Research Article Open Access
Voltammetric Resolution of Dopamine in Presence of Ascorbic Acid and Uric Acid at Poly (Brilliant Blue) Modified Carbon Paste Electrode
Ganesh PS and Kumara Swamy BE*
Department of PG Studies and Research in Industrial Chemistry, Kuvempu University, Jnana Sahyadri, Shankaraghatta-577451, Shimoga, Karnataka, India
- *Corresponding Author:
- Kumara Swamy BE
Department of PG Studies and Research in Industrial Chemistry
Kuvempu University, Jnana Sahyadri,Shankaraghatta-577451
Shimoga, Karnataka, India
Tel: +91 82822562251
Fax: +91 8282 256255
E-mail: kumaraswamy21@yahoo.com
Received Date: November 03, 2014; Accepted Date: December 05, 2014; Published Date: December 10, 2014
Citation: Ganesh PS, Kumara Swamy BE (2014) Voltammetric Resolution of Dopamine in Presence of Ascorbic Acid and Uric Acid at Poly (Brilliant Blue) Modified Carbon Paste Electrode. J Anal Bioanal Tech 5:229 doi: 10.4172/2155-9872.1000229
Copyright: © 2014 Ganesh PS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Poly (brilliant blue) modified carbon paste electrode was fabricated for the detection of dopamine in the presence of large excess of ascorbic acid and uric acid in phosphate buffer solution of pH 7.4. The redox peaks obtained at poly (brilliant blue) modified carbon paste electrode shows good electrocatalytic activity towards the oxidation of dopamine. From the study of scan rate variation the electrode process was found to be adsorption controlled. The limit of detection of dopamine was found to be 6.7 × 10-7 M. and the simultaneous study shows the good result with peak to peak separation between dopamine and other two analytes ascorbic acid and uric acid by both cyclic voltammetry and differential pulse voltammetric techniques
Keywords
Poly (brilliant blue); Dopamine; Ascorbic acid; Uric acid; Electrocatalytic oxidation; Cyclic voltammetry; Differential pulse voltammetry
Introduction
Dopamine (DA), Ascorbic acid (AA), and Uric acid (UA) are the compounds that play a very important role in the field of biomedicine and neurochemistry. DA is an important neurotransmitter molecule of catecholamine which is widely distributed in the mammalian central nervous system for message transfer. It plays a very important role in the function of central nervous, renal, hormonal and cardiovascular system and medicament to drug addiction and Parkinson’s disease [1]. AA is a water soluble vitamin that takes part in many important life processes; it has been used in the prevention and treatment of common cold, mental illness and cancer [2]. It can be chemically or electrochemically oxidized to dehydroascorbic acid [3]. UA is primary product of purine metabolism [4] abnormal levels of UA are symptoms of several disease like gout, hyperuricamia [5] enhanced urate level also leads to phenumonia and leukaemia [6]. Therefore the monitoring the levels of these compounds are of great importance.
DA, AA and UA usually coexist in biological fluids, and also the direct electrochemical oxidation of DA and AA at bare electrode is reversible and requires high potential. Moreover the DA and AA get oxidized at the same potential [7-9]. Therefore the development of various techniques for detecting the DA, AA and UA of neurotransmitters in the central nervous system has attracted much attention of researchers during the past few decades. However the methods like electrophoresis [10], chromatography [11], and chemiluminescence [12] have been employed for the detection of AA, DA and UA. Among all these methods electrochemical methods have been found great applicability [13-17]. The electroanalytical methods using modified electrodes are more promising for the simultaneous detection of these electroactive species [18-21]. In recent days the modification of electrode by the electropolymerisation technique is of great importance because of its sensitivity, selectivity and more reproducible results. There were so many reports on the electropolymerisation of N, N-dimethylaniline [22] styrene sulphonic acid [23] aminobenzoic acid [19] sulfosalicylic acid [18] and glycine [24] to modify the electrode for the detection of DA in presence of AA.
The present work describes the fabrication of stable electrode by electropolymerising coomassie brilliant blue R-250(brilliant blue) on the surface of carbon paste electrode to achieve the challenge of simultaneous determination of DA in presence of high concentration of AA and UA at physiological pH. Brilliant blue is the name of triphenylmethane dye widely used for staining proteins in the analytical biochemistry [25,26] and also used as a food colourant. Although no examination of the detection of dopamine in presence of large excess of AA and UA in physiological pH at poly (brilliant blue) film coated carbon paste electrode has not been reported. The carbon paste was modified with the different quantity of brilliant blue was used for the electrochemical determination of DA in presence of AA [27] and this work mainly reports the electropolymerisation of brilliant blue on bare carbon paste electrode and used for the determination of DA in presence AA and UA. This modified carbon paste electrode shows very good enhancement when compared to brilliant blue modified carbon paste electrode. This work reports about sensitivity, selectivity, stability and reproducibility of neurotransmitter at poly (brilliant blue) film coated carbon paste electrode at physiological pH.
Experimental Section
Reagents
Dopamine hydrochloride (DA), Uric acid (UA), Ascorbic acid (AA), Coomassie Brilliant Blue R-250(Brilliant Blue) was purchased from Himedia. The stock solution 25 × 10-4 M DA, 25 × 10-3 M UA, 25 × 10-3 M AA was prepared in 0.1 M perchloric acid, 0.1 M NaOH, and double distilled water respectively. Buffer used was 0.2 M Phosphate buffer (PBS) solution of pH 7.4. Graphite powder of 50 μm particle size was purchased from Merck and silicone oil from Himedia was used to prepare Carbon Paste Electrode (CPE). All the chemicals mentioned were all of analytical grade used as received without any further purification.
Apparatus
The electrochemical experiments were carried out using a model CHI-660C (CH Instrument-660 electrochemical workstation). A conventional three electrode cell was used with a saturated calomel electrode (SCE) as a reference, a platinum counter electrode, and bare or poly (brilliant blue) modified electrode as working electrode.
Preparation of bare carbon paste electrode
The bare CPE was prepared by hand mixing of 70% graphite powder and 30% silicone oil in an agate mortar for about 45min until a homogeneous paste was obtained. The paste was then packed into a cavity of PVC tube of 3 mm internal Diameter and smoothened on a tissue paper. The electrical contact was provided by a copper wire connected to the end of the tube.
Preparation of poly (brilliant blue) modified carbon paste electrode
The paste packing procedure was same as that at bare carbon paste electrode (BCPE). Electrochemical polymerization of brilliant blue at the CPE was carried out using cyclic voltammetric method in aqueous solution containing 0.5mM brilliant blue in 0.1 M NaOH solution. The electropolymerisation was achieved by the formation of film that grew between -0.5 V to +1.5 V at the scan rate of 0.1 Vs-1 for 10 cycles. After that the electrode was rinsed thoroughly with double distilled water.
Result and Discussion
Electrochemical polymerization of brilliant blue on CPE
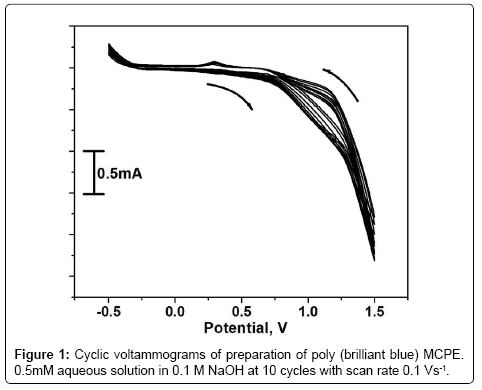
The poly (brilliant blue) modified carbon paste electrode (MCPE) was prepared by placing 0.5 mM brilliant blue with 0.1 M NaOH in an electrochemical cell. The potential window was maintained from -0.5 V to 1.5 V with scan rate 0.1 Vs-1 for 10 multiple cycles. Figure 1 shows during the process of multiple cycles the voltammogram has gradually descended with increase of cyclic time. This indicates that the poly (brilliant blue) film was formed and deposited on the surface of CPE [28,29] the structure of brilliant blue was shown in scheme 1.
Effect of multiple cycles on the preparation of poly (brilliant blue) MCPE
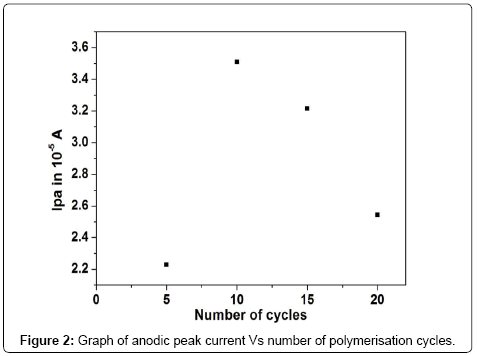
From the obtained experimental results the thickness of the film affects the electrocatalytic property of the carbon paste electrode. The coating was controlled by varying the multiple cycles on the CPE (from 5 to 20 multiple cycles) and corresponding electrocatalytic activity towards oxidation of 0.2mM DA in phosphate buffer solution (PBS) of pH 7.4 was investigated. The Figure 2 shows that at 10 multiple cycles the both anodic and cathodic peak currents was increased to almost 10 folds. Therefore ten cycles was chosen for the electropolymerisation of brilliant blue.
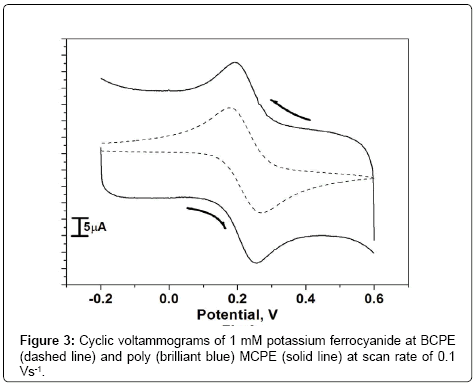
Electrochemical characterization of poly (brilliant blue) MCPE using standard potassium ferrocyanide system
The freshly prepared solution of 1 mM potassium ferrocyanide and 1 M KCl as supporting electrolyte was placed in the electrochemical cell. Figure 3 shows the cyclic voltammograms obtained for the 1 mM potassium ferrocyanide at both BCPE (dashed line) and poly(brilliant blue) MCPE ( solid line) at the scan rate 0.1 Vs-1. The low redox peak currents response was obtained at BCPE but at the poly (brilliant blue) MCPE exhibited stable enhancement of redox peak currents and also it shows the fast rate of electron transfer kinetics. The result obtained greatly improved the voltammetric response of potassium ferrocyanide at poly (brilliant blue) MCPE this suggests that the surface property of the modified electrode has been significantly changed and also the results proves that the electrocatalytic activity of our poly (brilliant blue) MCPE. The surface area available for reaction of species in solution can be estimated by the Randles-sevcik equation (1) [30,31].
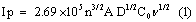
where, Ip is the peak current in A. C0 is the concentration of the electroactive species (mol cm-3), n is the number of electrons exchanged, D is the diffusion coefficient in cm2S-1, and υ is the scan rate (Vs-1), A is the electroactive area (cm2). For poly (brilliant blue) MCPE the electroactive surface area is maximum (0.03658 cm2) as compared with BCPE (0.02892 cm2).
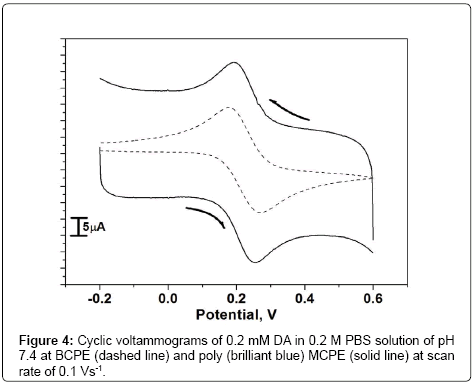
Electrochemical response of DA at poly (brilliant blue) MCPE
Figure 4 shows the cyclic voltammograms recorded for 0.2 mM DA at BCPE and poly (brilliant blue) MCPE in 0.2 M PBS solution of pH 7.4 with scan rate 0.1 Vs-1. At BCPE (dashed line) the dopamine shows oxidation and reduction potentials in the low current signals and at poly (brilliant blue) MCPE (solid line) shows significant increase in current signals and also it shows electrocatalytic activity of poly (brilliant blue) MCPE towards the detection of DA. The overall oxidation mechanism of DA could be explained on the basis of the dissociation ability of the –SO3H functional group of the poly (brilliant blue) at physiological pH of 7.4. The –SO3H group of poly (brilliant blue) could dissociate favorably into a negatively charged SO3 - group under this condition the –NH2 group of DA could obtain a proton and form the positive ion of DA. Therefore the negative charge –SO3 - on the surface of poly (brilliant blue) MCPE has a well affinity to the DA positive ions and could catalyse and promote the oxidation of DA [32-34].
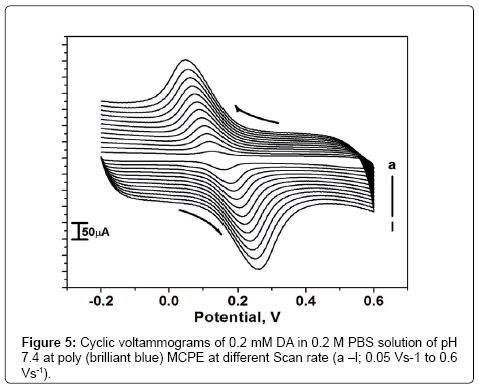
The effect of scan rate
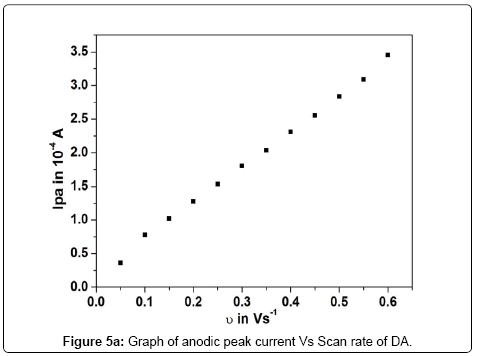
The effect of scan rate for 0.2 mM DA in PBS of pH 7.4 at poly (brilliant blue) MCPE was studied by cyclic voltammetric technique. According to Randles-sevciks equation the peak current is directly proportional to scan rate. Figure 5 shows the increase of redox peak currents with increase in scan rate from 0.05 Vs-1 to 0.6 Vs-1. The graph of anodic peak current (Ipa) vs. scan rate (υ) was plotted as shown in Figure 5a, the graph obtained was good linearity between the scan rate and Ipa in the range from 0.05 Vs-1 to 0.6 Vs-1. The correlation coefficient was r2=0.9989 which indicates the electrode reaction was adsorption-controlled [35,36]. According to an equation previously reported [37,38] for determining the value of k° form experimental (ΔEp) values equation (2) was a valid approximation of such curves for ΔEp>10 mV. The values of k° for the DA were determined from the experimental ΔEp values, the data are in Table 1. The values of k° indicates that strong adsorption of reactants and products are involved, here the k° is the heterogeneous rate constant, and ΔEp is the potential difference between the anodic and cathodic peak potentials. The heterogeneous rate constant (k°) was estimated using equation (2). The values of k° obtained at a scan rate of 0.35 Vs-1 for the poly (brilliant blue) MCPE exhibits larger heterogeneous rate constant compared with those determined in other scan rate variation studies. The calculated data’s are tabulate in Table 1.
| υ/ Vs-1 | ΔEp / V | k°/ s-1 |
|---|---|---|
| 0.05 | 0.0328 | 1.090 |
| 0.10 | 0.0667 | 1.480 |
| 0.15 | 0.0770 | 1.977 |
| 0.20 | 0.0913 | 2.234 |
| 0.25 | 0.1100 | 2.254 |
| 0.30 | 0.1265 | 2.244 |
| 0.35 | 0.1381 | 2.290 |
| 0.40 | 0.1544 | 2.172 |
| 0.45 | 0.1687 | 2.074 |
| 0.50 | 0.1830 | 1.954 |
| 0.55 | 0.1982 | 1.811 |
| 0.60 | 0.2137 | 1.655 |
Table 1: Variation of the voltammetric parameters gathered from the plots shown
in Fig 5 as a function of the potential scan rate.
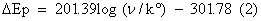
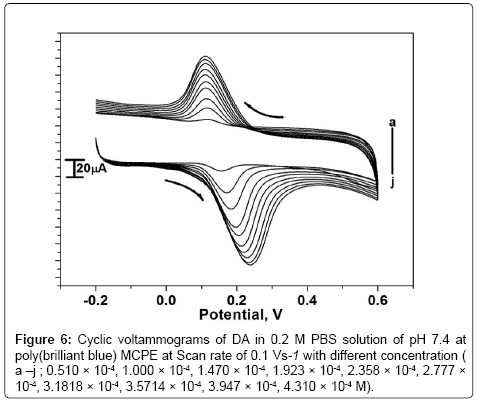
Effect of concentration of DA
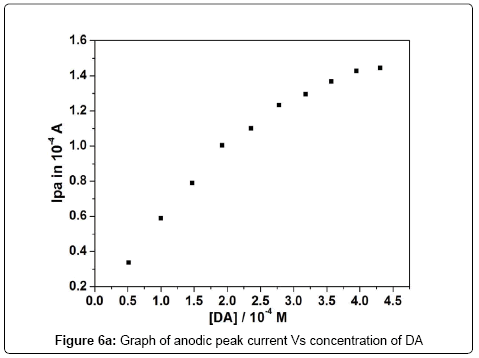
The electrocatalytic oxidation of DA was carried out by varying its concentration at poly (brilliant blue) MCPE. Figure 6 shows increasing the concentration of DA, the electrochemical anodic and cathodic peak current goes on increasing with shifting Epa towards more positive and Epc towards negative side. The concentration of DA from 0.510 × 10-4 M to 4.310 × 10-4 M showed that Epa was increased from 0.155 V to 0.238 V. The graph of anodic peak current Vs concentration of DA was plotted as shown in Figure 6a. The anodic peak current was linearly increasing upto the concentration of 2.358 × 10-4 M and further excess of increase of DA concentration shows less increase in Ipa and remains almost same this may due to the kinetic limitation [39].
Figure 6: Cyclic voltammograms of DA in 0.2 M PBS solution of pH 7.4 at poly(brilliant blue) MCPE at Scan rate of 0.1 Vs-1 with different concentration ( a –j ; 0.510 × 10-4, 1.000 × 10-4, 1.470 × 10-4, 1.923 × 10-4, 2.358 × 10-4, 2.777 × 10-4, 3.1818 × 10-4, 3.5714 × 10-4, 3.947 × 10-4, 4.310 × 10-4 M).
The limit of detection was calculated according to the previous reported literature [37] and the detection limit on the lower concentration range for DA was 6.7 × 10-7 M for the poly (brilliant blue) MCPE and limit of quantification was 22.3 × 10-7 M. The proposed electrode exhibited a relatively lower detection limit than those recently reported [40-43] as shown in Table 2.
| Electrode | Detection limit (mol/L) | Method | Reference |
|---|---|---|---|
| Bicopper complex modified GCE | 1.4 × 10-6 | DPV | [42] |
| Self-assembled gold nanoparticle modified Gold electrode | 9.0 × 10-5 | DPV | [43] |
| Ionic liquid modified Carbon paste electrode | 7.0 × 10-7 | CV | [44] |
| αCD/CNT/CE | 5.0 × 10-7 | DPV | [45] |
| Poly(brilliant blue) modified CPE | 6.7 × 10-7 | CV | This work |
Table 2: Comparision of detection limits of different modified electrodes.
Electrochemical oxidation of AA and UA at poly (brilliant blue) MCPE
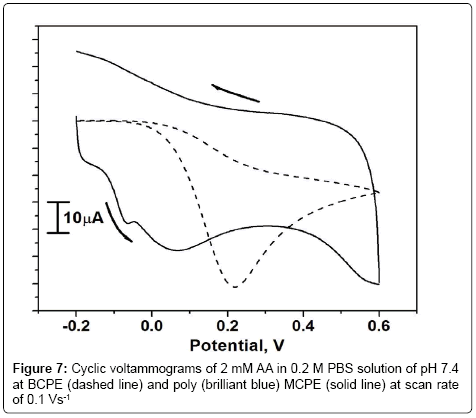
Figure 7 shows that the oxidation of 2 mM AA at BCPE and poly (brilliant blue) MCPE in 0.2 M PBS of pH 7.4 at the scan rate 0.1 Vs-1. From the Figure 7 it is observed that the oxidation potential of AA at BCPE was broad and poor in sensitivity (dashed line) the anodic peak potential was located at around 0.216 V. however, at poly (brilliant blue) MCPE the oxidation peak potential was shifted towards negative side by minimizing the over potential with enhancement in peak current, the anodic peak potential was located at -0.065 V. This shifting of oxidation peak potential and enhancement of peak current signal confirms the electrocatalytic activity of poly (brilliant blue) MCPE towards AA.
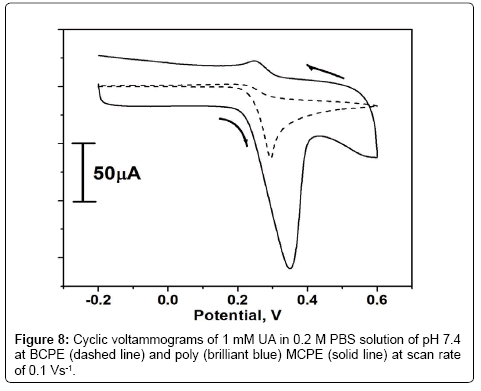
The oxidation of 1 × 10-3 M UA at BCPE and poly (brilliant blue) MCPE in 0.2 M PBS of 7.4 at the scan rate 0.1 Vs-1 is shown in Figure 8. At BCPE the oxidation peak potential was located at 0.293 V with less sensitivity. But in the same condition at poly (brilliant blue) MCPE the cyclic voltammogram obtained was with increase in current signal and oxidation peak potential was located at 0.350 V. Therefore the enhancement of current signal showed good sensor activity towards the detection of UA.
Simultaneous detection of DA, AA and UA
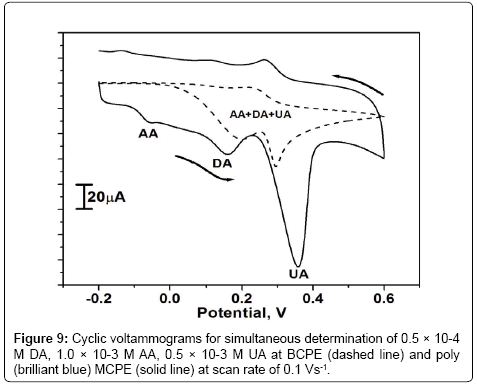
AA and UA were present along with DA in mammalian brain and the concentrations of these were much higher than that of DA. Since the oxidation potential of both AA and UA was nearly same as that of DA results in an overlapped voltammetric response at BCPE. The Figure 9 shows the cyclic voltammetric response of 0.5 × 10-4 M DA in presence of high concentration of 1.0 × 10-3 M AA and 0.5 × 10-3 M UA in PBS of pH 7.4 at the scan rate of 0.1 Vs-1 at both BCPE and poly (brilliant blue) MCPE. The cyclic voltammogram obtained for the mixture of DA and AA at BCPE was broad, less sensible and overlapped potential at 0.201 V (dashed line). However the poly (brilliant blue) MCPE has an ability to overcome this difficulty and achieved the separation of all three analytes in the mixture. The resulted cyclic voltammogram at poly (brilliant blue) MCPE has three well defined peak potential of DA, AA and UA at different potentials. The oxidation peak potential of DA, AA and UA were at 0.155 V, -0.060 V and 0.357 V respectively (solid line). The peak to peak separation of DA-AA was 0.251 V and that of DA-UA was 0.202 V. This result was more enough to identify DA in presence of High concentration of UA and AA at poly (brilliant blue) MCPE.
Interference study
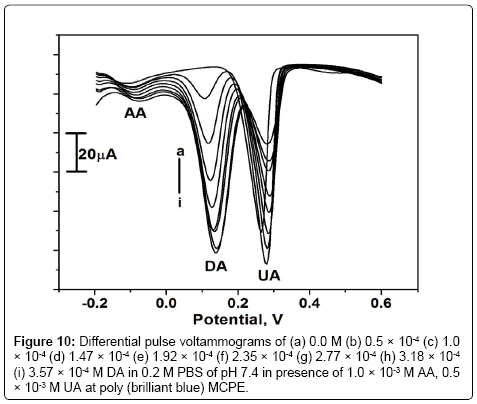
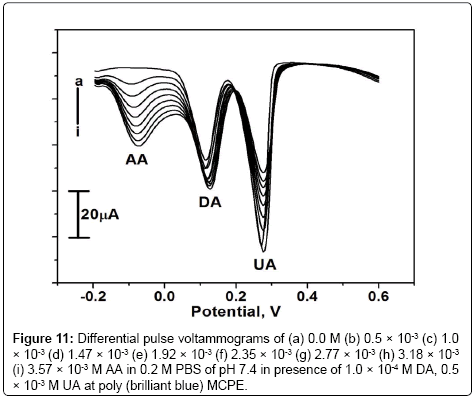
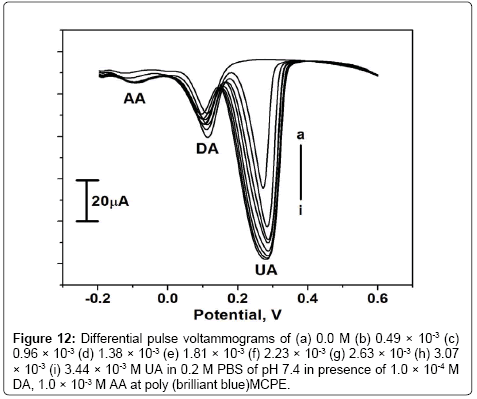
The interference study was carried out in the mixture of samples containing DA, AA and UA. In their mixtures was performed at the poly (brilliant blue) MCPE when the concentration of one species is changed, whereas the concentration of the other two species remained constant. From the Figure 10 it can be seen that the peak current of DA was increased from 0 to 3.57 × 10-4 M when keeping the concentration of AA and UA at 1.0 × 10-3 M and 0.5 × 10-3 M respectively. From the Figures 11 and 12 it is seen that by keeping the concentration of other two analytes constant the anodic peak current of AA or UA increased upto certain range.
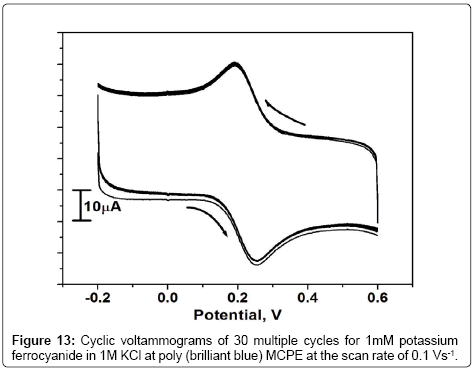
Stability of the poly (brilliant blue) MCPE
The stability of poly (brilliant blue) MCPE was studied for the reversible redox couple of Fe2+/Fe3+ in 1 M KCl at the scan rate of 0.1 Vs-1 by cyclic voltammetric technique. The result in Figure 13 shows that the redox peak current of potassium ferrocyanide remained virtually constant throught the 30 potential cycles and reflecting the stability of the poly (brilliant blue) MCPE.
Conclusion
The prepared poly (brilliant blue) MCPE shows excellent electrocatalytic activity towards the detection of DA electrochemically in the mixture of solutions contains large excess of AA and UA at physiological pH of 7.4 by using both CV and DPV techniques. The oxidation peak to peak separation of DA-AA was 0.251 V and that of DA-UA was 0.202 V. This result was more enough to identify DA in presence of large excess of AA and UA. The prepared modified electrode has detection limit of 6.7 × 10-7 M for DA by CV technique. It has excellent sensitivity, selectivity, stability, reproducibility and antifouling properties. This modified electrode can be applied for other neurotransmitters and also for some electroactive pharmaceutical compounds.
References
- Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122: 1437-1448.
- Dursun Z, Pelit L, Taniguchi I (2009) Voltammetric Determination of Ascorbic Acid and Dopamine Simultaneously at a Single Crystal Au(111) Electrode. Turk J Chem 33: 223-231.
- Wang Z, Liu J, Liang Q, Wang Y, Luo G (2002) Carbon nanotube-modified electrodes for the simultaneous determination of dopamine and ascorbic acid. Analyst 127: 653-658.
- Kaur H, Halliwell B (1990) Action of biologically-relevant oxidizing species upon uric acid. Identification of uric acid oxidation products. Chem Biol Interact 73: 235-247.
- Zen JM, Jou JJ, Ilangovan G (1998) Selective voltammetric method for uric acid detection using pre-anodized Nafion-coated glassy carbon electrodes. Analyst 123: 1345-1350.
- Miland E, Ordieres AJM, Blanco PT, Smyth MR, Fagian CO (1996) Poly(o-aminophenol)-modified bienzyme carbon paste electrode for the detection of uric acid. Talanta 43: 785-796.
- Lin X, Zhuang Q, Chen J, Zhang S, Zheng Y (2007) Electrocatalytic property of poly-chromotrope 2B modified glassy carbon electrode on dopamine and its application. Sensors Actuat B 125: 240-245.
- Zhang L, Lin X (2005) Electrochemical behavior of a covalently modified glassy carbon electrode with aspartic acid and its use for voltammetric differentiation of dopamine and ascorbic acid. Anal Bioanal Chem 382: 1669-1677.
- Deakin MR, Kovach PM, Stutts KJ, Wightman RM (1986) Heterogeneous mechanisms of the oxidation of catechols and ascorbic acid at carbon electrodes. Anal Chem 58: 1474-1480.
- Guan Y, Chu Q, Ye J (2004) Determination of uric acid in human saliva by capillary electrophoresis with electrochemical detection: potential application in fast diagnosis of gout. Anal Bioanal Chem 380: 913-917.
- Innoue K, Namiki T, Iwasaki Y, Yoshimura Y, Nakazawa H (2003) Determination of uric acid in human saliva by high-performance liquid chromatography with amperometric electrochemical detection. J Chromatogr B 785: 57-63.
- Hong HC, Huang HJ (2003) Flow injection analysis of uric acid with a uricase- and horseradish peroxidase-coupled Sepharose column based luminol chemiluminescence system. Anal Chim Acta 499: 41-46.
- Xue KH, Tao FF, Xu W, Yin SY, Liu JM (2005) Selective determination of dopamine in the presence of ascorbic acid at the carbon atom wire modified electrode. J Electroanal Chem 578: 323-329.
- Xue KH, Tao FF, Yin SY, Shen W, Xu W (2004) Investigation of the electrochemical behaviors of dopamine on the carbon atom wire modified electrode. Chem Phys Lett 391: 243-247.
- Zhang P, Wu FH, Zhao GC, Wei XW (2005) Selective response of dopamine in the presence of ascorbic acid at multi-walled carbon nanotube modified gold electrode. Bioelectrochem 67: 109-114.
- Razmi H, Agazadeh M, Habibi-A B (2003) Electrocatalytic oxidation of dopamine at aluminum electrode modified with nickel pentacyanonitrosylferrate films, synthesized by electroless procedure. J Electroanal Chem 547: 25-33.
- Swamy BEK, Venton BJ (2007) Carbon nanotube-modified microelectrodes for simultaneous detection of dopamine and serotonin in vivo. Analyst 132: 876-884.
- Zhao H, Zhang Y, Yuan Z (2001) Study on the electrochemical behavior of dopamine with poly(sulfosalicylic acid) modified glassy carbon electrode. Anal Chim Acta 441: 117-122.
- Xu F, Gao M, Wang L, Shi G, Zhang W, et al. (2001) Sensitive determination of dopamine on poly(aminobenzoic acid) modified electrode and the application toward an experimental Parkinsonian animal model. Talanta 55: 329-336.
- Vasantha VS, Chen SM (2006) Electrocatalysis and simultaneous detection of dopamine and ascorbic acid using poly(3,4-ethylenedioxy)thiophene film modified electrodes. J Electroanal Chem 592: 77-87.
- Chen Y, Yuan J, Wang X, Tian C (2004) Simultaneous determination of dopamine and ascorbic acid at a poly(toluidine blue) modified electrode. Anal Sci 20: 1725-1728.
- Roy PR, Okajima T, Ohsaka T (2003) Simultaneous electroanalysis of dopamine and ascorbic acid using poly (N,N-dimethylaniline)-modified electrodes. Bioelectrochem 59: 11-19.
- Zhang Y, Cai Y, Su S (2006) Determination of dopamine in the presence of ascorbic acid by poly(styrene sulfonic acid) sodium salt/single-wall carbon nanotube film modified glassy carbon electrode. Anal Biochem 350: 285-291.
- Gilbert O, Swamy BEK, Chandra U, Sherigara BS (2009) Simultaneous detection of dopamine and ascorbic acid using polyglycine modified carbon paste electrode: A cyclic voltammetric study. J Electroanal Chem 636: 80-85.
- De St Groth SF, Webster RG, Datyner A (1963) Two new staining procedures for quantitative estimation of proteins on electrophoretic strips. Biochem Biophys Acta 71: 377-391.
- Chrambach A, Reisfeld RA, Wyckoff M, Zaccari J (1967) A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem 20: 150-154.
- Ganesh PS, Swamy BEK (2014) Electrochemical Determination of Dopamine in Presence of Ascorbic Acid at Brilliant Blue Modified Carbon Paste Electrode: A Voltammetric Study Journal of Chemical Engineering and Research 2: 113-120.
- Yao H, Sun Y, Lin X, Tang Y, Liu A, et al. (2007) Selective Determination of Epinephrine in the Presence of Ascorbic Acid and Uric Acid by Electrocatalytic Oxidation at Poly(eriochrome Black T) Film-modified Glassy Carbon Electrode. Anal Sci 23: 677-682.
- Lin X, Li W, Yao H, Sun Y, Huang L, et al. (2007) Electrocatalytic Response and Determination of Noradrenaline in the Presence of L-Ascorbic and Uric Acids with Poly(Eriochrome Black T)-Modified Electrode. Collect Czech Chem Commun 72: 1177-1188.
- Pacios M, Valle MD, Bartroli J, Esplandiu MJ (2008) Electrochemical behavior of rigid carbon nanotube composite electrodes. J Electroanal Chem 619: 117-124.
- Salinas-Torres D, Huerta F, Montilla F, Morallon E (2011) Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim Acta 56: 2464-2470.
- Yao H, Sun Y, Lin X, Tang Y, Huang L (2007) Electrochemical characterization of poly(eriochrome black T) modified glassy carbon electrode and its application to simultaneous determination of dopamine, ascorbic acid and uric acid. Electrochim Acta 52: 6165-6171.
- Chandra U, Swamy BEK, Gilbert O, Sherigara BS (2010) Voltammetric resolution of dopamine in the presence of ascorbic acid and uric acid at poly (calmagite) film coated carbon paste electrode. Electrochimica Acta 55: 7166-7174.
- Chandra U, Swamy BEK, Gilbert O, Reddy S, Shankar SS, et al. (2011) Simultaneous Detection of Dopamine and Uric Acid at Poly (Fast Sulfone Black F) Film Coated Graphite Pencil Electrode. Anal Bioanal Electrochem 3: 316-326.
- Avendano SC, Angles GA, Silva MTR, Pina GR, Romo MR, et al. (2007) On the electrochemistry of dopamine in aqueous solution. Part I: The role of [SDS] on the voltammetric behavior of dopamine on a carbon paste electrode. J Electroanal Chem 609: 17-26.
- Gosser Jr DK (1993) Cyclic Voltammetry Simulation and Analysis of Reaction Mechanisms. 1st edition, VCH-Weinheim.
- Reddy S, Swamy BEK, Jayadevappa H (2012) CuO nanoparticle sensor for the electrochemical determination of dopamine. Electrochem Acta 61: 78-86.
- Mahanthesha KR, Swamy BEK (2013) Pretreated/Carbon paste electrode based voltammetric sensors for the detection of dopamine in presence of ascorbic acid and uric acid. J Electroanal Chem 703: 1-8.
- Nicholson RS (1965) Theory and Application of Cyclic Voltammetry for Measurement of Electrode Reaction Kinetics. Anal Chem 37: 1351-1355.
- Wang M, Xu X, Gao J (2007) Voltammetric studies of a novel bicopper complex modified glassy carbon electrode for the simultaneous determination of dopamine and ascorbic acid. J Appl Electrochem 37: 705-710.
- Raoof JB, Kiani A, Ojani R, Valiollahi R, Nadimi SR (2010) Simultaneous voltammetric determination of ascorbic acid and dopamine at the surface of electrodes modified with self-assembled gold nanoparticle films. J Solid State Electrochem 14: 1171-1176.
- Sun W, Yang M, Jiao K (2007) Electrocatalytic oxidation of dopamine at an ionic liquid modified carbon paste electrode and its analytical application. Anal Bioanal Chem 389: 1283-1291.
- Ge-Yun W, Xiu-Juan L, Guo-An L, Zong-Hua W (2005) a-Cyclodextrin Incorporated Carbon Nanotube-coated Electrode for the Simultaneous Determination of Dopamine and Epinephrine. Chin J Chem 23: 297-302.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16225
- [From(publication date):
February-2015 - Apr 21, 2025] - Breakdown by view type
- HTML page views : 11472
- PDF downloads : 4753