Weight Loss is Associated with Changes in Gut Microbiome: A Randomized, Cross-Over Trial Comparing a Mediterranean and a Low-Fat Vegan Diet in Overweight Adults
Received: 10-May-2021 / Accepted Date: 24-May-2021 / Published Date: 31-May-2021 DOI: 10.4172/2165-7904.1000443
Abstract
Background: Mediterranean and vegan diets improve body weight and gut microbiome composition. The aim of this study was to compare both diets head-to-head.
Methods: Randomized cross-over trial, conducted February-October 2019. Sixty-two overweight adults were assigned to each diet for 16-week periods in random order, separated by a 4-week washout. Body weight was the primary outcome. Secondary measures included changes in gut microbiome which was measured using 16S rRNA sequencing.
Results: Body weight decreased on the vegan compared with the Mediterranean diet (treatment effect -6.0 kg [95% CI -7.5 to -4.5]; p<0.001). The relative abundance of Bacteriodetes decreased (p<0.001 for both diets) and Eubacteria increased on both diets (p<0.001 for the Mediterranean and p=0.009 for the vegan diet). The relative abundance of Lachnospiraceae increased (p=0.03), the Firmicutes to Bacteroidetes ratio increased (p=0.04) and the butyrate-producing bacteria decreased (p=0.02) on the Mediterranean diet. The relative abundance of Proteobacteria decreased (p<0.001), and Enterobacteria and Ruminococcus increased on the vegan diet (p=0.04 and p<0.001, respectively). Changes in body weight correlated positively with changes in relative abundance of Firmicutes both on the Mediterranean (r=+0.36; p=0.01) and the vegan diet (r=+0.41; p<0.001) and with changes in relative abundance of Lachnospiraceae both on the Mediterranean (r=+0.40; p<0.001) and the vegan diet (r=+0.44; p<0.001). In addition, the changes in body weight correlated negatively with changes in relative abundance of Enterobacteria on the Mediterranean diet (r=-0.32; p=0.02) and Eubacteria on the vegan diet (r=- 0.49; p<0.001).
Conclusions: A low-fat vegan diet led to a greater weight loss compared with a Mediterranean diet. This may be partly explained by the difference in gut microbiome composition.
Trial registration: ClinicalTrials.gov number, NCT03698955.
Keywords: Diet; Gut microbiome; Mediterranean; Nutrition; Vegan; Weight
Abbreviations
BMI, Body Mass Index; HbA1c: Glycated Hemoglobin; HOMA-IR: Homeostasis Model Assessment Insulin Resistance; OGIS: Oral Glucose Insulin Sensitivity; PREDIM: Predicted Insulin Sensitivity Index
Introduction
The gut microbiome plays an important role in human physiology and health. Dysbiosis of the gut microbiota has been linked to metabolic syndrome, obesity, and type 2 diabetes [1]. Evidence from previous studies suggest that obese people have a reduced ratio of Bacteroidetes to Firmicutes [2]. Importantly, abundance, richness, and the types of bacteria present are influenced by diet [3]. However, the most effective dietary approach is a topic of debate. Research has demonstrated a beneficial effect to gut microbiota for plant-based dietary patterns, particularly vegan and Mediterranean diets, both of which have been shown to increase the short chain fatty acid-producing bacteria [4,5]. Plant foods, which are high in fiber, are staples in both the Mediterranean and vegan diet. The high fiber content in both diets selects for bacteria that ferment fiber as a precursor to producing short chained fatty acids. These microbiota-mediated short chained fatty acids seem to play an important role in cardiometabolic health [6].
Both Mediterranean and vegan diets emphasize the consumption of plant-based foods, including fruits, vegetables, whole grains, and legumes. However, while the Mediterranean diet includes fish and white meat, dairy products, eggs, and wine, and favors olive oil as the primary source of fat [7], a vegan diet eliminates all animal-derived products. While both diets tend to promote a normal BMI [8,9], vegan diets seem to have a greater weight loss effect than Mediterranean diets when compared head to head [10]. This difference may be partly explained by differences in gut microbiota. While higher Mediterranean diet scores are associated with greater abundance of Prevotella [11], Faecalibacterium prausnitzii is typically noted in greater abundance in vegans and vegetarians than omnivores [12].
In this secondary analysis of a randomized crossover trial, which compared a Mediterranean and low-fat vegan diet head to head in overweight adults [13], we predicted that a 16-week dietary intervention would result in 1) a greater abundance of Bacteroidetes relative to Firmcutes on both diets, 2) a greater abundance of Prevotella on the Mediterranean diet, and 3) a greater abundance of Faecalibacterium prausnitzii on the vegan diet. Furthermore, we anticipated that 4) these changes would be associated with changes in body weight.
Materials and Methods
Study design and Eligibility
The methods have been described in detail previously [13]. Briefly: This randomized, cross-over trial took place between February and October 2019 in Washington, DC. We enrolled adults, age 30-76 years, with a body mass index between 28-40 kg/m2. Exclusion criteria included type I diabetes, smoking, pregnancy or lactation, alcohol or drugs abuse, or already following a vegan or Mediterranean diet. The study protocol was approved by the Advarra Instititutional Revew Board, located in Columbia, MD, USA, on September 20, 2018 (protocol identification number Pro00029777). The study was registered on ClinicalTrials.gov (ID: NCT03698955). All participants gave informed, written consent.
Randomization and study groups
Participants were randomized in a 1:1 ratio into 2 groups. Group 1 started with a Mediterranean diet for 16 weeks, followed by a 4-week wash-out period, and then switched to a low-fat vegan diet for 16 weeks. Group 2 followed a low-fat vegan diet for 16 weeks, and after a 4-week wash-out period, they adopted the Mediterranean diet for 16 weeks. Participants were assessed at weeks 0, 16, 20, and 36.
The Mediterranean diet was based on the PREDIMED protocol [14], which includes ≥ 2 servings/day of vegetables, ≥ 2-3 servings/ day of fresh fruits, ≥ 3 servings/week of legumes, ≥ 3 servings/week of fish or shellfish, and ≥ 3 servings/week of nuts or seeds, and selects lean white meats over red meats. Participants were discouraged from consuming cream, butter, margarine, processed meats, sweetened beverages, pastries, and processed snacks. Participants were instructed to use 50 g of extra virgin olive oil as their main culinary fat.
The low-fat vegan diet consisted of fruits, vegetables, grains, and legumes. Animal products and added fats were excluded and vitamin B12 was supplemented (500 μg/day).
Both diets were ad libitum diets, with no meals provided for either intervention. Alcohol was limited to one beverage/day for women, and two beverages/day for men. Participants were instructed to keep their physical activity and prescribed medications constant, unless otherwise directed by their personal physicians. Participants attended weekly classes specific to their assigned diets for the whole intervention period.
Participants submitted a 3-day diet record at weeks 0, 16, 20, and 36. Dietary data was reviewed and analyzed by a Registered Dietitian or a team member certified in Nutrition Data System for Research version 2018 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) [15]. Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) [16].
Outcomes
The following measurements were completed at weeks 0, 16, 20, and 36 after participants fasted overnight for 10-12 hours.
Anthropometrics: A scale calibrated for accuracy to 0.1 kg was used to measure body weight. Dual energy x-ray absorptiometry (Lunar iDXA, GE Healthcare; Madison, WI) with Encore® 2005 v.9.15.010 software, equipped with the CoreScan module (GE Healthcare, Madison, WI) was used to measure body composition.
Gut microbiota composition
The participants received uBiome ExplorerTM kits (uBiome, Inc., San Francisco, CA) and provided a small stool sample into the collection tube that contained a lysis and stabilization buffer. The uBiome’s laboratory performed DNA extraction, next-generation sequencing of the V4 region of the 16S rRNA gene, and analysis. The methods have been described in detail previously [17]. Briefly: For DNA extraction, bead-beating was used to lyse the samples. universal primers (515F: GTGCCAGCMGCCGCGGTAA and 806R: GGACTACHVGGGTWTCTAAT) were used to amplify the V4 variable region of the 16S rRNA gene, using PCR. qPCR was used for quantification with the Kapa Bio-Rad iCycler qPCR kit on a BioRad MyiQ. Pair-end Illumina NextSeq 500 was used for sequencing, giving 2 x 150 bp paired- end sequences.
Using the BCL2FASTQ software, the samples were demultiplexed and fastq files were generated. Only reads with Q-score ≥ 30 were included in the analysis. The primers were removed and forward and reverse reads were put together and clustered, using the Swarm algorithm, version 2.1.5 [18]. The most abundant sequence per cluster was considered the real biological sequence and was assigned the count of all reads in the cluster. Chimera sequences were removed using the VSEARCH algorithm [19]. Reads passing all the filtered reads were aligned against a database of target 16S rRNA gene sequences and taxonomic annotations derived from the SILVA database, version 132 [20,21]. The relative abundance of each taxon was counted by dividing the number linked to that taxa by the total amount of filtered reads. An abundance-weighted phylogenetic diversity measure [22] was used to calculate alpha diversity.
Statistical analysis
The statistical analysis was performed in all participants with complete data across all timepoints. A cross-over ANOVA model was used with between-subject and within-subject factors and interactions. Factors diet (Mediterranean and vegan), subject, time (week), period (1 and 2) were included in the model. Period-specific estimates of treatment effects were also determined for certain outcomes. Within each intervention, paired comparison t-tests were calculated to test whether the changes from baseline to 16 weeks in each treatment period were statistically significant. The statistician was blinded to the theorized effects of interventions and group assignment. Results are presented as means with dual 95% confidence intervals (CI). The treatment difference is the mean difference between outcomes on the vegan versus the Mediterranean diet.
Results
Participant characteristics
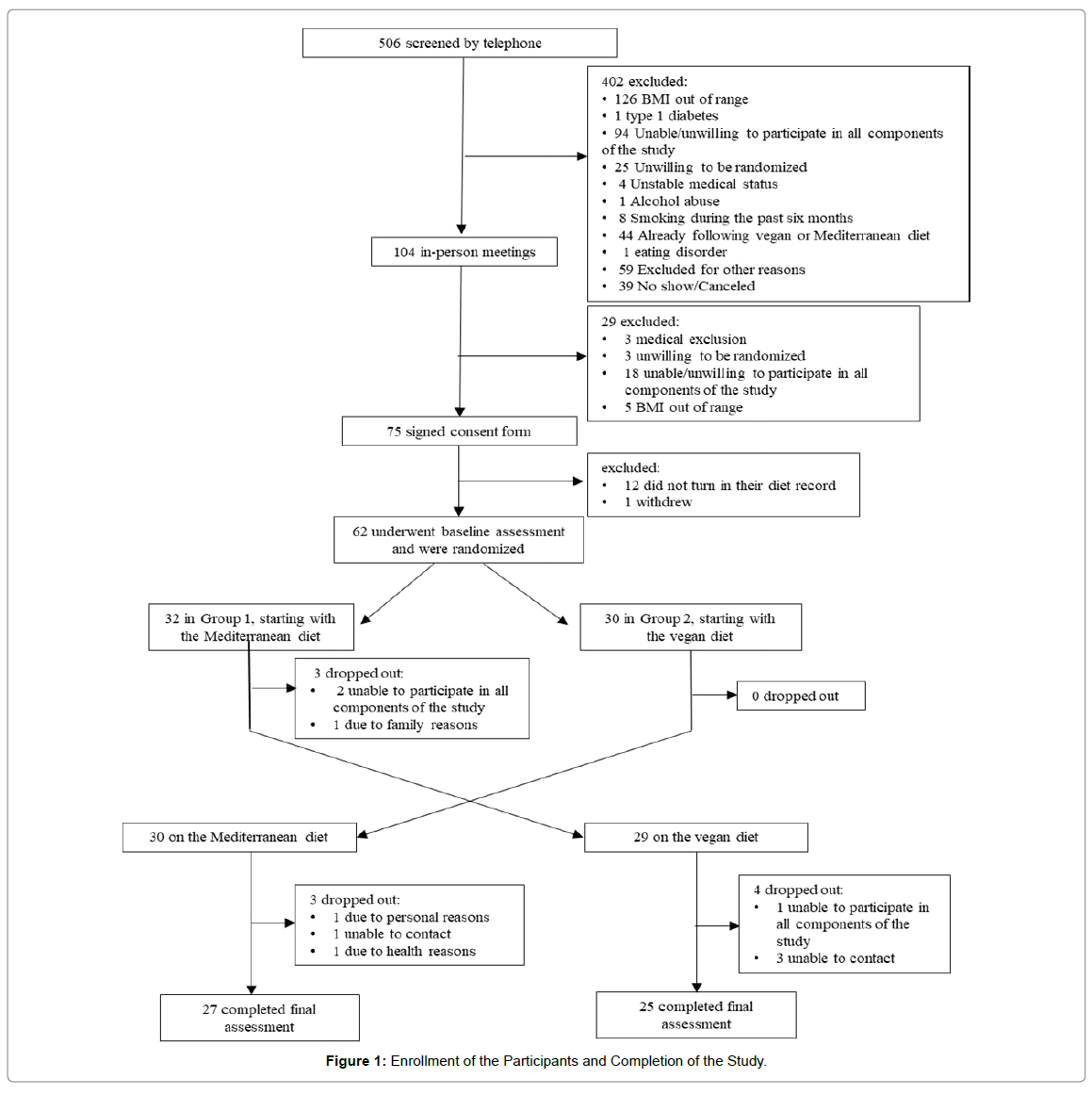
Of 506 people screened by telephone, 62 met participation criteria and were randomly assigned to start with the Mediterranean (n=32) or the vegan diet (n=30) diet (Figure 1). Demographic characteristics are listed in Table 1. There were no significant differences between the groups. Fifty-two participants (84%) study completed the whole study. Changes in dietary intake, physical activity, body weight, and gut microbiome are presented in Table 2.
| Characteristic | Group 1 (n=32) |
Group 2 (n=30) |
P-Value |
|---|---|---|---|
| Age (years) | 56.6 | 58.3 | 0.50 |
| Sex (number, %) | |||
| Female | 26 (81.3) | 22 (73.3) | 0.46 |
| Male | 6 (18.8) | 8 (26.7) | |
| Race, (number, %) | |||
| White | 15 (46.9) | 16 (53.3) | 0.90 |
| Black | 16 (50.0) | 14 (46.7) | |
| Asian, Pacific Islander | 0 (0.0) | 0 (0.0) | |
| American Indian, Eskimo, Aleut | 1 (3.1) | 0 (0.0) | |
| Not disclosed | 0 (0.0) | 0 (0.0) | |
| Ethnicity, (number, %) | |||
| Non-Hispanic | 23 (71.9) | 23 (76.7) | 0.14 |
| Hispanic | 3 (9.4) | 0 (0.0) | |
| Not disclosed | 6 (18.8) | 7 (23.3) | |
| Marital status | |||
| Not married | 15 (46.9) | 15 (50.0) | 0.71 |
| Married | 17 (53.1) | 14 (46.7) | |
| Not disclosed | 0 (0.0) | 1 (3.3) | |
| Education | |||
| High school | 0 (0.0) | 0 (0.0) | 0.28 |
| Associates | 7 (21.9) | 5 (16.7) | |
| College | 13 (40.6) | 9 (30.0) | |
| Graduate degree | 12 (37.5) | 16 (53.3) | |
| Occupation | |||
| Service occupation | 7 (21.9) | 4 (13.3) | 0.29 |
| Technical, sales, administrative | 8 (25.0) | 9 (30.0) | |
| Professional or managerial | 2 (6.3) | 7 (23.3) | |
| Retired | 7 (21.9) | 6 (20.0) | |
| Other | 8 (25.0) | 4 (13.3) | |
| Medications | |||
| Lipid-lowering therapy (%) | 12 (37.5) | 11 (36.7) | 0.95 |
| Antihypertensive therapy (%) | 16 (50.0) | 14 (46.7) | 0.79 |
| Thyroid medications (%) | 3 (9.4) | 1 (3.3) | 0.61 |
| Physical Activity (METs) | 2289.7 | 2665.5 | 0.70 |
| Energy intake (kcals) | 1825.8 | 1911.8 | 0.54 |
| Anthropometrics | |||
| Body weight (kg) | 97.6 | 98.4 | 0.80 |
| BMI (kg/m2) | 34.3 | 33.7 | 0.42 |
| Fat mass (g) | 43.9 | 41.5 | 0.17 |
| Lean mass (g) | 51.5 | 54.1 | 0.25 |
| VAT volume (cm3) | 2017.4 | 2126.7 | 0.68 |
| Lipids | |||
| Total cholesterol (mg/dL) | 203.3 | 202.2 | 0.93 |
| LDL-cholesterol (mg/dL) | 119.9 | 116.6 | 0.76 |
| HDL-cholesterol (mg/dL) | 58.8 | 56.4 | 0.53 |
| HbA1c | 5.8 | 5.8 | 0.93 |
Table 1: Baseline Characteristics of the Study Population. Data are means ± SD, or number (%). P-values refer to t-tests for continuous variables and χ2 or Fisher’s exact test for categorical variables. The P-value calculated for ethnicity distribution is for the comparison between Hispanic vs. non-Hispanic categories and all other comparisons also exclude undisclosed datapoints. Group 1 started with the Mediterranean diet and Group 2 started with the vegan diet.
| Variable | Mediterranean Baseline |
Mediterranean Final |
DMediterranean | Vegan Baseline | Vegan Final | DVegan | Treatment Effect | P-value |
|---|---|---|---|---|---|---|---|---|
| Dietary intake | ||||||||
| Energy (kcal) | 1776 (1625 to 1928) | 1855 (1699 to 2011) | +79 (-120 to +277) | 1815 (1649 to 1982) | 1315 (1191 to 1440) | -500 (-639 to -362)*** | -579 (-801 to -357) | <.001 |
| % Calories from Fat | 35 (33 to 38) | 43 (40 to 45) | +7 (+4 to +10)*** | 38 (36 to 40) | 17 (15 to 19) | -21 (-24 to -18)*** | -28 (-32 to -24) | <.001 |
| % Calories from Carbohydrate | 47 (43 to 50) | 40 (37 to 42) | -7 (-10 to -4)*** | 42 (40 to 45) | 69 (66 to 71) | +26 (+23 to +29)*** | +33 (+29 to +37) | <.001 |
| % Calories from Protein | 16 (15 to 18) | 15 (14 to 16) | -1 (-3 to 0) | 18 (17 to 19) | 12 (12 to 13) | -6 (-7 to -4)*** | -5 (-6 to -3) | <.001 |
| Alcohol (g) | 5 (2 to 8) | 5 (3 to 7) | 0 (-3 to +3) | 5 (3 to 8) | 3 (2 to 5) | -2 (-4 to 0)* | -2 (-5 to +2) | 0.31 |
| Cholesterol (mg) | 242 (188 to 296) | 217 (179 to 256) | -25 (-93 to +43) | 292 (251 to 334) | 19 (1 to 37) | -273 (-317 to -230)*** | -248 (-331 to -166) | <.001 |
| % Calories from SFA | 10 (9 to 11) | 9 (8 to 9) | -1 (-2 to 0)* | 11 (10 to 12) | 4 (3 to 4) | -8 (-9 to -6)*** | -6 (-8 to -5) | <.001 |
| % Calories from MUFA | 14 (13 to 16) | 22 (20 to 24) | +8 (+6 to +10)*** | 15 (14 to 16) | 6 (5 to 7) | -9 (-11 to -8)*** | -17 (-20 to -14) | <.001 |
| % Calories from PUFA | 9 (8 to 9) | 9 (9 to 10) | +1 (-0 to +2) | 9 (8 to 10) | 6 (6 to 7) | -3 (-4 to -2)*** | -4 (-5 to -2) | <.001 |
| Total Fiber (g) | 23 (22 to 28) | 29 (26 to 32) | +5 (+2 to +7)*** | 22 (19 to 26) | 33 (29 to 37) | +10 (+8 to +13)*** | +6 (+3 to +9) | <.001 |
| Soluble Fiber (g) | 7 (6 to 8) | 7 (7 to 8) | 0 (0 to +1) | 6 (5 to 7) | 9 (7 to 10) | +2 (+1 to +3)*** | +2 (+1 to +3) | 0.002 |
| Insoluble Fiber (g) | 18 (16 to 20) | 22 (20 to 24) | +4 (+2 to +6)*** | 16 (14 to 18) | 24 (21 to 27) | +8 (+6 to +10)*** | +4 (+1 to +7) | 0.007 |
| Physical activity | ||||||||
| Physical Activity (MET) | 2384 (1693 to 3074) | 2740 (2073 to 3406) | +356 (-127 to +839) | 2585 (1466 to 3705) | 2952 (1822 to 4081) | +366 (-711 to +1444) | +10 (-1251 to +1271) | 0.99 |
| Anthropometric variables and body composition | ||||||||
| Body weight (kg) | 94.5 (90.9 to 98.1) | 94.5 (90.7 to 98.3) | +0.0 (-0.9 to +0.9) | 97.3 (93.6 to 101.0) | 91.3 (87.4 to 95.3) | -6.0 (-7.2 to -4.9)*** | -6.0 (-7.5 to -4.5) | <.001 |
| But Microbiota | ||||||||
| Prevotella (%) | 2.6 (0.2 – 5.1) | 5.7 (1.8 – 9.5) | +3.0 (-1.3 to +7.4) | 4.0 (1.0 – 7.0) | 4.2 (0.94 – 7.5) | +0.20 (-3.7 to +4.1) | -2.8 (-10.2 to +4.6) | 0.45 |
| Akkermansia (%) | 1.2 (0.49 – 1.9) | 1.6 (0.11 – 3.1) | +0.43 (-0.68 to +1.5) | 2.2 (0.0 – 4.3) | 1.3 (0.47 – 2.1) | -0.85 (-2.6 to +0.91) | -1.3 (-3.9 to +1.3) | 0.33 |
| Faecalibacterium prausnitzii (%) | 9.2 (4.4 – 14.0) | 7.4 (6.0 – 8.9) | -1.7 (-6.6 to +3.2) | 6.0 (4.8 – 7.2) | 7.2 (5.8 – 8.7) | +1.2 (-0.15 to +2.61) | +3.0 (-2.3 to +8.2) | 0.26 |
| Firmicutes (%) | 50.9 (47.0 – 54.9) | 55.8 (48.9 – 62.6) | +4.8 (-2.9 to +12.6) | 49.0 (44.4 – 53.7) | 54.4 (49.0 – 59.9) | +5.4 (-2.5 to +13.3) | +0.59 (-14.0 to +15.1) | 0.94 |
| Bacteroidetes (%) | 30.8 (27.3 – 34.3) | 20.2 (16.8 – 23.7) | -10.6 (-14.2 to -6.9)*** | 33.0 (29.5 – 36.5) | 22.4 (19.2 – 25.6) | -10.6 (-13.9 to -7.3)*** | -0.04 (-5.8 to +5.8) | 0.99 |
| Enterobacteriaceae (%) | 0.61 (0.07 – 1.2) | 0.62 (-0.04 – 1.3) | +0.01 (-0.55 to +0.56) | 0.06 (0.01 – 0.12) | 0.35 (0.07 – 0.63) | +0.28 (+0.01 to +0.56)* | +0.28 (-0.36 to +0.92) | 0.38 |
| Bacteroides Fragilis (%) | 0.17 (0.05 – 0.29) | 0.10 (0.02 – 0.17) | -0.08 (-0.15 to -0.002)* | 0.28 (0.05 – 0.50) | 0.22 (0.07 – 0.37) | -0.06 (-0.21 to +0.09) | +0.02 (-0.14 to +0.17) | 0.82 |
| Clostridium (%) | 0.65 (0.36 – 0.95) | 0.40 (0.14 – 0.66) | -0.25 (-0.64 to +0.13) | 0.64 (0.38 – 0.89) | 0.76 (0.33 – 1.18) | +0.12 (-0.38 to +0.62) | +0.37 (-0.37 to +1.1) | 0.32 |
| Methanobrevibacter (%) | 0.71 (0.19 – 1.2) | 0.91 (-0.09 – 1.9) | +0.20 (-0.44 to +0.84) | 0.56 (0.05 – 1.1) | 0.78 (-0.08 – 1.6) | +0.21 (-0.26 to +0.69) | +0.01 (-0.47 to +0.50) | 0.96 |
| Eubacterium (%) | 0.19 (0.09 – 0.29) | 0.82 (0.40 – 1.2) | +0.63 (+0.16 to +1.1)** | 0.16 (0.06 – 0.26) | 0.96 (0.57 – 1.3) | +0.80 (+0.37 to +1.2)*** | +0.18 (-0.61 to +0.96) | 0.65 |
| E Coli (%) | 0.00 (0.00 – 0.00) | 0.59 (-0.08 – 1.3) | +0.59 (-0.08 to +1.3) | 0.00 (0.00 – 0.01) | 0.04 (0.00 – 0.08) | +0.04 (-0.01 to +0.08) | -0.55 (-1.2 to +0.12) | 0.11 |
| Bifidobacterium (%) | 1.7 (0.9 – 2.6) | 1.2 (0.61 – 1.9) | -0.49 (-1.1 to +0.08) | 1.2 (0.62 – 1.8) | 1.9 (0.83 – 3.0) | +0.72 (-0.06 to +1.5) | +1.2 (+0.25 to +2.2) | 0.02 |
| Proteobacteria (%) | 3.3 (2.4 – 4.1) | 2.6 (1.7 – 3.6) | -0.64 (-1.6 to +0.28) | 4.0 (2.8 – 5.1) | 1.7 (1.2 – 2.1) | -2.3 (-3.3 to -1.4)*** | -1.7 (-3.2 to -0.23) | 0.02 |
| Actinobacteria (%) | 2.3 (1.1 – 3.4) | 2.0 (1.1 – 2.9) | -0.27 (-2.0 to +1.5) | 1.7 (1.0 – 2.5) | 2.8 (1.4 – 4.1) | +1.0 (-0.84 to +2.9) | +1.3 (-2.1 to +4.6) | 0.44 |
| Ruminococcaceae (%) | 16.7 (12.1 – 21.3) | 21.0 (17.9 – 24.1) | +4.3 (-0.4 to +9.0) | 14.9 (12.7 – 17.0) | 21.5 (18.9 – 24.1) | +6.6 (+3.6 to +9.7)*** | +2.3 (-3.6 to +8.2) | 0.43 |
| Lachnospiraceae (%) | 17.8 (15.5 – 20.2) | 25.3 (20.0 – 30.6) | +7.5 (+0.88 to +14.0)* | 19.3 (16.0 – 22.7) | 24.6 (19.9 – 29.3) | +5.3 (-1.4 to +12.0) | -2.2 (-14.7 to +10.3) | 0.73 |
| Roseburia (%) | 3.6 (2.4 – 4.8) | 3.5 (2.6 – 4.4) | -0.08 (-1.3 to +1.1) | 4.7 (3.3 – 6.0) | 4.3 (3.3 – 5.2) | -0.40 (-1.5 to +0.72) | -0.32 (-2.1 to +1.4) | 0.72 |
| Anaerostipes (%) | 1.7 (1.2 – 2.3) | 1.3 (0.77 – 1.9) | -0.37 (-0.85 to +0.11) | 1.6 (0.98 – 2.2) | 1.8 (1.3 – 2.2) | +0.15 (-0.45 to +0.74) | +0.52 (-0.31 to +1.3) | 0.22 |
| Megasphaera (%) | 0.18 (-0.08 – 0.44) | 0.09 (0.01 – 0.18) | -0.09 (-0.32 to +0.15) | 0.15 (0.03 – 0.27) | 0.12 (-0.08 – 0.33) | -0.03 (-0.15 to +0.10) | +0.06 (-0.30 to +0.42) | 0.74 |
| Firmicutes:Bacteroidetes Ratio | 2.3 (1.0 – 3.5) | 3.9 (2.9 – 4.9) | +1.6 (+0.05 to +3.2)* | 2.7 (0.5 – 4.8) | 3.3 (2.6 – 4.0) | +0.60 (-1.5 to +2.7) | -1.0 (-3.7 to +1.7) | 0.45 |
| Butyrate Producing Bacteria | 29864 (24808 – 34920) | 22588 (18919 – 26256) | -7277 (-13141 to -1412)* | 25988 (21189 – 30788) | 25688 (20013 – 31362) | -300.7 (-6869 to +6267) | +6976 (-1255 to +15206) | 0.09 |
| Diversity | 1.8 (1.7 – 1.9) | 1.5 (1.4 – 1.6) | -0.34 (-0.49 to -0.18)*** | 1.7 (1.6 – 1.8) | 1.5 (1.4 – 1.6) | -0.23 (-0.39 to -0.07)** | +0.11 (-0.12 to +0.33) | 0.34 |
Table 2: Changes in outcomes during the study comparing a Mediterranean and low-fat vegan diet, using a standard crossover-trial model, comparing outcome changes on each diet while taking within-subject correlation into account. Data are means with 95% confidence intervals. Listed P values are for interaction between group and time assessed by repeated measures ANOVA. *p<0.05, **p<0.01 and ***p<0.001 for within-group changes from baseline assessed by paired comparison t tests. The treatment difference is the mean (average) difference between participant outcomes on the vegan versus the Mediterranean diet.
Dietary intake
Dietary intake has been described in detail previously [13]. Briefly: Based on the self-reported diet records, energy intake slightly decreased on the vegan diet (p<0.001) compared with no change on the Mediterranean diet. The percentage of energy coming from carbohydrates increased on the vegan (p<0.001) and decreased on the Mediterranean diet (p<0.001). Energy consumed from fat decreased on the vegan (p<0.001) but increased on the Mediterranean diet (p<0.001), mainly coming from monounsaturated fat (p<0.001). We observed an increase in fiber intake on both diets, which was greater on the vegan diet (p<0.001).
Body weight and body composition
The participants lost 6.0 kg on the vegan diet on average, compared with no mean weight loss on the Mediterranean diet (between-group p<0.001), most of the weight loss coming from the reduction in fat (Table 2).
Gut microbiome
Changes in gut microbiome composition are summarized in Table 2. The α-diversity, which is the measure of microbial diversity within each sample, decreased on both diets (p<0.001 for the Mediterranean and p=0.006 for the vegan diet). The relative abundance of Bacteriodetes decreased (p<0.001 for both diets) and Eubacteria increased on both diets (p<0.001 for the Mediterranean and p=0.009 for the vegan diet). The relative abundance of Bacteriodes fragilis decreased on the Mediterranean diet (p=0.04). The relative abundance of Lachnospiraceae increased (p=0.03), the Firmicutes to Bacteroidetes ratio increased (p=0.04) and the butyrate-producing bacteria decreased (p=0.02) on the Mediterranean diet. The relative abundance of Proteobacteria decreased (p<0.001), and Enterobacteria and Ruminococcus increased on the vegan diet (p=0.04 and p<0.001, respectively).
Changes in body weight correlated positively with changes in relative abundance of Firmicutes both on the Mediterranean (r=+0.36; p=0.01) and the vegan diet (r=+0.41; p<0.001) and with changes in relative abundance of Lachnospiraceae both on the Mediterranean (r=+0.40; p<0.001) and the vegan diet (r=+0.44; p<0.001). All these correlations remained significant and at the same magnitude even after adjustment for changes in energy intake: r=+0.36; p=0.01 for Firmicutes on the Mediterranean and r=+0.36; p=0.02 on the vegan diet; r=+0.39; p=0.01 for Lachnospiraceae on the Mediterranean and r=+0.36; p=0.02 on the vegan diet. In addition, the changes in body weight correlated negatively with changes in relative abundance of Enterobacteria on the Mediterranean diet (r=-0.32; p=0.02) and Eubacteria on the vegan diet (r=-0.49; p<0.001), even after the adjustment for changes in energy intake (r=-0.34; p=0.02; and r=-0.42; p<0.001, respectively).
Discussion
In this 36-week randomized cross-over trial, a low-fat vegan diet led to greater reductions in body weight compared with a Mediterranean diet. The relative abundance of Bacteriodetes decreased and Eubacteria increased on both diets. The relative abundance of Lachnospiraceae increased, the Firmicutes to Bacteroidetes ratio increased and the butyrate-producing bacteria decreased on the Mediterranean diet. The relative abundance of Proteobacteria decreased, and Enterobacteria and Ruminococcus increased on the vegan diet. Changes in body weight correlated positively with changes in relative abundance of Firmicutes and Lachnospiraceae on both diets. In addition, changes in body weight correlated negatively with changes in relative abundance of Enterobacteria on the Mediterranean diet and Eubacteria on the vegan diet.
We hypothesized a greater increase in the abundance of Bacteroidetes relative to Firmicutes on both diets. However, in the present study, we found Bacteroidetes to significantly decrease in both groups and the ratio of Firmicutes to Bacteroidetes to increase. This contradicts the current literature which shows a higher abundance of Bacteroidetes in vegans and vegetarians compared to omnivores [23– 25]. For instance, in one study, the bacterial composition of Indian adults was compared to that of Chinese adults. Both groups ate a diet that emphasized whole, plant-based foods, however, the Chinese adults ate more animal fat and protein. The microbiomes of the Indian adults were found to have nearly four times the percentage of Bacteroidetes than in the Chinese adults, 16.39% versus 4.27%, respectively (p=0.001). This may be explained by their lower intake of animal products [25], which supports our initial hypothesis.
Additionally, it has been shown that the Bacteroidetes to Firmicutes ratio correlates negatively with BMI, meaning a greater abundance of Bacteroidetes relative to Firmicutes is associated with a lower BMI [26]. Compared to non-obese individuals, obese individuals have been shown to have a three-fold less relative abundance of the Bacteroidetes phylum and a greater abundance of Firmicutes [27]. Although we observed the opposite: the body weights of the vegan participants decreased significantly while the ratio of Firmicutes to Bacteroidetes increased, we did note a moderate, positive association between changes in body weight with changes in relative abundance of Firmicutes on both diets.
In this study, the relative abundance of Prevotella increased insignificantly under both interventions, with a greater increase under the Mediterranean diet. This finding supports our second hypothesis as well as the current literature. Plant-based foods are high in a type of carbohydrate called polysaccharides. Prevotella is a polysaccharidedegrading bacteria, and thus, a diet high in polysaccharides is beneficial for bacterial substrate utilization [28,29]. Additionally, significant associations between vegetable-based diets and the abundance of Prevotella have been shown [30].
Our third hypothesis, which predicted an increase in the abundance of Faecalibacterium prausnitzii on the vegan diet, was also correct, although the increase was insignificant. Faecalibacterium prausnitzii has been shown to be more abundant on vegetarian and vegan diets [31]. Populations that consume higher amounts of resistant starch in place of protein and fat, which reflects the diet composition of this study’s vegan intervention, have greater abundance of Faecalibacterium prausnitzii [32]. High-fiber diets most likely support the growth of Faecalibacterium prausnitzii due to the role that this species plays in degrading plant polysaccharides and starch to produce health-promoting short chain fatty acids [33,34]. While we observed a decrease in Faecalibacterium prausnitzii under the Mediterranean diet, a Mediterranean-style diet, in addition to a high-fiber diet and a vegetable-rich macrobiotic diet, has been associated with an increased in Faecalibacterium among individuals with type 2 diabetes [34].
We also observed significant changes in the relative abundance of butyrate producing bacteria, Enterobacteria, Ruminococcace and Proteobacteria. Based on the current literature, we would expect to see an increase in butyrate producing bacteria, however, we observed a significant decrease on the Mediterranean diet. Butyrate, along with acetate and propionate, is a short chain fatty acid that has been shown to increase in individuals with the highest adherence to a Mediterranean diet [35] and in individuals following a strict vegan or vegetarian diet [30].
The significant increase in Enterobacteria under the vegan diet is also in opposition to previous research. Zimmer et al. noted lower Enterobacteriaceae species in vegans than controls [36]. Kim et al. also observed a decrease with a strict vegetarian diet [28]. However, we did observe a negative correlation between changes in body weight and changes in relative abundance of Enterobacteria on the Medtierranean diet (r=-0.32; p=0.02) that remained significant after adjusting for changes in energy intake (r=-0.34, p=0.02).
It is less clear as to how to results of Ruminococcace line up against previous research as there is less of a clear trend. In one study examining changes in microbiota on an animal-based diet versus a plant based diet, the animal based diet decreased Ruminococcus [37]. Conversely, Ruminococcus was associated with omnivorous diets, as opposed to vegan vegetarian diets, in another study [30]. Lastly, our Protobacteria results are supported by other studies. A study examining the microbiome of undernourished and obese children in Mexico found a positive correlation between proteobacteria and fat intake, in addition to greater abundance of Rroteobacteria in obese children. This is consistent with our study findings, with Proteobacteria being decreased after the low-fat vegan dietary intervention, and is in line with the significant weight loss in this group [38]. In another study, which examined microbiota in relation to Mediterranean diet adherence, lower intake of polysaccharides was related to a higher relative abundance of Rroteobacteria (P=0.028) [35].
Study strengths and limitations
The strengths of the study include the randomized cross-over design and the reasonably long study duration provided sufficient time for adaptation to the diet and to capture microbiome changes. The weekly classes provided for participants also were a strength in facilitating adherence. Despite the long trial length, we achieved a high level of retention (84%), in accordance with our previous findings [39]. Importantly, the results of this free-living study are applicable for general population.
We also need to admit some limitations. Dietary adherence was high, but it has been shown previously that self-reported data may not always accurately reflect real dietary intake [40]. However, it is reassuring that the reported changes in dietary intake were accompanied by changes in body weight and gut microbiome. Furthermore, our participants were generally health-conscious individuals who were willing to change their diet. Therefore, they may not be representative of the general population, but rather of a population seeking advice how to lose weight.
Conclusion
In conclusion, this 36-week randomized cross-over trial showed that a low-fat plant-based diet reduced body weight compared with a Mediterranean diet. This may be partly explained by the difference in gut microbiome composition.
Declarations
Ethics approval and consent to participate: The study protocol was approved by the Advarra Instititutional Revew Board, located in Columbia, MD, USA, on September 20, 2018 (protocol identification number Pro00029777). The study was registered on ClinicalTrials.gov (ID: NCT03698955). All participants gave informed, written consent.
Consent for publication: All authors had full access to the data and approved the final version of the manuscript for publication.
Availability of data and materials: The deidentified data will be available upon request at hkahleova@pcrm.org
Competing Interests: All authors except for MA, RC, and RH work for the Physicians Committee for Responsible Medicine in Washington, DC, a nonprofit organization providing educational, research, and medical services related to nutrition. Dr. Barnard is an Adjunct Professor of Medicine at the George Washington University School of Medicine. He serves without compensation as President of the Physicians Committee for Responsible Medicine and the Barnard Medical Center in Washington, DC. He writes books and articles and gives lectures related to nutrition and health and has received royalties and honoraria from these sources.
Funding: This work was funded by the Physicians Committee for Responsible Medicine.
Authors’ contributions: HK and NDB designed the research study and drafted the manuscript. ER, JA, and AN wrote sections of the manuscript. HK, ER, JA, and AN conducted the research study. MA and RC analyzed the gut microbiome samples and helped with the data interpretation. RH analyzed the data and performed statistical analyses. HK and NDB reviewed and approved the submitted version. All authors had full access to data and revised and approved the manuscript for publication.
References
- Khan MT, Nieuwdorp M, Bäckhed F. Microbial Modulation of Insulin Sensitivity. Cell Metab. 2014 Nov 4;20(5):753–60.
- Arora T, Sharma R. Fermentation potential of the gut microbiome: implications for energy homeostasis and weight management. Nutr Rev. 2011 Feb;69(2):99–106.
- Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016 Jul 7;535(7610):56–64.
- Glick-Bauer M, Yeh M-C. The Health Advantage of a Vegan Diet: Exploring the Gut Microbiota Connection. Nutrients. 2014 Oct 31;6(11):4822–38.
- Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front Microbiol [Internet]. 2018 [cited 2020 Jun 30];9. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2018.00890/full
- Chambers ES, Preston T, Frost G, Morrison DJ. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr Nutr Rep. 2018;7(4):198–206.
- Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev. 2006 Feb;64(2 Pt 2):S27-47.
- D’Innocenzo S, Biagi C, Lanari M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients [Internet]. 2019 Jun 9 [cited 2020 Jul 7];11(6). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6627690/
- Spencer EA, Appleby PN, Davey GK, Key TJ. Diet and body mass index in 38 000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes. 2003 Jun;27(6):728–34.
- Rogerson D, Maçãs D, Milner M, Liu Y, Klonizakis M. Contrasting Effects of Short-Term Mediterranean and Vegan Diets on Microvascular Function and Cholesterol in Younger Adults: A Comparative Pilot Study. Nutrients [Internet]. 2018 Dec 3 [cited 2020 Jul 7];10(12). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6316028/
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016 Nov;65(11):1812–21.
- Matijašić BB, Obermajer T, Lipoglavšek L, Grabnar I, Avguštin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr. 2014 Jun;53(4):1051–64.
- Barnard ND, Alwarith J, Rembert E, Brandon L, Nguyen M, Goergen A, et al. A Mediterranean Diet and Low-Fat Vegan Diet to Improve Body Weight and Cardiometabolic Risk Factors: A Randomized, Cross-over Trial. J Am Coll Nutr. 2021 Feb 5;1–13.
- Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med. 2018 21;378(25):e34.
- Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988 Oct;88(10):1268–71.
- Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006 Sep;9(6):755–62.
- Almonacid DE, Kraal L, Ossandon FJ, Budovskaya YV, Cardenas JP, Bik EM, et al. 16S rRNA gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome. PLOS ONE. 2017 May 3;12(5):e0176555.
- Mahé F, Rognes T, Quince C, de Vargas C, Dunthorn M. Swarm: robust and fast clustering method for amplicon-based studies. PeerJ. 2014 Sep 25;2:e593.
- Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016 Oct 18;4:e2584.
- Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007 Dec 1;35(21):7188–96.
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013 Jan;41(Database issue):D590–6.
- McCoy CO, Iv FAM. Abundance-weighted phylogenetic diversity measures distinguish microbial community states and are robust to sampling depth. PeerJ. 2013 Sep 12;1:e157.
- Matijašić BB, Obermajer T, Lipoglavšek L, Grabnar I, Avguštin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr. 2014 Jun;53(4):1051–64.
- Liszt K, Zwielehner J, Handschur M, Hippe B, Thaler R, Haslberger AG. Characterization of bacteria, clostridia and Bacteroides in faeces of vegetarians using qPCR and PCR-DGGE fingerprinting. Ann Nutr Metab. 2009;54(4):253–7.
- Jain A, Li XH, Chen WN. Similarities and differences in gut microbiome composition correlate with dietary patterns of Indian and Chinese adults. AMB Express [Internet]. 2018 Jun 23 [cited 2019 Feb 25];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6015586/
- Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity: Obese Gut Microbiota and Inflammation. Obesity. 2013 Dec;21(12):E607–15.
- Koliada A, Syzenko G, Moseiko V, Budovska L, Puchkov K, Perederiy V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017 May 22;17(1):120.
- Kim M-S, Hwang S-S, Park E-J, Bae J-W. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ Microbiol Rep. 2013 Oct;5(5):765–75.
- Glick-Bauer M, Yeh M-C. The health advantage of a vegan diet: exploring the gut microbiota connection. Nutrients. 2014 Nov;6(11):4822–38.
- De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–21.
- Ferrocino I, Cagno RD, Angelis MD, Turroni S, Vannini L, Bancalari E, et al. Fecal Microbiota in Healthy Subjects Following Omnivore, Vegetarian and Vegan Diets: Culturable Populations and rRNA DGGE Profiling. PLOS ONE. 2015 Jun 2;10(6):e0128669.
- Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013 Jul;98(1):111–20.
- Abell GCJ, Cooke CM, Bennett CN, Conlon MA, McOrist AL. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol Ecol. 2008 Dec;66(3):505–15.
- Candela M, Biagi E, Soverini M, Consolandi C, Quercia S, Severgnini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr. 2016;116(1):80–93.
- Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front Microbiol. 2018;9:890.
- Zimmer J, Lange B, Frick J-S, Sauer H, Zimmermann K, Schwiertz A, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012 Jan;66(1):53–60.
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014 Jan 23;505(7484):559–63.
- Méndez-Salazar EO, Ortiz-López MG, Granados-Silvestre M de los Ã, Palacios-González B, Menjivar M. Altered Gut Microbiota and Compositional Changes in Firmicutes and Proteobacteria in Mexican Undernourished and Obese Children. Front Microbiol [Internet]. 2018 [cited 2020 Jul 30];9. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02494/full
- Barnard ND, Gloede L, Cohen J, Jenkins DJA, Turner-McGrievy G, Green AA, et al. A low-fat vegan diet elicits greater macronutrient changes, but is comparable in adherence and acceptability, compared with a more conventional diabetes diet among individuals with type 2 diabetes. J Am Diet Assoc. 2009 Feb;109(2):263–72.
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, et al. Relative Validity of Nutrient Intakes Assessed by Questionnaire, 24-Hour Recalls, and Diet Records Compared With Urinary Recovery and Plasma Concentration Biomarkers: Findings for Women. Am J Epidemiol. 2017 Oct 4;
Citation: Kahleova H, Rembert E, Alwarith J, Nowak A, Agnello M, et al. (2021) Weight Loss is Associated with Changes in Gut Microbiome: A Randomized, Cross-Over Trial Comparing a Mediterranean and a Low-Fat Vegan Diet in Overweight Adults. J Obes Weight Loss Ther 11: 443. DOI: 10.4172/2165-7904.1000443
Copyright: © 2021 Kahleova H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5213
- [From(publication date): 0-2021 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 4307
- PDF downloads: 906