- Xin YL (1994) Advances in the treatment of malignant tumors by electrochemical therapy (ECT). Eur J Surg 574: 31-36.
- Matsushima Y, Takahashi E, Hagiwara K, Konaka C, Miura H, et al. (1994) Clinical and experimental studies of anti-tumoral effects of electrochemical therapy (ECT) alone or in combination with chemotherapy. Eur J Surg 574: 59-67.
- Chou CK, Vora N, Li JR, Yen Y, Ren RL,et al. (1999) Development of electrochemical treatment at the City of Hope (USA). Electricity and Magnetism in Biology and Medicine 927-930.
- Xin Y, Xue F, Ge B, Zhao F, Shi B, et al. (1997) Electrochemical treatment of lung cancer. Bioelectromagnetics 18: 8-13.
- Robertson GS, Wemyss-Holden SA, Dennison AR (1998) Experimental study of electrolysis-induced hepatic necrosis. Br J Surgery 85: 1212-1216.
- Jaroszeski MJ, Coppola D, Pottinger C, Benson K, Gilbert RA, et al. (2001) Treatment of hepatocellular carcinoma in a rat model, using electrochemotherapy. Eur J Cancer 37: 422-430.
- Holandino C, Veiga VF, Rodriques ML, Morales MM, Capella MA, et al. (2001) Direct current decreases cell viability but not P-glucoprotein expression and function in human multidrug resistant leukemic cells. Bioelectromagnetics 22: 470-478.
- Susil R, Semrov D, Miklavcic D (1998) Electric field-induced transmembrane potential depends on cell density and organization. Electro- and Magnetobiology 17: 391-399.
- Watson BW (1991) Reappraisal: the treatment of tumors with direct electric current. Med Sci Res 19: 103-105.
- Samuelsson L, Jonsson L, Stahl E (1983) Percutaneous treatment of pulmonary tumors by electrolysis. Radiologie 23: 284-287.
- Miklavcic D, Sersa G, Kryzanowski M, Vodovnik L (1993) Tumor treatment by direct electric current, tumor temperature and pH, electrode materials and configuration. Bioelectr Bioeng 30: 209-220.
- Szasz A, Szasz N, Szasz O (2010) Oncothermia-Principles and practices. Springer Science, Heidelberg, Germany.
- Moros EG (2012) Physics of Thermal Therapy, Fundamentals and Clinical Applications, CRC Press, Taylor and Francis Group, UK.
- Pang CLK (2015) Hyperthermia in Oncology. CRC Press, Taylor and Francis Group, UK.
- Kufe D (2003) Cancer Medicine. BC Decker Inc., Hamilton.
- Perez CA, Brady LW, Halperin EC (2004) Principles and Practice of Radiation Oncology. 4th ed, Lippincott Williams and Wilkins, Philadelphia, USA.
- DeVita VT, Hellman S, Rosenberg SA (2004) Cancer: Principles and Practice of Oncology. 7th ed, Lippincott Williams and Wilkins, Philadelphia, USA.
- van der Zee J (2002) Heating the patient: a promising approach? Ann Oncol 13: 1173-1184.
- Szasz A, Szasz N, Szasz O (2013) Local hyperthermia in oncology-to choose or not to choose? In: Huilgol N (ed.) Hyperthermia. InTech.
- Roussakow S (2013) Critical analysis of electromagnetic hyperthermia randomized trials: dubious effect and multiple biases, Conf Papers in Med.
- Storm FK (1993) What happened to hyperthermia and what is its current status in cancer treatment? J Surg Oncol 53: 141-143.
- Bath C (2014) Using hyperthermia for cancer treatment: proofs, promises, and uncertainties. ASCO Post.
- Calaprice A (2005) The New Quotable Einstein, Princeton University Press, Princeton, USA.
- Szasz A (2013) “Quo Vadis” oncologic hyperthermia? Conf Papers Med.
- Milligan AJ (1984) Whole-body hyperthermia induction techniques. Cancer Res 44: 4869-4872.
- Heckel M (1990) Ganzkörperhyperthermie und Fiebertherapie-Grundlagen und Praxis, Hippokrates, Stuttgart.
- Ardenne VA, Wehner H (2005) Extreme Whole-Body Hyperthermia with Water-Filtered Infrared-A Radiation, NCBI Bookshelf, Landes Bioscience. Madame Curie Bioscience Database.
- Srobl B (2006) Jahre ganzkörperhyperthermie kombiniert mit chemotherapie beim ovarialkarzinom, Dolphin-0-und-1-Studie. Hyperthermia Symposium pp: 22-23.
- Kelleher DK, Thews O, Rzeznik J (1999) Water-filtered infrared-a radiation: a novel technique for localized hyperthermia in combination with bacteriochlorophyll-based photodynamic therapy. J Hyp 15: 467-474.
- Ash SR, Steinhart CR, Curfman MF (1997) Extracorporeal whole body hyperthermia treatments for HIV infection and AIDS. ASAIO J 43: 830-838.
- Suvernev AV, Ivanov GV, Efremov AV (2006) Whole body hyperthermia at 43.5-44°C: dream or reality? In: Baronzio GF, Hager ED (eds.) Hyperthermia in Cancer Treatment: A Primer, Landes Biosceince. Springer Science 18: 228-232.
- Weiss TF (1996) Cellular Biophysics. Bradford Book, MIT Press, Cambridge.
- Oleson N (1985) The role of magnetic inductiontechniques for producing hyperthermia. In: Anghileri LJ, Robert J (eds.) Hyperthermia in Cancer Treatment. Boca Roton 2:141-154.
- Nishide SM, Ueno S (1993) A method of localized hyperthermia by using a figure-of-eight coil and short-circuit rings. Eng Med Biol Soc Proc pp: 1447-1448.
- Jojo M, Murakami A, Sato F (2001) Consideration of handy excitation apparatus for the inductive hyperthermia. IEEE Trans Magn 37: 2944-2946.
- Gilchrist RK, Medal R, Shorey WD (1957) Selective inductive heating of lymph nodes. Ann Surg 146: 596-606.
- Rand RW, Snow HD, Brown WJ (1982) Thermomagnetic surgery for cancer. J Surg Res 33: 177-183.
- Matsuki H, Satoh T, Murakami K (1990) Local hyperthermia based on soft heating method utilizing temperature sensitive ferrite-rod. IEEE Trans Magn 26: 1551-1553.
- Jordan A, Scholz R, Wust P, Fakhling H, Felix R (1999) Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible supermegnetic nanoparticles. J Magn Magn Mat 201: 413-419.
- Turner PF (1984) Regional hyperthermia with an annular phase array. IEEE Trans Biomed Eng BME 31: 106-111.
- Taylor LS (1978) Devices for microwave hyperthermia. In: Streffer C, van Beuningen D, Dietzel F (eds.) Cancer Therapy by Hyperthermia and Radiation. Urban & Schwarzenberg, Baltimore, Munich pp: 115-121.
- Gonzalez-Gonzalez D, van Dijk JDP, Oldenburger F (1992) Results of combined treatment with radiation and hyperthermia in 111 patients with large of deep-seated tumours. In: Germeg EW (ed.) Hyperthermia Oncology 1: 415.
- Myerson RJ, Leybovich L, Emami B, Grigsby PW, Straube W, et al. (1991) Phantom studies and preliminary clinical experience with the BSD2000. Int J Hyp 7: 937-951.
- Wust P, Fahling H, Wlodarczyk W, Seebass M, Gellermann J, et al. (2001) Antenna arrays in the sigma-eye applicator: interactions and transforming networks. Med Phys 28: 1793-1805.
- Wust P, Felix R, Deuflhard P (1999) Kunstliches fieber gegen krebs. Spektrum der Wissenschaft pp: 78-84.
- Issels RD, Adbel-Rahman S, Wendtner CM, Falk MH, Kurze V, et al. (2001) Neoadjuvant chemotherapy combined with regional hyperthermia (RHT) for locally advanced primary or recurrent high-risk adult soft-tissue sarcomas (STS) of adults: long-term results of a phase II study. Eur J Cancer 37: 1599-1608.
- Fenn AJ (2007) Breast cancer treatment by focused microwave thermotherapy. Sudbury, MA: Jones and Barlett pp: 54-56.
- LeVeen HH (1976) Tumor eradication by radiofrequency therapy. JAMA 235: 2198-2200.
- Short JG, Turner PF (1980) Physical hyperthermia and cancer therapy. Proc IEEE 68: 133-142.
- Storm FK, Morton DL, Kaiser LR (1982) Clinical radiofrequency hyperthermia: a review. Natl Cancer Inst Monogr 61: 343-350.
- Jo S, Sugahara T, Yamamoto I (1994) Clinical response of hyperthermia using heating equipment thermotron-RF8 in Japan. Biomed Eng-Appl Basis Commun 6: 340-362.
- Hiraki Y, Nakajo M, Miyaji N, Takeshita T, Churei H, et al. (1998) Effectiveness of RF capacitive hyperthermia combined with radiotherapy for stages III and IV oro-hypopharyngeal cancers: a non-randomized comparison between thermoradiotherapy and radiotherapy. Int J Hyp 14: 593-594.
- Masunaga SI, Hiraoka M, Akuta K, NishimuraY, Nagata Y, et al. (1994) Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int J Hyp 10: 31-40.
- Szasz A (2015) Bioelectromagnetic Paradigm of Cancer Treatment Oncothermia. In: Paul J. Rosch edn. Bioelectromagnetic and subtle energy medicine. CRC Press, Taylor & Francis Group 2015: 323-336.
- Szasz A, Szasz O, Szasz N (2006) Physical background and technical realization of hyperthermia. In: Baronzio GF, Hager ED edn. Locoregional Radiofrequency-Perfusional- and Wholebody- Hyperthermia in Cancer Treatment: New clinical aspects, Chapter 3, Springer, NY, USA pp: 27-59.
- ITU Radio Regulations (2012) CHAPTER II-Frequencies, ARTICLE 5 Frequency allocations, Section IV-Table of Frequency Allocations.
- Gonzalez-Gonzalez D (1996) Thermo-radiotherapy for tumors of the lower gastro-instenstinal tract. In: Seegenschmiedt MH, Fessenden P, Vernon CC (eds.) Thermo-Radiotherapy and Thermo-Chemiotherapy. Biol Physiol Phys 1: 105-119.
- Oleson JR (1995) Review Eugene Robertson special lecture, hyperthermia from the clinic to the laboratory: a hypothesis. Int J Hyp 11: 315-322.
- Overgaard J (1989) The current and potential role of hyperthermia in radiotherapy. Int J Rad Oncol Biol Phys 16: 535-549.
- van der Zee J, Truemiet-Donker AD, The SK, Seldenrath JJ, Meerwaldt JH, et al. (1988) Low-dose reirradiation in combination with hyperthermia: a palliative treatment for patients with breast cancer recurring in previously irradiated areas. Int J Rad Oncol Biol Phys 15: 1407-1413.
- Vernon CC, Harrison M (1990) Hyperthermia with low-dose radiotherapy for recurrent breast carcinoma. The Lancet 336: 1383.
- Bicher JI, Al-Bussam N, Wolfstein RS (2006) Thermotherapy with curative intent-breast, head, and neck, and prostate tumors. Deutsche Zeitschrift fur Onkologie 38: 116-122.
- Molls M (1992) Hyperthermia-the actual role in radiation oncology and future prospects. Strahlenterapie und Onkologie 168: 183-190.
- Seegenschmiedt MH, Feldmann HJ, Wust P (1995) Hyperthermia-its actual role is radiation oncology. Strahlentherapie und Onkologie 171: 560-572.
- Wust P, Rau B, Gemmler M, Schlag P, Jordan A, et al. (1995) Radio-thermotherapy in multimodal surgical treatment concepts. Onkologie 18: 110-121.
- Takahashi M, Hiraoka M, Nishimura Y, Takahashi M, Abe M, et al. (1993) Clinical results of thermoradiotherapy for deep-seated tumors. In: Matsuda T ed. Cancer Treatment by Hyperthermia, Radiation and Drugs. Taylor & Francis pp: 227-239.
- Hiraoka M, Jo S, Akuta K, Takahashi M, Abe M, et al. (1987) Radiofrequency capacitive hyperthermia for deep-seated tumors-I. Studies on Thermometry. Cancer 60: 121-127.
- Tsukiyama I (1993) Clinical results of thermoradiotherapy for superfizial and shallow-seated tumors. In: Matsuda T. edn. Cancer treatment by hyperthermia, radiation and drugs, Taylor & Francis, London-Washington, DC, USA pp: 216-228
- Myerson RJ, Scott CB, Emami B, Sapozink MD, Samulskiet TV, et al. (1996) A phase I/II study to evaluate radiation therapy and hyperthermia for deep-seated tumors: a report of RTOG 89-08. Int J Hyp 4: 449-459.
- Urano M, Kuroda M, Nishimura Y (1999) For the clinical application of thermochemotherapy given at mild temperatures. Int J Hyp 15: 79-107.
- Wiedermann GJ, Feyerabend T, Mentzel M (1994) Thermochemotherapie: grunde fur die kombinationsbehandlung mit hyperthermia und chemotherapie. Focus Mul 11: 44-50.
- Ohno T, Sakagami T, Shiomi M (1993) Hyperthermai therapy for deep-regional cancer: thermochemotherapy, a combination of hyperthermia with chemotherapy. In: Matsuda T (ed.) Cancer treatment by hyperthermia, radiation and drugs, Taylor & Francis, London-Washington, DC, USA pp: 303-316.
- Issels R (1999) Hyperthermia combined with chemotherapy-biological rationale, clinical application, and treatment results. Onkologie 22: 374-381.
- Issels RD, Lindner LH, Weweij J, Wust P, Reichardt P, et al. (2010) Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomized phase 3 multicentre study. Lancet Oncol 11: 561-570.
- LeVeen HH, Rajagopalan PR, Vujic I, Gobien RP, O'Brien PH, et al. (1984) Radiofrequency thermotherapy, local chemotherapy, and arterial occlusion in the treatment of non-resectable cancer. Am Surg 50: 61-65.
- http://www.isshin.or.jp/okamura/awaji2004/awaji1.html
- Franchi F, Grassi P, Ferro D, Pigliucci G, De Chicchis M, et al. (2007) Antiangiogenic metronomic chemotherapy and hyperthermia in the palliation of advanced cancer. Eur J Cancer Care 16: 258-262.
- Ohtsubo T, Kano E, Hayashi S (2001) Enhancement of cytotoxic effects of chemotherapeutic agents with hyperthermia in vitro. In: Kosaka M, Sugahara T, Schmidt KL, et al. (eds.) Thermotherapy for Neoplasia, Inflammation, and Pain. Springer Verlag, Tokyo pp: 451-455.
- Kawasaki S, Asaumi JI, Shibuya K, Kosaka M, Sugahara T, et al. (2001) Recent aspects of elucidating the cellular basis of thermochemotherapy. Thermotherapy Neoplasia Inflam Pain pp: 424-432.
- Masunaga S, Hiraoka M, Akuta K, Nishimura Y, Nagata Y (1990) Non-randomized trials of thermoradiotherapy versus radiotherapy for preoperative treatment of invasive urinary bladder cancer. J Jpn Soc Ther Radiol Oncol 2: 313-320.
- Rau B, Wust P, Hohenberger P (1998) Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer-a phase II clinical trial. Ann Surg 227: 380-389.
- Kodama K, Doi O, Higashyama M (1993) Long-term results of postoperative intrathoracic chemo-thermotherapy for lung cancer with pleural dissemination. Cancer 72: 426-431.
- Pearson AS, Izzo F, Fleming RY, Ellis LM, Delrio P, et al. (1999) Intraoperative radiofrequency ablation of cryoablation for hepatic malignances. Amer J Surg 178: 592-598.
- Kouloulias VE, Kouvaris JR, Nikita KS, Golematis BC, Uzunoglu NK, et al. (2002) Intraoperative hyperthermia in conjunction with multi-schedule chemotherapy (pre- intra- and post operative), by-pass surgery, and post-operative radiotherapy for the management of unresectable pancreatic adenocarcinoma. Int J Hyp 18: 233-252.
- Stahl H, Wust P, Maier-Hauff K, Seebass M, Mischel M, et al. (1995) The use of an early postoperative interstitial-hyperthermia combination therapy in malignant gliomas. Strahlenther Onkol 171: 510-524.
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, et al. (1998) Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/- hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 40: 287-295.
- Hulshof MC, Raaymakers BW, Lagendijk JJ, Koot RW, Crezee H, et al. (2004) A feasibility study of interstitial hyperthermia plus external beam radiotherapy in glioblastoma multiforme using the Multi Electrode Current Source (MECS) system. Int J Hyp 20: 451-463.
- Pontiggia P, Duppone Curto F, Rotella G, Sabato A, Rizzo S (1995) Hyperthermia in the treatment of brain metastases from lung cancer. Experience on 17 cases. Anticancer Res 15: 597-601.
- Ohtsuru A, Braiden V, Cao Y, Kosaka M, Sugahara T, et al. (2001) Cancer gene therapy in conjunction with hyperthermia under the control of heat-inducible promoter. Thermotherapy Neoplasia Inflam Pain pp: 464-470.
- Balckburn LV, Galoforo SS, Corry PM, Lee YJ (1998) Adenoviral-mediated transfer of heat-inducible double suicide gene into prostate carcinoma cells. Cancer Res 58: 1358-1362.
- Huang Q, Hu JK, Lohr F, Zhang L, Braun R, et al. (2000) Heat-induced gene expression as a novel targeted cancer gene therapy strategy. Cancer Res 60: 3435-3439.
- Gaber MH, Wu NZ, Hong K, Huang SK, Dewhirst MW, et al. (1996) Thermosensitive liposomes: extravasation and release of contents in tumor microvascular networks. Int J Radiat Oncol Biol Phys 36: 1177-1187.
- Yerushalmi A, Shani A, Fishelovitz Y, Arielly J, Singer D, et al. (1986) Local microwave hyperthermia in the treatment of carcinoma of the prostate. Oncology 43: 299-305.
- Piantelli M, Tatone D, Castrilli G, Savini F, Maggiano N, et al. (2001) Quercetin and tamoxifen sensitize human melanoma cells to hyperthermia. Melanoma Res 11: 469-476.
- Oleson JR, Calderwood SK, Coughlin CT, Dewhirst MW, Gerweck LE, et al. (1988) Biological and clinical aspects of hyperthermia in cancer. Ther Am J Clin Oncol 11: 368-380.
- Henderson BW, Waldow SM, Potter WR, Dougherty TJ (1985) Interaction of photodynamic therapy and hyperthermia: tumour response and cell survival studies after treatment of mice in vivo. Cancer Res 45: 6071-6077.
- Lohr F, Hu K, Huang Q, Zhang L, Samulski TV, et al. (2000) Enhancement of radiotherapy by hyperthermia-regulated gene therapy. Int J Rad Oncol Biol Phys 48: 1513-1518.
- Skitzki JJ, Repasky EA, Evans SS (2009) Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Invest Drugs 10: 550-558.
- Vertrees RA, Jordan JM, Zwischenberger JB (2007) Hyperhtermia and chemotherapy: the science. In: Hlem CW, Edwards RP (eds.) Current Clinical Oncology, Intraperitoneal Cancer Therapy. Humana Press, Totowa.
- Fatehi D, van der Zee J, van der Wal E (2006) Temperature data analysis for 22 patients with advanced cervical carcinoma treated in Rotterdam using radiotherapy, hyperthermia and chemotherapy: a reference point is needed. Int J Hyp 22: 353-363.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC (2000) Comparison of radio- therapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 355: 1119-1125.
- Vasanthan A, Mitsumori M, Part JH (2005) Regional hyperthermia combined with radiotherapy for uterine cervical cancers: a multiinstitutional prospective randomized trial of the international atomic energy agency. Int J Rad Oncol Biol Phys 61: 145-153.
- Heilbrunn LV (1923) The colloid chemistry of protoplasm. Am J Physiol 64: 481-498.
- Yatvin MB, Dennis WH, Streffer C, van Beuningen D, Dietzel F, et al. (1978) Membrane lipid composition and sensitivity to killing by hyperthermia, procaine and radiation. Cancer Therapy Hyperthermia Radiation. Urban & Schwarzenberg, Baltimore, Munich.
- Bowler K, Duncan CJ, Gladwell RT (1973) Cellular heat injury. Comp Biochem Physiol 45A: 441-450.
- Wallach DFH, Streffer C, van Beuningen D, Dietzel F, et al. (1978) Action of hyperthermia and ionizing radiation on plasma membranes. Cancer Therapy Hyperthermia Radiation. Urban & Schwarzenberg, Baltimore, Munich.
- Nishida T, Akagi K, Tanaka Y (1997) Correlation between cell killing effect and cell-membrane potential after heat treatment: analysis using fluorescent dye and flow cytometry. Int J Hyp 13: 227-234.
- Weiss TF (1996) Cellular Biophysics. Electrical Properties, MIT Press, Cambridge 2.
- Ricardo R, Gonzalez-Mendez R, Hahn GM (1989) Effects of hyperthermia on the intercellular pH and membrane potential of Chinese hamster ovary cells. Int J Hyp 5: 69-84.
- Mikkelsen RB, Verma SP, Wallach DFH, Streffer C, van Beuningen D, et al. (1978) Hyperthermia and the membrane potential of erythrocyte membranes as studied by Raman spectroscopy. Cancer Therapy Hyperthermia Radiation pp: 160-162.
- Hahn GM (1990) The heat-shock response: effects before, during and after gene activation. In: Gautherie M ed. Biological Basis of Oncologic Thermotherapy. Springer Verlag, Berlin pp: 135-159.
- Vaupel PW, Kelleher DK, Seegenschmiedt MH, Fessenden P Vernon CC (1996) Metabolic status and reaction to heat of normal and tumor tissue. Thermo-Radiotherapy and Thermo-Chemiotherapy. Biol Physiol Phys 1: 157-176.
- Kabakov AE, Gabai VL (1997) Heat Shock Proteins and Cytoprotection: ATP-Deprived Mammalian Cells. (Series: Molecular Biology Intelligence Unit). Springer Verlag, New York, Berlin, Heidelberg,
- Vernon CC, Hand JW, Field SB (1996) Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. Int J Rad Oncol Biol Phys 35: 731-744.
- Emami B, Scott C, Perez CA (1996) Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumours: a prospectively controlled randomized study by the radiation therapy oncology group. Int J Rad Oncol Biol Phys 34: 1097-1104.
- Jones EL, Oleson JR, Prosnith LR, Samulski TV, Vujaskovic Z, et al. (2005) Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 23: 3079-3085.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, et al. (2000) Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Lancet 355: 1119-1125.
- Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, et al. (2001) A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyp 17: 97-105.
- Sharma S, Patel FD, Sandhu AP, Gupta BD, Yadav NS (1989) A prospective rendomized study of local hyperthermia as a supplement and radiosensitiser in the treatment of carcinoma of the cervix with radiotherapy. Endocurietherapy Hypertherm Oncol 5: 151-159.
- Vasanthan A, Mitsumori M, Park JH, Zhi-Fan Z, Yu-Bin Z, et al. (2005) Regional hyperthermia combined with radiotherapy for uterine cervical cancers: a multiinstitutional prospective randomized trial of the international atomic energy agency. Int J Rad Oncol Biol Phys 61: 145-153.
- Fatehi D, van der Zee J, van der Wal E, Van Wieringen WN, Van Rhoon GC (2006) Temperature data analysis for 22 patients with advanced cervical carcinoma treated in Rotterdam using radiotherapy, hyperthermia and chemotherapy: a reference point is needed. Int J Hyp 22: 353-363.
- Mitsumori M, Zeng ZF, Oliynychenko P, Park JH, Choi IB, et al. (2007) Regional hyperthermia combined with radiotherapy for locally advanced non-small cell lung cancers: a multi-institutional prospective randomized trial of the International Atomic Energy Agency”. Int J Clin Oncol 12: 192-198.
- Moritz AR, Henriques FC (1947) Studies of thermal injury. ii. the relative importance of time and surface temperature in the causation of cutaneous burns. Am J Pathol 23: 695-720.
- Henriques FC (1947) Studies of thermal injury v. the predictability and the significance of thermally induced rate processes leading to irreversible epidermal injury. Arch Pathol 43: 489-502.
- Hand JW, Ledda JL, Evans TS (1982) Temperature distribution in tissues subjected to local hyperthermia by rf induction heating. Br J Cancer 45: 31-35.
- Ohguri T, Imada H, Yahara K, Kakeda S, Tomimatsu A, et al. (2004) Effect of 8-MHz radiofrequency-capacitive regional hyperthermia with strong superficial cooling for unresectable or recurrent colorectal cancer. Int J Hyp 20: 465-475.
- Doi O, Kodama K, Higashiyama M (1993) Postoperative chemothermotherapy for locally advanced lung cancer with carcinomatous pleuritis. Cancer Treatment by Hyperther Radiation Drugs 31: 338-352.
- Itazawa T (2006) Hyperthermia Combined with Chemoradiotherapy for treatment of locally advanced head and neck cancer with bulky lymph node metastasis; Jap J Hyperthermic Oncol 22: 151-158.
- Thrall DE, Rosner GL, Azuma C, Larue SM, Case BC, et al. (2000) Using units of CEM43oC T90 local hyperthermia thermal dose can be delivered as prescribed. Int J Hyp 16: 415-428.
- Fenn AJ, King GA (1994) Adaptive radiofrequency hyperthermia-phased array system for improved cancer therapy: phantom target measurements. Int J Hyp 10: 189-208.
- van der Zee J (2005) Renewing oncological hyperthermia-oncothermia. Presentation in Mumbay, India.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ (2003) Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyp 19: 267-294.
- Dewey WC (1994) Arrhenius relationships from the molecule and cell to the clinic. Int J Hyp 10: 457-483.
- Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE (1977) Cellular Response to Combination of Hyperthermia and Radiation. Radiology 123: 463-474.
- Sapareto SA, Dewey WC (1984) Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 10: 787-800.
- Pearce JA (2009) Relationship between Arrhenius models of thermal damage and the CEM 43 thermal dose. Proc. SPIE 7181. Energy-based Treatment of Tissue and Assessment.
- Weaver JA, Stoll AM (1969) Mathematical Model of Skin Exposed to Thermal Radiation. Aerosp Med 40: 24-30.
- Takata AN (1974) Development of Criterion for Skin Burns. Aerosp Med 45: 634-637.
- Stoll AM (1967) Heat transfer in biotechnology. In: Hartnett JP, Irvine TF (eds.) “Advances in Heat Transfer Academic Press Inc. 4: 65-139.
- Leophold KA, Dewhirst MW, Samulsky TV, Dodge RK, Georg SL (1993) Cumulative minutes with T90 greater than temindex is predictive of response of superficial malignancies to hyperthermia and radiation. Int J Radiat Oncol Biol Phys 25: 841-847.
- Perez CA, Sapareto SA (1984) Thermal dose expression in clinical hyperthermia and correlation with tumor response/control. Cancer Res 44: 4818-4825.
- Thrall DE, LaRue SM, Yu D, Samulski T, Sanders L (2005) Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Canc Res 11: 5206-5214.
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, et al. (2005) Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 23: 3079-3085.
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB et al. (1996) Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer. Resuts from five randomized controlled clinical trials. Int J Rad Oncol Biol Phys 35: 731-744.
- Maguire PD (2001) A phase II trial testing the thermal dose parameter CEM43oCT90 as a predictor of response in soft tissue sacomas treated with pre-operative thermorasiotherapy. Int J Hyp 17: 283-290.
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D (2005) Re-setting the biologic rationale for thermal therapy. Int J Hyp 21: 779-790.
- de Bruijne M, van der Holt B, van Rhoon GC, van der Zee J (2010) Evaluation of CEM43°CT90 thermal dose in superficial hyperthermia; a retrospective analysis. Strahlenther & Onkol (Radiotherapy and Oncology) 186: 436-443.
- Assi H (2009) A new cem43 thermal dose model based on Vogel-Tammann-Fulcher behavior in thermal damage processes; Ryerson University, Canada.
- Jones E, Thrall D, Dewhirst MW, Vujaskovic Z (2006) Prospective thermal dosimetry: the key to hyperthermia's future. Int J Hyp 22: 247-253.
- Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and microenvironment of human tumors: a review. Cancer Res 49: 6449-6465.
- Dudar TE, Jain RK (1984) Differential response of normal and tumor microcirculation to hyperthermia. Cancer Res 44: 605-612.
- Song CW, Lokshina A, Rhee JG (1984) Implication of blood-flow in hyperthermic treatment of tumors. IEEE Trans Biomed Eng 31: 9-16.
- Song CW, Choi IB, Nah BS (1995) Microvasculature and persfusion in normal tissues and tumors. In: Seegenschmiedt MH, Fessenden P, Vernon CC (eds.) Thermoradiometry and Thermochemotherapy 1: 139-156.
- Song CW, Park H, Griffin RJ (2001) Theoretical and experimental basis of hyperthermia. Springer Verlag, Tokyo pp: 394-407.
- Takana Y (2001) Thermal responses of microcirculation and modification of tumor blood flow in treating the tumors. In: Kosaka M, Sugahara T, Schmidt KL, et al. (eds.) Theoretical and Experimental Basis of Hyperthermia. Thermotherapy for Neoplasia, Inflammation, and Pain. Springer Verlag, Tokyo pp: 408-419.
- Baronzio GF, Gramaglia A, Baronzio A (2006) Influence of tumor microenvironment on thermoresponse: biologic and clinical implications. In: Baronzio GF, Hager ED (eds.) Hyperthermia in Cancer Treatment: A Primer. Landes Biosceince, Springer Science, NY, USA pp: 62-86.
- Roca C, Primo L (2006) Hyperthermia and angiogenesis, results and perspectives. In: (Eds) Baronzio GF, Hager ED. Hyperthermia in Cancer Treatment: A Primer. Landes Biosceince, Springer Science, NY, USA pp: 87-93.
- Kelleher DK, Vaupel P (2006) Vascular effects of localized hyperthermia. In: Baronzio GF and Hager ED (eds.) Hyperthermia in Cancer Treatment: A Primer. Landes Biosceince, Springer Science, NY, USA pp: 94-104.
- Shoji H, Motegi M, Osawa K, Okonogi N, Okazaki A, et al. (2015) A novel strategy of radiofrequency hyperthermia (neothermia) in combination with preoperative chemoradiotherapy for the treatment of advanced rectal cancer: a pilot study. Cancer Med 4: 834-843.
- Fatehi D (2007) Technical Quality of Deep Hyperthermia, Using the BSD-2000, PhD thesis. Uitgeverij Box Press, Oisterwijk, The Netherlands.
- Jones E, Dewhirst M, Vujaskovic Z (2003) Hyperthermia improves the complete response rate for superficial tumors treated with radiation: results of a prospective randomized trial testing the thermal dose parameter CEM 43°T90. Int J Rad Oncol Biol Phys 57: 253-254.
- https://www.intechopen.com/books/hyperthermia/local-hyperthermia-in-oncology-to-choose-or-not-to-choose-
- Szasz A (2013) Electromagnetic effects in nanoscale range. Cellular Response to Physical Stress and Therapeutic Applications. eds. Tadamichi Shimizu, Takashi Kondo, Nova Science Publishers Inc.
- Warburg O (1996) Oxygen, The Creator of Differentiation, Biochemical Energetics. Academic Press, NY, USA.
- Szentgyorgyi A (1968) Bioelectronics, A Study on Cellular Regulations, Defense and Cancer. Academic Press, London.
- Schwan HP (1963) Determination of biological impedances. In: Physical Techniques in Biological Research. Vol. 6, Academic Press, NY, USA pp: 323-406.
- Lovelady DC, Richmond TC, Maggi A (2007) Distinguishing cancerous from non-cancerous cells through analysis of electrical noise. Phys Rev E Stat Nonlin Soft Matter Phys 76: 041908.
- Bassingthwaighte JB, Leibovitch LS, West BJ (1994) Fractal Physiology. Oxford University Press, New York, Oxford.
- Szasz O, Andocs G, Meggyeshazi N (2013) Modulation effect in oncothermia. Conference Papers in Medicine.
- http://ieeexplore.ieee.org/document/6930100/
- http://www.hindawi.com/journals/cpis/2013/938689/
- Szasz A, Szasz O, Szasz N (2005) Physical background and technical realization of hyperthermia. Landes Biosceince, Springer Science, NY, USA.
- Szasz A (2015) Bioelectromagnetic paradigm of cancer treatment-oncothermia. In: Rosch PJ (ed.) Bioelectromagnetic and Subtle Energy Medicine. CRC Press, Taylor and Francis Group, NY, USA pp: 323-336.
- Wismeth C, Dudel C, Pascher C, Ramm P, Pietsch T, et al. (2010) Transcranial electro-hyperthermia combined with alkylating chemotherapy in patients with relapsed high-grade gliomas-Phase I clinical results. J Neurooncol 98: 395-405
- Sahinbas H, Groenemeyer DHW, Boecher E, Szasz A (2007) Retrospective clinical study of adjuvant electro-hyperthermia treatment for advanced brain-gliomas. Deutsche Zeitschrift fuer Onkologie 39: 154-160.
- Szasz A (2014) Current status of oncothermia therapy for lung cancer. Korean J Thorac Cardiovasc Surg 47: 77-93.
- Lee DY, Park JS, Jung HC, Byun ES, Haam SJ (2015) The outcome of the chemotherapy and oncothermia for far advanced adenocarcinoma of the lung: case reports of four patients. Adv Lung Cancer 4: 1-7.
- Yeo SG (2015) Definitive radiotherapy with concurrent oncothermia for stage IIIB non-small-cell lung cancer: A case report. J Adv Phys 10: 2538-2559.
- Lee DY, Haam SJ, Kim TH, Lim JY, Kim EJ, et al. (2013) Oncothermia with chemotherapy in the patients with Small Cell Lung Cancer. Hindawi Publishing Corporation Conference Papers in Medicine.
- Jeung TS, Ma SY, Choi JH, Yu J, Lee SY (2015) Results of oncothermia combined with operation, chemotherapy and radiation therapy for primary, recurrent and metastatic sarcoma. Case Rep Clin Med 4: 157-168.
- Volovat C, Volovat SR, Scripcaru V, Miron L, Lupascu C (2014) The results of combination of ifosfamid and locoregional hyperthermia (EHY 2000) in patients with advanced abdominal soft-tissue sarcoma after relapse of first line chemotherapy. Romanian Rep Phys 1: 175-181.
- Hager ED, Dziambor H, Höhmann D, Gallenbeck D, Stephan M (1999) Deep hyperthermia with radiofrequencies in patients with liver metastases from colorectal cancer. Anticancer Res 19: 3403-3408.
- Gadaleta-Caldarola G, Infusino S, Galise I, Ranieri G (2014) Sorafenib and locoregional deep electro-hyperthermia in advanced hepatocellular carcinoma: A phase II study. Oncol Lett 8: 1783-1787.
- Fiorentini G, Milandri C, Dentico P, Giordani P, Catalano V (2012) Deep electro-hyperthermia with radiofrequencies combined with thermoactive drugs in patients with liver metastases form colorectal cancer (CRC) a phase II clinical study. 31st Conference of International Clinical Hyperthermia Society (ICHS), Budapest, Hungary pp: 12-15.
- Ferrari VD, De Ponti S, Valcamonico F (2007) Deep electro-hyperthermia (EHY) with or without thermo-active agents in patients with advanced hepatic cell carcinoma: phase II study. J Clin Oncol 25: 18S.
- Hager ED, Süsse B, Popa C, Schritttwieser G, Heise A, et al. (1994) Complex therapy of the not in sano respectable carcinoma of the pancreas-a pilot study. J Cancer Res Clin Oncol 120: R47.
- Volovat C, Volovat SR, Scripcaru V, Miron L (2014) Second-line chemotherapy with gemcitabine and oxaliplatin in combination with loco-regional hyperthermia (EHY-2000) in patients with refractory metastatic pancreatic cancer-preliminary results of a prospective trial. Romanian Rep Phys 66: 166-174.
- Jeung TS, Ma SY, Lim S, Sangwook L (2013) Cases That Respond to Oncothermia Monotherapy. Hindawi Publishing Corporation Conference Papers in Medicine.
- Szasz O, Szasz A (2016) Oncothermia-Nano-heating paradigm. J Cancer Sci Ther 6: 4
- Szasz A (2013) Electromagnetic effects in nanoscale range. In: Shimizu T, Kondo T (eds.) Cellular Response to Physical Stress and Therapeutic Applications. Chapter 4. Nova Science Publishers.
- Andocs G, Renner H, Balogh L, Fonyad L, Jakab C, et al. (2009) Strong synergy of heat and modulated electro- magnetic field in tumor cell killing, Study of HT29 xenograft tumors in a nude mice model. Strahlenther Onkol 185:120-126.
- Vincze Gy, Szigeti Gy, Andocs G, Szasz A (2015) Nanoheating without artificial nanoparticles. Biol Med 7: 4
- Andocs G, Rehman MU, Zhao YL (2015) Nanoheating without artificial nanoparticles Part II, Experimental support of the nanoheating concept of the modulated electro-hyperthermia method, using U37 cell suspension model. Biol Med 7: 4.
- Vancsik T, Andocs G, Kovago CS, Papp E, Meggyeshazi N, et al. (2015) Electro-hyperthermia may target tumor-cell membranes, presentation at the 33rd Annual Conference of the International Clinical Hyperthermia Society (ICHS), Nidda, Germany.
- Andocs G, Szasz O, Szasz A (2009) Oncothermia treatment of cancer: from the laboratory to clinic. Electromagn Biol Med 28: 148-165.
- Tsang YW, Huang CC, Yang KL (2015) Improved immunological tumor microenvironment by combined electro-hyperthermia followed by dendritic cell immunotherapy; cancer immunology, immunotherapy. BMC Cancer 15: 708.
- Pardoll DM (2012) Immunology beats cancer: a blueprint for successful translation. Nat Immunol 13: 12.
- June C (2012) T-cell therapy at the threshold. Nat Biotechnol 30 :7.
- Couzin-Frankel J (2013) Cancer immunotherapy. Science 242: 1432-1433.
- Meggyeshazi N, Andocs G, Balogh L, Balla P, Kiszner G, et al. (2014) DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther Onkol 190: 815-822.
- Andocs G, Meggyeshazi N, Balogh L, Spisak S, Maros ME, et al. (2015) Upregulation of heat shock proteins and the promotion of damage-associated molecular pattern signals in a colorectal cancer model by modulated electrohyperthermia. Cell Stress Chap 20: 37-46.
- Andocs G, Meggyeshazi N, Okamoto Y, Balogh L, Szasz O (2013) Bystander Effect of Oncothermia. Conference Papers in Medicine pp 1-6.
- Andocs G, Szasz A, Iluri N (2012) Tumor vaccination patent.
- Qin W, Akutsu Y, Andocs G, Sugnami A, Hu X, et al. (2014) Modulated electro-hyperthermia enhances dendritic cell therapy through an abscopal effect in mice. Oncol Rep 32: 2873-2379.
- Tsang YW, Huang CC, Yang, KL (2015) Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer 15:708.
- Kovago CS, Meggyeshazi N, Andocs G, Szasz O (2014) Abscopal effect by modulated electro-hyperthermia. Society of Thermal Medicine Annual Meeting, Minneapolis, Minnesota pp: 6-10.
- Meggyeshazi N, Kovago CS, Vancsik T (2015) Tumor cell death induced by modulated electrohyperthermia in combination with Marsdenia tenacissima in murine colorectal allograft tumor model, 33rd Annual Conference of the International Clinical Hyperthermia Socitey. Salzausen, Nidda, Germany pp: 10-12.
- Yoon SM, Lee JS (2011) Case of abscopal effect with metastatic non-small-cell lung cancer. Oncothermia J 5: 53-57.
- Hegyi G, Vincze Gy, Szasz A (2012) On the dynamic equilibrium in homeostasis. Open J Biophys 2: 64-71.
Relevant Topics
- Acupuncture Therapy
- Advances in Naturopathic Treatment
- African Traditional Medicine
- Australian Traditional Medicine
- Chinese Acupuncture
- Chinese Medicine
- Clinical Naturopathic Medicine
- Clinical Naturopathy
- Herbal Medicines
- Holistic Cancer Treatment
- Holistic health
- Holistic Nutrition
- Homeopathic Medicine
- Homeopathic Remedies
- Japanese Traditional Medicine
- Korean Traditional Medicine
- Natural Remedies
- Naturopathic Medicine
- Naturopathic Practioner Communications
- Naturopathy
- Naturopathy Clinic Management
- Traditional Asian Medicine
- Traditional medicine
- Traditional Plant Medicine
- UK naturopathy
Recommended Journals
Article Tools
Article Usage
- Total views: 5159
- [From(publication date):
May-2017 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 4202
- PDF downloads : 957