Visual Impairment in HIV Negative Tuberculosis Meningitis
Received: 30-Dec-2015 / Accepted Date: 18-May-2016 / Published Date: 25-May-2016 DOI: 10.4172/2572-2050.1000107
Abstract
Objective: Visual impairment is a common problem in tuberculous meningitis (TBM). Present study has been conducted to evaluate the prevalence of visual impairment at presentation and at 3 months.
Methods: Twenty seven consecutive HIV negative patients with TBM were included. Visual acuity, colour vision, field of vision, and visual evoked potentials (VEP) were recorded at baseline and at 3 months. The criteria for visual impairment were: visual acuity <6/12 and <N/10, defective color vision, and visual field abnormality either alone / in combination. Fundus was examined by a single examiner using slit lamp biomicroscopic examination with 90 D lens and by indirect ophthalmoscopy with 2.2D lens.
Results: Twelve patients out of 27 had visual impairment at presentation and the causes were optochiasmatic arachnoiditis (n=6), optic atrophy (n=2), occipital infarct (n=1) and unremarkable (n=3). Three patients showed improvement in visual acuity, 6 patients had no change and 3 patients expired at 3 months. On multivariate analysis papilloedema, optic atophy, temporal disc pallor and hydrocephalus were predictors of visual impairment at 3months (p< 0.001 ).
Conclusion: Visual impairment in TBM is observed in half the patients. It may be predicted by the presence of hydrocephalus on computerized tomography (CT)/ magnetic resonance imaging (MRI), while a simple bedside fundus examination can predict the visual impairment at three months. VEP helps in detecting sub-clinical visual impairment.
Keywords: Tuberculous meningitis; Opticatrophy; Optochiasmatic arachnoiditis; Visual impairment; Visual evoked potentials
5639Abbreviations
TBM: Tuberculous Meningitis; VEP: Visual Evoked Potentials; CT: Computerized Tomography; MRI: Magnetic Resonance Imaging; CSF: Cerebrospinal Fluid; ATT: Anti-Tubercular Treatment; PCR: Polymerase Chain Reaction; GCS: Glasgow Coma Scale; MMSE: Mini- Mental State Examination; MRC: Medical Research Council; WHO: World Health Organization; AFB: Acid Fast Bacilli; FSE: Fast Spin Echo; SE: Spin Echo; FLAIR: Fluid Attenuated Inversion Recovery.
Introduction
Tuberculous meningitis (TBM) is the most common form of meningitis in the developing countries. It still carries a high morbidity and mortality despite the availability of computerised tomography (CT) scan, magnetic resonance imaging (MRI) scan and effective chemotherapy. Several recent studies involving multivariate regression analysis have suggested that stage of TBM, age, focal weakness, cranial nerve palsy and hydrocephalus are significant prognostic factors for a poor outcome at 3 months [1-6].
Visual impairment, especially blindness is a devastating outcome of TBM, occurring in 26-72% [1-9]. It can occur due to a lesion anywhere in the visual pathway because of the disease process per se, complications occurring during its course or as a result of side effects of the drugs given for its treatment. It may result from papillitis, papilloedema, primary or secondary optic atrophy, optochiasmatic arachnoiditis and occipital infarct [10]. In addition ethambutol toxicity may also contribute to visual impairment. Sub-clinical visual impairment can be detected by visual evoked potentials [11].
Despite the fact that visual abnormalities in TBM were first reported more than fifty years ago, it has not been studied systematically and is still the subject of isolated case reports [12-16]. A study on tuberculous meningoencephalitis in children found neuro-ophthalmic features in 67% [9]. Optic nerve involvement was the most common finding and there was a significant association of raised intracranial tension with neuro-ophthalmological features. The patients were seen only at presentation and no outcome assessment was done. As there is a paucity of prospective studies regarding visual impairment in TBM, the present study has been undertaken to assess the prevalence and predictors of visual impairment in TBM, and to assess its prognosis at 3 months [5,7,9].
Material And Methods
Plan and design of the study
The present study was undertaken at a tertiary care centre. It was a prospective observational study, of one year duration. Enrollment of patients was done for 8 months, with a minimum follow-up of 3 months.
The study included HIV negative subjects between the age group 18-60 years and were followed up for three months. The study was approved by the Institutional Ethics Committee and an informed consent as per tenets of Helsinki’s Declaration was taken from the patient or relative (in case the patient was in altered sensorium) prior to the start of the study.
Selection of patient: The patients were evaluated for HIV using ELISA (Meril, J.Mitra, New Delhi, India). The inclusion criteria were based on the clinical and supportive criteria of TBM described by Shankar et al. [17]. The clinical criteria included fever, headache and neck stiffness of >2 weeks duration. The supportive criteria included Cerebrospinal fluid (CSF) cells >20/dl with a lymphocytic predominance, CSF protein >100 mg/dl, negative Gram’s stain, negative India ink preparation for Cryptococcus neoformans, CT scan findings of hydrocephalus and basal exudates, evidence of extra-neural tuberculosis and response to anti-tubercular treatment (ATT).
The patients were divided into four diagnostic categories, on the basis of the above clinical and supportive criteria. The definitive criteria included clinical criteria along with bacterial isolation from CSF or diagnosis at autopsy. The highly probable included clinical criteria along with three supportive criteria. Probable included clinical criteria along with two supportive criteria and possible included clinical criteria along with one supportive criteria.
These criteria have been validated in a study involving bacterial isolation, polymerase chain reaction (PCR), response to treatment and necropsy [18].
Patients who had taken ATT for >2 weeks before enrollment, those who were not-cooperative for visual assessment or having noncorrectable visual impairment prior to the present illness, visual impairment due to nutritional causes, diabetes mellitus and hypertension were excluded from the study.
Plan of study: A detailed history and examination was performed using a structured proforma and the following variables were recorded, age, gender, stage of TBM, duration of illness, convulsions, diplopia, presence of extra-neural tuberculosis, level of consciousness by Glasgow Coma Scale (GCS), mini-mental state examination (MMSE), cranial nerve involvement, fundus abnormality, limb weakness, and micturition abnormality. The staging of TBM was done using the Medical Research Council (MRC) staging [19]. Folstein’s MMSE was done to evaluate the cognitive functions. A cut off value of <19 for uneducated, <23 for high school , <27 for intermediate and <29 for graduates were taken as abnormal MMSE [20]. GCS was used to assess the level of consciousness. A GCS score of <10 was taken as abnormal.
Visual assessment: Visual assessment was done at presentation (within 7 days of enrollment) and at 3 months follow-up. All the patients underwent a detailed neuro-ophthalmic examination by one of the authors (SS). This included corrected visual acuity, color vision, peripheral and central visual field examination and ophthalmoscopic examination. Visual acuity for distance was tested by Snellen’s chart and for near vision by near vision charts. Color vision was evaluated by Ishihara’s test. Diplopia was assessed by using Hess chart. Field of vision was examined by doing visual field using the Humphrey visual field analyser (Humphrey Instruments, USA). Interpretation of the visual field chart was done by one of the authors (SS).
A detailed ophthalmoscopic examination was performed by one of the authors (SS) to assess the presence/absence of papilloedema, primary/secondary optic atrophy, presence/absence of choroid tubercle. For the purpose of differentiation between primary and secondary optic atrophy, fundus appearance was taken into consideration as following: when the disc was chalky white with sharp border, and arteries and veins were reduced in size, it was considered to be primary optic atrophy. A gray disc with blurred borders, thin arteries and dilated veins was considered as secondary optic atrophy.
Visual evoked potentials (VEP) was recorded on Neuropack four evoked potential machine (Nihon Kohden, Tokyo, Japan) according to the method of Halliday [21]. The VEP was assessed only in those patients having a visual acuity of ≥ 6/36. A single channel recording was done using the Oz-Fz montage. Visual stimulation was done with a check size of 16 and pattern type stimulation. The checks were made to reverse at a rate of 2 Hz and 200 responses were averaged with a low frequency fitter of 1Hz and high frequency filter of 100 Hz. At least two trials were obtained to ensure replicability of the VEP. Patients were given short breaks between sets of stimuli to avoid loss of concentration. Smoothening of the response was done. Cursors were placed at N1-P1 and N2. The absolute latency of the P100 response was recorded in msec. Amplitude of the P100 wave was measured in relation to the preceding and following negative waves in uV (N1-P1 and P1-N2). The P100 latency and amplitude were recorded for 20 controls (10 male and 10 female). The criteria for abnormal P100 latency was taken as mean of control+2 SD. Taking this criteria the cut off for abnormal P100 latency for male patients was 122.1 ms for right eye and 121.1 ms for left eye. For female patients the cut off was 113.2 ms for right eye and 112.9 ms for left eye. Amplitude was not considered as a criteria for abnormality because mean - 2 SD of amplitude values were around zero.
Criteria for visual impairment: As WHO has not given any specific criteria for visual impairment and they have categorized it into low vision and blindness, for the purpose of this study we have considered visual impairment as :
Visual acuity of <6/12 (20/40) for far vision and <N/10 for near vision which is not correctible by glasses, as this is associated with functional disability in activities of daily living.
Defective colour vision: Visual field was considered abnormal, if there was constriction of visual field or a scotoma was present. Blindness defined by WHO criteria as visual acuity of less than 3/60, or corresponding visual field loss to less than 10 degrees, in the better eye with best possible correction
Criteria for visual improvement: Improvement was said to be present if a patient who had impairment in any of the above mentioned criteria and at three months follow up the visual acuity for distance vision became ≥ 6/12 (20/40) for far vision, ≥ N/10 for near vision or there was improvement in color/field of vision.
Cerebrospinal fluid examination: Ten ml of cerebrospinal fluid (CSF) was collected by a lumbar p
suncture in left lateral decubitus. In addition to routine biochemistry and cytology, India ink preparation and Gram’s staining was also done. The sediment of centrifuged CSF was examined for acid fast bacilli (AFB) by Zeihl-Nelson method. CSR PCR for tuberculosis and CSF culture was also performed.
Radiological investigations: CT and MRI were done at the time of enrollment in the study. A plain and contrast CT scan of the brain was done on III generation spiral CT scanner of Wipro GE using a slice thickness of 10mm. The CT scan was done in all the 27 patients while; MRI scan was possible in only 18 patients. MRI of the brain was performed on whole body 1.5 Tesla MRI system (Signa General Electric Medical Systems, Milwaukee, USA) equipped with an actively shielded whole body magnetic field gradient set allowing upto 33 mt/m using a quadrative head coil.
Treatment
Patients without visual impairment were prescribed 4-drug antitubercular therapy, rifampicin, isoniazid, pyrazinamide, ethambutol (RHZE). In those patients presenting with visual impairment or developing it while taking ATT, ethambutol was replaced by streptomycin. In patients who developed hepatotoxicity, modified ATT was given. Dexamethasone was given intravenously as 0.4 mg/kg/d for week one, 0.3 mg/kg/d for week two, 0.2 mg/kg/d for week three, 0.1 mg/kg/d for week four followed by 4 mg of oral drug, tapered each week by 1 mg [22]. Corticosteroids were given in stage II and III patients. Decongestive therapy in the form of I/V mannitol was given in those having signs of raised intracranial tension.
Outcome: The outcome of TBM patients was defined on the basis of 3 month Barthel index score [23]. A score below 12 was defined as poor and 12 or more as a good outcome measure. Death was included in the poor outcome group for statistical analysis.
Statistical analysis: Statistical analysis was done using MS-Excel and SPSS software. The values are given as Mean+SD. Univariate analysis was performed by Chi square test for non-parametric data and students ‘t’ test for independent variables for parametric data. Multivariate analyses were done using the dichotomous dependent variable visual impairment on presentation and at three months The variables (y) were assigned the value one when present and 0 when absent [1]. The independent variables found to be significant by univariate analysis were also categorized as follows:
MMSE normal=0, abnormal=1;
Paresis absent=0, present=1;
CT Scan normal=0, abnormal=1;
Hydrocephalus absent=0, present=1;
Fundus normal=0, abnormal=1.
If x1, x2, x3…………………. Xp characteristics related to the event then the logistic regression model specified that the probability of positive predictions was as follows:
P (y=1/X1, X2, X3………….. Xp)=1/ 1+exp [- (A+Σ Bj×j)]
The β coefficients values and p values were calculated for each independent variable. In the predictive model only the parameters with least p value and also clinically relevant were taken as significant.
Results
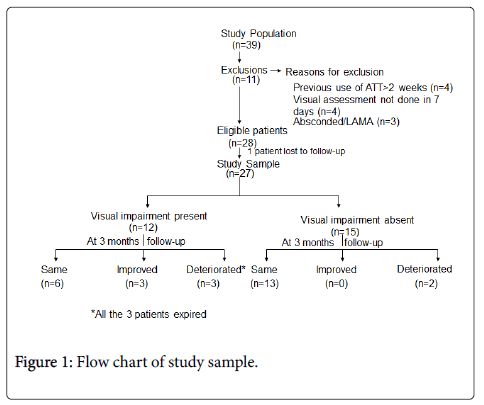
Out of the 28 patients enrolled in the study, one patient was lost to follow up, so the data of only 27 patients has been analyzed. The study population, visual findings and their follow-up at 3 months are depicted in Figure 1. The baseline demographic, clinical profiles and CSF findings of all 27 patients are depicted in Table 1.
| Patient characteristics | Mean ± SD/ % |
|---|---|
| Age (years) | 23 ± 1.9 |
| Male sex | 9/27 (33.3%) |
| Duration of illness (mth) | 2.2 ± 2.3 |
| Highly probable TBM | 26/27 (96.2%) |
| MRC Staging | |
| Stage I TBM | 8/27 (29.6%) |
| Stage II TBM | 17/27 (62.9%) |
| Stage III TBM | 2/27 (7.4%) |
| Cranial Nerve Abnormality | |
| VI nerve palsy | 13/27 (48.1%) |
| VII nerve palsy | 3/27 (11.1%) |
| III nerve palsy | 1/27 (3.7%) |
| Fundus Abnormalities | |
| Papilloedema | 6/27 (22.2%) |
| Secondary optic atrophy | 2/27 (7.4%) |
| Choroid tubercle | 1/27 (3.7%) |
| First line ATT | 25/27 (92.5%) |
| Steroid therapy | 22/27 (81.4%) |
| CSF Changes | |
| Cells (/ cumm) | 186.52 ± 210.45 |
| Lymphocytes % | 86.26 ± 10.72 |
| Protein (mg/dl) | 207.85 ± 280.46 |
| Glucose (mg/dl) | 39.63 ± 21.2 |
| CSF/ Blood Glucose Ratio | 37.12 ± 21.2 |
| CSF PCR for tuberculosis | 18/27 (66.6%) |
Table 1: Baseline characteristics of patients * CSF culture for Mycobacterium tuberculosis and Zeihl Nelson stain for AFB was negative in all the patients.
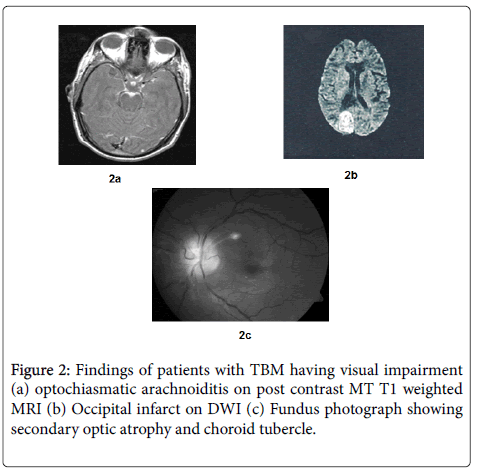
Visual impairment was present in 12/27 (44.44%) of patients at presentation. Decreased visual acuity was present in all the patients, out of which 6 patients also had impairment of near vision and 1 patient had defective color vision. Five patients in addition to the decrease in visual acuity had a field defect on perimetry in the form of presence of scotoma and 2 patients out of them in addition had constriction of peripheral field. The median visual acuity was 6/9 (range 6/6-PL/PR absent). Three patients out of 12 had blindness. At presentation the commonest cause of visual impairment was Optochiasmatic arachnoiditis (6/12), followed by secondary optic atrophy (2/12) and occipital infarct (1/12) (Figure 2). In 3 patients the findings were unremarkable (3/12).
Three patients died during course of illness so follow-up examination at 3 months was possible in 24 patients (24/27). 33.33% patients had visual impairment at 3 months. In 15 patients with normal vision at baseline 2 patients had visual impairment at 3 months follow up and ethambutol was stopped in these 2 patients. Out of 12 patients with visual impairment at presentation, 3 patients died during the course of illness so only 9 patients were followed up. Out of this three patients had improvement in visual acuity. During follow up three patients with blindness expired.
CT scan was abnormal in 9/27 patients. Basal exudates was the commonest finding n=5 (18.5%), followed by hydrocephalus n=4 (14.8%), tuberculoma n=3 (11.1%) and infarction n=3 (11.1%). Although MRI could be done in only 18 patients, it was abnormal in all, in the form of meningeal enhancement (100%). Infarction n=11 (61.9%), tuberculoma n=7 (38.9%), optochiasmatic arachnoiditis n=6 (33.3%), vasculitis n=6 (33.3%) and hydrocephalus n=5 (27.8%) were the other findings.
The VEP was not possible in 4 patients because visual acuity was less than 6/36. Out of the rest 23 patients the VEP was done in 12 patients because the other patients were not cooperative. The mean ± SD of P100 latency in control group (right eye 112.4 ± 4.72 msec, left eye 111.7 ± 4.55 msec) and patients with TBM (right eye 117.5 ± 7.94 msec, left eye 117.33 ± 9.4 msec) showed no statistically significant difference. The mean ± SD of amplitude in control group (right eye 10.77 ± 6.49 uv, left eye 10.19 ± 4.77 uv) and patients with TBM (right eye 10.19 ± 4.77 uv, left eye 10.12 ± 5.0 uv) showed no statistically significant difference. Six out of 12 patients had abnormal VEP based on the absolute value of the latency (Mean+2 SD control data).
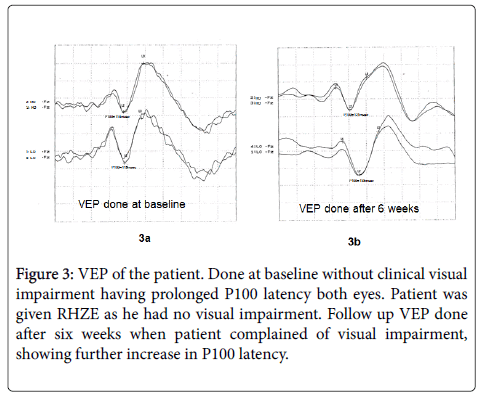
Figure 3: VEP of the patient. Done at baseline without clinical visual impairment having prolonged P100 latency both eyes. Patient was given RHZE as he had no visual impairment. Follow up VEP done after six weeks when patient complained of visual impairment, showing further increase in P100 latency.
Comparison of the clinical features and investigations at baseline in the two groups (visual impairment present at three months vs visual impairment absent at three months) revealed that in those with visual impairment, fundus abnormality and hydrocephalus were significantly more common (Figure 3). On univariate analysis abnormal MMSE, presence of paresis, abnormal CT scan, abnormal MRI scan and presence of hydrocephalus were significantly correlated with visual impairment at presentation while on multivariate analysis only hydrocephalus was significantly correlated with visual impairment (Tables 2 and 3). Visual impairment at 3 months was significantly correlated with presence of baseline fundus abnormality and hydrocephalus both on univariate and on multivariate analysis (Tables 2 and 3). Five patients had a poor outcome (based on BI <12 or death) and all 5 patients had visual impairment.
| Baseline Variables | Visual Impairment | |||
|---|---|---|---|---|
| At presentation | At 3 months‡ | |||
| Absent n=15 | Present n=12 | Absent n=16 | Present n=8 | |
| Mean Age (year)† | 23.3 ± 9.8 | 22.6 ± 10.5 | 22.4 ± 6.8 | 23.0 ± 13.6 |
| Female | 5 | 4 | 11 | 5 |
| Stage III | 0 | 2 | 0 | 0 |
| Duration of illness (mths)† | 1.8 ± 1.6 | 2.7 ± 2.9 | 1.7 ± 1.5 | 2.1 ± 1.7 |
| Seizure | 6 | 9 | 6 | 6 |
| Diplopia | 7 | 6 | 6 | 5 |
| H/o TB Contact | 1 | 2 | 1 | 1 |
| Abnormal MMSE | 2 | 6** | 2 | 3 |
| Extrapulmonary TB | 3 | 2 | 3 | 1 |
| Cranial Nerve Palsy | 7 | 8 | 7 | 6 |
| Paresis | 0 | 5* | 1 | 2 |
| Micturition abnormality | 1 | 1 | 0 | 1 |
| Fundus abnormality | 4 | 7 | 3 | 7* |
| ESR†(mm in first hour) | 18.7 ± 10.1 | 17.5 ± 7.7 | 20.1 ± 8.8 | 13.8 ± 8.2 |
| CSF Cells†( cubic mm) | 209.4 ± 276.1 | 157.9 ± 77.9 | 204.9 ± 264.3 | 180.9 ± 97.1 |
| Protein†(mg/dl) | 140.3 ± 48.8 | 292.3 ± 410.9 | 131.7 ± 43.9 | 369.8 ± 480.1 |
| Glucose†(mg/dl) | 37.6 ± 20.4 | 42.2 ± 21.8 | 37.8 ± 19.4 | 44.0 ± 21.7 |
| CT Abnormal | 2 | 7** | 3 | 4 |
| MRI Abnormal# | 6 (n=10) | 8** (n=8) | 6 | 5 |
| Evidence of Hepatitis | 1 | 1 | 1 | 0 |
| Requirement of shunt surgery | 0 | 1 | 0 | 0 |
| Hydrocephalus | 7 | 5* | 0 | 2** p<0.05 |
| Steroid therapy | 11 | 11 | 11 | 8 |
Table 2: Univariate analysis of baseline variable with visual impairment.
| Variables | ‘ß’ coefficient | p value |
|---|---|---|
| Visual Impairment at Presentation | ||
| Abnormal MMSE | 0.2476 | 0.29 |
| Hydrocephalus | 0.4258 | 0.08 |
| Constant | 0.2427 | |
| Visual Impairment at 3 months | ||
| Fundus Abnormality | 0.4203 | 0.02 |
| Hydrocephalus | 0.4724 | 0.05 |
| Constant | 0.0788 | |
Table 3: Multivariate logistic regression model showing the most significant parameters at baseline in TBM for visual impairment at presentation (n=12) and at 3 months (n=8).
Discussion
The findings of the present prospective study suggest that visual impairment was present in 44% patients at presentation and 33% patients at 3 months follow-up. The presence of visual impairment is a predictor of poor outcome in patients with TBM. Multivariate logistic regression analysis suggests that presence of hydrocephalus is a significant predictor of visual impairment at baseline while presence of fundus abnormality in addition to hydrocephalus is a significant predictor of visual impairment at 3 months [24]
Previous studies have reported visual impairment ranging from 27%-32% [5,7,9]. In a study of TBM evaluating the role of corticosteroids on intracranial pressure, CT findings and clinical outcome in children, visual impairment was present in only 16 out of 119 patients and there was no difference in its occurrence whether the patients were given steroids or not [25]. In the present study also there was no significant difference in the occurrence of visual impairment whether the patient were taking steroid or not.
In the present study the most common finding on fundus examination was papilloedema, followed by secondary optic atrophy and temporal pallor. According to Kalra and Ghose, primary optic atrophy was reported in 19 out of 50 children with TBM [8]. Choroid tubercles in addition to evidence of military tuberculosis have been reported in 25% patients of TBM by Mooney [7]. In the present study choroid tubercles were observed in only 1 out of 27 patients on fundus examination. Kalita and Misra in a clinico-radiological study of 165 cases with TBM observed 20 cases with military tuberculosis and none of them had any evidence of choroid tubercles [5]. In a recent study by Sinha and Garg, predictors of vision deterioration in patients with tuberculous meningitis were found to be papilledema, cranial nerve palsies, raised CSF fluid protein (>1 g/L) and optochiasmatic arachnoiditis in MRI.
The etiology and mechanism of optic nerve changes in TBM is not very well understood it is thought that the optic nerve involvement may be post inflammatory. Basal arachnoiditis, exudates and adhesions may produce primary optic atrophy by pressure and traction on the nerve [26]. The mechanism operating in a given case may be difficult to determine. It is possible that more than one factor may be playing a role in a given patient. Hydrocephalus is a well-known cause of visual impairment [7,8]. Edema of the nerve head may be caused by raised intracranial pressure and may be accentuated by inflammatory adhesions of the nerve sheath to the nerve, optic atrophy was definitely more frequent in cases with proved hydrocephalus. The frequency and etiology of primary optic atrophy including temporal pallor is well known in hydrocephalus subjects, and it was observed in 28.5% of cases in a large series [27]. Thus it appears that the risk of optic nerve changes in TBM may be enhanced by the presence of hydrocephalus.
In the present study, cause of visual impairment could be determined in 9 out of 12 patients and optochiasmatic arachnoidditis on MRI was found in 6 out of those 9 patients. So MRI of the brain is especially helpful in determining the cause of visual impairment as it was abnormal in all the cases and 3 patients where the cause of visual impairment could not be determined, a MRI had not been done.
In our study VEP was abnormal in 6/12 (50%) patients, none of them had clinical visual impairment. There is no comparative study in adults about VEP abnormality in TBM. In a recent study on VEP abnormality in pediatric TBM patients an abnormal VEP has been reported in 28% [28]. However, the data is not exactly comparable as flash VEP was used and pediatric group of patients are not cooperative for checker board VEP. The VEP abnormality in absence of clinical impairment is reported due to extrinsic compression of anterior visual pathway [21]. A case report of optochiasmatic arachnoiditis in TBM showed improvement in VEP following surgery which was associated with improvement in post-operative visual acuity [29].
Two of our patients developed visual impairment on treatment and ethambutol was replaced with streptomycin in them. Both of them had a normal fundus examination and normal MRI with normal vision at baseline. Both had a prolonged VEP latency even at baseline and had further increase in P100 latency on treatment. It reestablishes the effect of ethambutol on optic nerve and usefulness of VEP in detecting subclinical optic nerve involvement. Similar effect of ethambutol on VEP and visual acuity were reported by other authors [11,30] Ethambutol is known to produce a dose related optic neuropathy [31].
The present study had its limitations because of small sample size and short duration follow up of 3 months. MRI and VEP could not be performed in all the patients. Despite these limitations it is the first observational study of visual impairment in TBM in which both clinical as well as electrophysiological evaluation of patients were done. Further studies with larger sample size and longer duration of follow up are needed to evaluate the causes and outcome of visual impairment in TBM [32].
Conclusions
Our findings in the present prospective study of HIV negative cases of TBM, suggest that, visual impairment at presentation is seen in nearly half the patients, and it can be predicted by presence of hydrocephalus. Visual impairment at 3 months follow-up is seen in one third of patients. An abnormal fundus examination on initial presentation is a significant predictor of visual impairment at 3 months after presentation.
References
- Misra UK, Kalit AJ, Srivastava M, Mandal SK (1996) Prognosis of tubercular meningitis : a multivariate analysis. J NeurolSci 137: 57-61.
- Misra UK, Kalita J, Roy AK, Mandal SK, Srivastava M (2000) Role of clinical, radiological and neurophysiological changes in predicting the outcome of tuberculous meningitis: a multivariate analysis. J NeurolNeurosurg Psychiatry 68: 300-303.
- Mahadevan B, Mahadevan S, Serane VT (2002) Prognostic factors in childhood tuberculosis meningitis. J Trop Pediatr 48: 362-365.
- Lu Ch, Chang WN, Chang HW (2001) The prognostic factors of adult tuberculous meningitis. Infection 29: 299-304.
- Kalita J, Misra UK (1999) Outcome of tuberculous meningitis at 6 and 12 months: a multiple regression analysis. Int J Tuber Lung Dis 3: 261-265.
- Humphries MJ, Teoh R, Lau J, Gabriel M (1990) Factors of prognostic significance in Chinese children with tuberculous meningitis. Tubercle 71: 161-168.
- Mooney AJ (1956) Some ocular sequelae of tuberculous meningitis: a preliminary survey. Am J Ophthal 41: 753-768.
- Kalra V, Ghose S (1985) Optic nerve involvement in tuberculous meningitis. Indian Pediatr 22: 783-785.
- Amitava AK, Alam S, Hussain R (2000) Neuro-ophthalmic features in pediatric tubercular meningoencephalitis. J PediatrOphthalmol Strabismus 38: 229-234.
- Yiannikas C, Walsh JC, Mcleod JG (1983) Visual evoked potentials in the detection of subclinical optic toxic effects secondary to ethambutol. Arch Neurol 40: 645-648.
- Scott RM, Sonntag VK, Wilcox LM, Adelman LS, Rockel TH (1977) Visual loss from optochiasmaticarachnoiditis after tuberculous meningitis: Case report. J Neurosurg 46: 524-526.
- Poon WS, Ahuja A, Li AK (1993) Optochiasmatictuberculoma causing progressive visual failure: where has medical treatment failed? Postgrad Med J 69: 147-149.
- Silverman IE, Liu GT, Bilaniuk LT, Volpe NJ, Galetta SL (1995) Tuberculous meningitis with blindness and perichiasmal involvement on MRI. PediatrNeurol 12: 65-67.
- Tsai MH, Huang YC, Lin TY (2004) Development of tuberculoma during therapy presenting as hemianopia. PediatrNeurol 31: 360-363.
- Sunbul M, Leblekicioglu H, Turan D (2007) Permanent visual loss despite appropriate therapy in tuberculous meningitis. South Med J 100: 228-229.
- Shankar P, Manjunath DV, Mohan KK (1991) Rapid diagnosis of tuberculous meningitis by polymerase chain reaction. Lancet 337:5-7.
- Ahuja GK, Mohan KK, Prasad K, Behari M (1994) Diagnostic criteria for tuberculous meningitis and their validation. Tuber Lung Dis 75 1994; 149-152.
- Medical Research Council Report (1948) Streptomycin treatment of tuberculous meningitis. Lancet 528-596.
- Crum RM, Anthony JC, Bassell SS, Folstein MF (1993) Population based norms for the Mini Mental Status Examination by age and education level. JAMA 269: 2386-2391.
- Halliday AM, Kriss A (1993) The visual evoked potential and electroratinogram in the investigation of diseases of the eye. In: Halliday AM, edn. Evoked Potentials in Clinical Testing . 2nd ed Edinburgh Churchill Livingstone 141-194.
- The 10TH revision of the WHO international statistical classification of diseases (1993) Injuries and causes of death. WHO, Geneva.
- Thwaites GE, Nguyen DB, Nguyen HD (2004) Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 351: 1741-1751.
- Mahoney FI, Barthel DW (1967) Functional evaluation: the Barthel index. Md State Med 14: 61-65.
- Schoeman JF, Van Zyl Le, Laubscher JA, Donald PR (1997) Effect of corticosteroids on intracranial pressure, computed tomographic findings and clinical outcome in young children with tuberculous meningitis. Pediatrics 99: 226-231.
- Lamba PA, Bhalla JS, Mullick DN (1986) Ocular manifestations of tubercular meningitis: a clinico- biochemical study. J PediatrOphthalmol Strabismus 23: 123-125.
- Ghose SB (1983) Optic nerve changes in hydrocephalus. Trans OpthalmolSoc (UK) 103: 217- 220.
- Arulprakash S, Verma SP, Bhardwaj PK, Mishra SS, Chansoria M (2006) Brain stem auditory evoked response and visual evoked responses in children with tubercular meningitis. Indian Pediatrics 43: 631-634.
- Wilcox LM Jr, Scott RM (1976) Arachnoiditis and VECP change. J PediatrOpthalmol 13: 346-349.
- Srivastava AK, Goel UC, Bajaj S, Singh KJ, Dwivedi NC, et al. (1997) Visual evoked responses in ethambutol induced optic neuritis. J Assoc Physicians India 45:847-849.
- Liebold JE (1966) The ocular toxicity of ethambutol and its relation to dose. Ann NY AcadSci 135: 904.
- SinhaMK, Garg RK, Anuradha HK, Agarwal A, Singh MK et al. (2010) Visual impairment in tuberculous meningitis: Predictors and Prognosis. J NeurolSci 290: 27-32.
Citation: Abbas A, Shukla R, Ahuja RC, Gupta RK, Singh KD, et al. (2016) Visual Impairment in HIV Negative Tuberculosis Meningitis. J Meningitis 1:107. DOI: 10.4172/2572-2050.1000107
Copyright: ©2016 Abbas A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 15079
- [From(publication date): 6-2016 - Jul 19, 2025]
- Breakdown by view type
- HTML page views: 14004
- PDF downloads: 1075