What are the Safety and Efficacy of Calcium Hydroxyapatite and Polycaprolactone in Adults for Facial Rejuvenation?
Received: 12-Jul-2017 / Accepted Date: 08-Sep-2017 / Published Date: 29-Sep-2017
Abstract
Facial rejuvenation is a beautifying treatment which targets to repair the skin and restore a youthful appearance to the human face. Calcium Hydroxyapatite is naturally occurring mineral form of Calcium whereas Polycaprolactone (PCL) is a synthetic biodegradable polymer. Here the use of Calcium Hydroxyapatite and Polycaprolactone in facial rejuvenation is discussed.
Keywords: Calcium hydroxylapatite; Facial rejuvenation; Polycaprolactone; Aesthetic improvement; Hyaluronic acid
Abbreviations
CaHA: Calcium Hydroxyapatite; HA: Hyaluronic Acid; NASHA: Non-Animal Stabilized Hyaluronic Acid; PCL: Polycaprolactone; GAIS: Global Aesthetic Improvement Scale; WSRS: Wrinkle Severity Rating Scale; LRS: Lemperle Rating Scale; RCT: Randomized Controlled Trial
Introduction
As per the Cochrane collaboration, a systematic review evaluates and compiles the findings of multiple clinical trials and provides best evidence to address the chosen research question.
Conclusion can then be made and future recommendations suggested. Below are the steps in a systematic review as per Cochrane:
• Identify your research question.
• Search for studies.
• Define inclusion and exclusion criteria.
• Extract studies that fulfill the above criteria.
• Perform data analysis of selected studies.
• Evaluate the degree of bias of the above.
• Present findings and assess the quality and level of evidence.
Discussion
Research question
• Target population-Adults, Intervention-Facial Rejuvenation with Polycaprolactone and Calcium hydroxyapatite fillers, Outcome(s)- Safety, Efficacy
• What are the Safety and Efficacy of Calcium Hydroxyapatite and Polycaprolactone in Adults for Facial Rejuvenation?
Search strategy
A comprehensive search of the current literature was undertaken using 3 databases, Cochrane Library, Medline via the PubMed interface and Google scholar for an extensive review. The search strategy was designed to select all relevant articles via Mesh terms combined with key text terms.
Different constructs of search terms were formed by the implementation of truncation of the following terms:
Search parameters
• Radiesse or collagen inducing agent or collagen inducer collagen stimulants or CAHA or Calcium Hydroxylapatite and facial rejuvenation-67 results on PubMed
• Search radiesse and safety or efficacy Sort by: Relevance Filters: published in the last 10 years; Humans-14 results on PubMed
• Search polycaprolactone and safety or efficacy Sort by: Relevance Filters: published in the last 10 years; Humans-106 results on PubMed
• Search polycaprolactone and safety or efficacy and "last 10 years"[PDat] and Humans[Mesh]) and facial rejuvenation Sort by: Relevance Filters: published in the last 10 years; Humans-1 result on PubMed
• Polycaprolactone or PCL or Ellanse and facial rejuvenation-2 results on PubMed
• Polycaprolactone or PCL or Ellanse and dermal filler-7 results on PubMed
• Radiesse or polycaprolactone-49 results on Google Scholar
• Radiesse or polycaprolactone-23 results on Cochrane
The search parameters were limited to all English Language articles from 2006 to 2016, excluding animal and in vitro studies. Manuscripts that related to non-cosmetic interventions were excluded.
Search outcome
Initial search yielded 67 articles on PubMed for CaHA, 7 articles on PubMed for PCL, 49 Papers from Google Scholar for PCL and radiesse, 7 on Cochrane for radiesse, 16 on Cochrane for PCL were also identified.
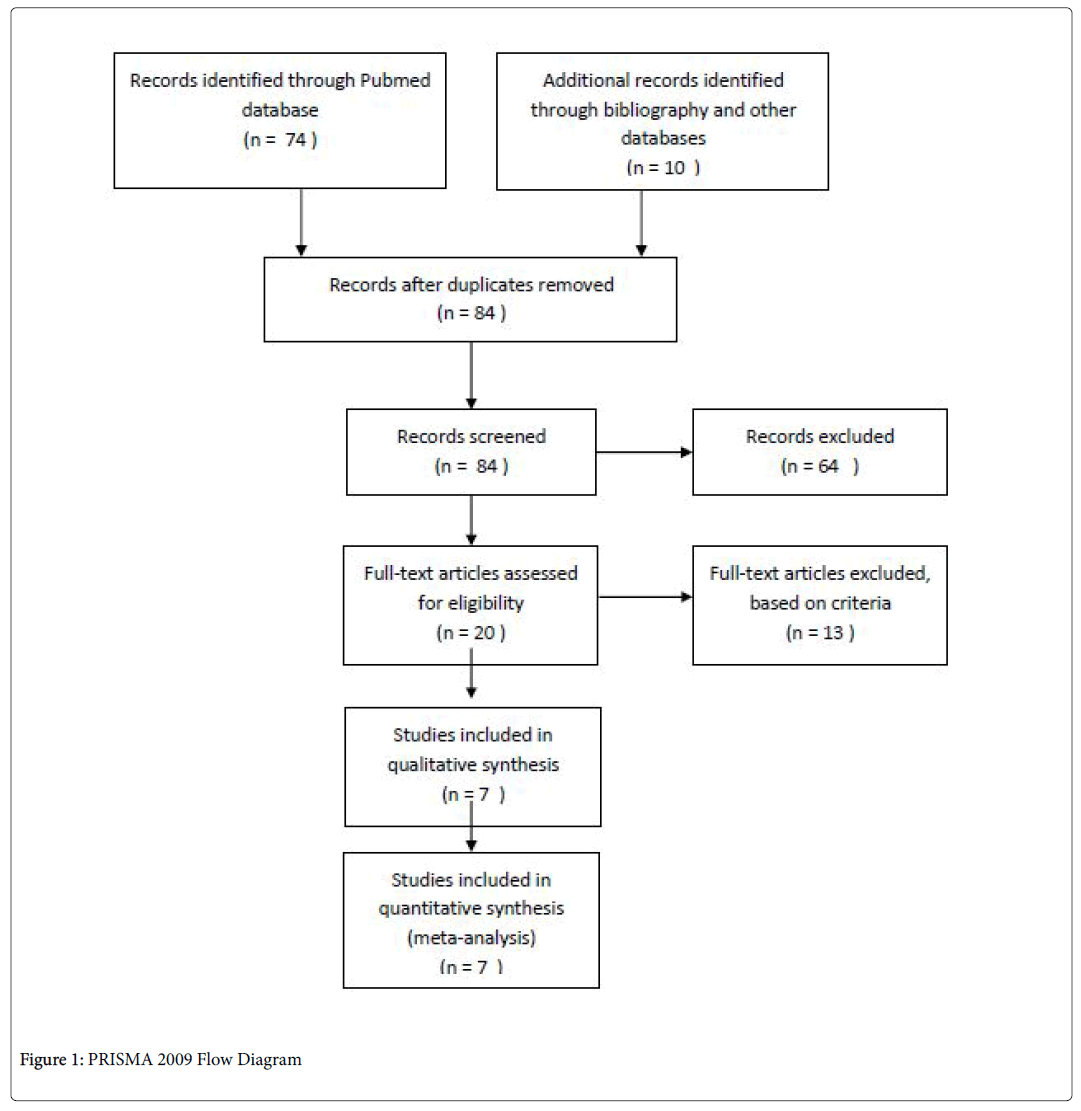
Scanning the references of these papers yielded further studies. The search term was refined further to extract relevant articles. A review of the title and abstract of these papers was undertaken to determine their relevance to our research question and this then yielded 22 potentially relevant papers (Figure 1).
Next an in-depth analysis of these papers was carried out to ascertain the level of evidence, quality and whether they met all of our inclusion and exclusion criteria (Table 1).
Inclusion criteria
• Adults>19 years old
• Human studies
• Facial rejuvenation
• English language publications from 2006 to 2016
• Highest quality papers with good evidence
Exclusion criteria
• Animal studies
• In vitro studies
Data Analysis
| Author and date | Intervention | Sample size | Outcome measurement | Evidence level | Critique |
|---|---|---|---|---|---|
| Smith et al. [1] | Nasolabial folds (CaHA vs. collagen) | 117 | LRS and GAIS | 1 | Good randomized, split face comparative study. |
| Limitation: Study was funded by Bio form Medical. | |||||
| Moers et al. [2] | Nasolabial folds (CaHA vs. 2HA, Juvederm and Perlane) | 205 | GAIS and WSRS and patient satisfaction form. | 1 | Good study design, multicenter high powered RCT Large sample size. |
| Limitation: Study funded by Bio Form Medical. | |||||
| Moers et al. [3] | Nasolabial folds (CaHA NASHA) | 60 | GAIS and WSRS through blinded evaluator. | 2 | Good study design, multi centered high powered RCT. Statistical analysis was clear and significant. |
| Marmur et al. [4] | Nasolabial folds (CaHA in dark skin patients) | 100 | Patient and doctor evaluation. Nil report of adverse events in people with dark skin. | 2 | Good study design, multi centered high powered RCT. Research question was appropriate and focused. |
| Limitation: Study funded by Bio form Medical. Follow-up of only 6 months cannot address long term complications. | |||||
| Moers et al. [5] | Cheek CaHA | 116 | GAIS through blinded independent evaluators and MRI scans | 1 | Good study design, high powered RCT. Use of objective measurement endpoint (MRI scan) |
| Limitation: Study funded by Merz. | |||||
| Galadari et al. [6] | Nasolabial folds (PCL vs. NASHA) | 40 | WSRS and GAIS via doctor evaluation. | 2 | Good study design of blinded split face study. Follow-up of 12 months. |
| Limitation: This was a single center study. | |||||
| Moers et al. [7] | Nasolabial fold (PCL 24 month follow-up) | 40 | WSRS and GAIS blinded evaluation. | 2 | Good follow-up period of 24 months in a high powered RCT. |
| Limitation: Funding for study by AQTIS Medical, which may introduce outcome bias. |
Table 1: Analysis of data.
Conclusion
CaHA safety and efficacy
The search involving Calcium Hydroxylapatite produced 15 best evidence papers supporting the safety and efficacy of CaHA dermal fillers for facial rejuvenation. These included 12 clinical trials and 2 expert consensus reviews. The best evidence for efficacy and safety of Calcium Hydroxylapatite was found in the treatment of naso-labial folds with 2 levels I studies [1,2], 2 level II studies [3,4] and several lower level studies. Additionally, 1 level I study [5], demonstrated good results on the efficacy and safety of CAHA fillers for cheek and midface augmentation. In my opinion, this is because the naso-labial fold has traditionally been an ideal site for split face, randomized controlled trials, and has historically been the most augmented area for the use of fillers.
The safety and efficacy of Calcium Hydroxylapatite was good, comparable to hyaluronic acid fillers as demonstrated in the studies by Moers et al. [2,3]. Majority of these trials demonstrated no serious adverse events apart from nodules, which could be attributed to treatment technique. Of note, several studies have noted longevity averaging 12 to 18 months, and even up to 30 months post injection in select cases.
PCL safety and efficacy
The search involving the use of polycaprolactone produced 5 studies, of which only 2 small studies of best evidence on the Safety and Efficacy of PCL based fillers for facial rejuvenation, both of which were based on naso-labial fold treatment, because in my opinion, this is the most commonly treated site for patients. Both studies demonstrated promising results and durability with no adverse side effects of nodule or granuloma formation. PCL based dermal filler is relatively new and not as popular as hyaluronic acid based fillers, hence the lack of clinical trials evaluating it.
PCL, otherwise known as Ellanse, is available in different grades, representing different degrees of durability as per manufacturer’s claim, Ellanse-S, Ellanse-M, Ellanse-L and Ellanse-E, with longevity of 12, 24, 36 and 48 months respectively. A level II study by Moers et al. found the durability of PCL-1 to be up to 12 months and PCL-2, up to 24 months. However, from my literature search, I did not find published data supporting PCL formulations with claimed longevities of 3 and 4 years. Additionally, the use of PCL is not prevalent in Southeast Asia; hence I do not find anecdotal evidence to substantiate this.
Clinical Application
PCL potentially has good evidence to be used for patients who seek greater longevity with their treatments. And in terms of comfort, patients would prefer it to radiesse as it comes pre mixed with lidocaine. However, as CaHa has been around far longer, at this point in time, there exists greater evidence for the safety and efficacy of CaHa fillers over PCL fillers. Hence future recommendations would be for large robust RCTs to be conducted, with a split face intervention as a control using hyaluronic acid or CaHa to further support its safety and efficacy. Hence, CaHa filler presents as a good alternative to hyaluronic acid fillers for patients who seek greater longevity with their treatments. Although with newer collagen stimulating agents in the market, like Polycaprolactone fillers for example, options for the consumer who seek more durable treatments are increasing.
References
- Smith S, Busso M, McClaren M, Bass LS (2007) A randomized, bilateral, prospective comparison of Calcium Hydroxylapatite microspheres versus human-based collagen for the correction of nasolabial folds. Dermatol Surg 33: S112-S121.
- Moers M, Vogt S, Santos BM, Planas J, Vallve SR, et al. (2007) A multicentre, randomized trial comparing Calcium Hydroxylapatite to two Hyaluronic Acids for Treatment of nasolabial folds. Dermatol Surg 33: S144-S151.
- Moers MM, Tufet JO (2008) Calcium Hydroxylapatite versus non animal stabilized Hyaluronic Acid for the correction of nasolabial folds : A 12-month, multicentre, prospective, randomized, controlled, split-face trial. Dermatol Surg 34: 210-215.
- Marmur ES, Taylor SC, Grimes PE, Boyd CM, Porter JP, et al. (2009) Six-month safety results of Calcium Hydroxylapatite for treatment of nasolabial folds in fitzpatrick skin types IV to VI. Dermatol Surg 35: 1641-1645.
- Moers M, Storck R, Howell DJ, Ogilvie P, Ogilvie A (2012) Physician and patient satisfaction after use of Calcium Hydroxylapatite for cheek augmentation. Dermatol Surg 38: 1217-1222.
- Galadari H, Van Abel D, Al Nuami K, Al Faresi F, Galadari IA (2014) A randomized, prospective, blinded, split face, single-centre study comparing Polycaprolactone to Hyaluronic Acid for treatment of nasolabial folds. J Cosmet Dermatol 14: 27-32.
- Moers MM, Sherwood S (2013) Polycaprolactone for the correction of nasolabaial folds: A 24-month, prospective, randomized, controlled clinical trial. Dermatol Surg 39: 457-463.
Citation: Chang D (2017) What are the Safety and Efficacy of Calcium Hydroxyapatite and Polycaprolactone in Adults for Facial Rejuvenation? Cosmetol & Oro Facial Surg 3: 124.
Copyright: © 2017 Chang D. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 4388
- [From(publication date): 0-2017 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 3533
- PDF downloads: 855