Research Article Open Access
Viral Infections in Renal Transplant Recipients in Belarus
| Tamara Vasil’evna Amvrosieva1*, Zoya Fedorovna Bogush1, Natallia Uladzimirauna Paklonskaya1, Oleg Valentinovich Kalachik2, Elena Petrovna Kishkurno3 and Kanstantsin Leanidavich Dziadziulia1 | ||
| 1The Republican Research and Practical Center for Epidemiology and Microbiology, Minsk, Belarus | ||
| 2The Republican Scientific and Practical Center of Organ and Tissue Transplantation, Minsk, Belarus | ||
| 3Belarusian Medical Academy of Post-Graduate Education, Minsk, Belarus | ||
| Corresponding Author : | Dr. Tamara Amvrosieva The Republican Research and Practical Center for Epidemiology and Microbiology 23, Philimonov str., 220114, Minsk, Republic of Belarus Tel: +375172650896 E-mail: amvrosieva@gmail.com |
|
| Received February 20, 2015; Accepted April 24, 2015; Published April 30, 2015 | ||
| Citation: Amvrosieva TV, Bogush ZF, Paklonskaya NU, Kalachik OV, Kishkurno EP, et al. (2015) Viral Infections in Renal Transplant Recipients in Belarus. J Infect Dis Ther 3: 212. doi: 10.4172/2332-0877.1000212 | ||
| Copyright: © 2015 Amvrosieva T et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
The presented study had its aim in establishing the prevalence and etiological structure of post-transplant viral complications and the kinetics of infection development. Two groups of recipients were examined: Group 1 comprised patients without any clinical signs of infection (n=84) monitored for a year, Group 2 included recipients with a clinical presentation of infection (n=92). 591 blood samples and 446 urine samples from 196 patients (20 donors and 176 renal transplant recipients) were analyzed. All donor-recipient pairs were tested for CMV, EBV, and HSV-1, 2. Latent viral infection reactivation was detected by the presence of viraemia (DNAemia) induced by CMV, EBV, HSV-1, 2, HHV-6, VZV, PVB19, or viruria (DNAuria) induced by BKV.
The percentage of Group 1 patients with viral DNA detected amounted to 41.9% for CMV, 30.4% for EBV, 17.4% for BKV, 5.6% for HHV-6, 1.9% for AdV, and 1.7% for PVB19. HSV-1, 2 and VZV infections in this group were not observed. The rate of CMV, EBV, and BKV infection reactivation, determined by the viraemic patients percentage, increased with time and peaked 1, 1.5 and 2 months after transplantation for EBV, BKV, and CMV, respectively. Three to six months after transplantation, the percentage of patients with active CMV, EBV, or BKV infection decreased significantly; and later on, viraemia was only occasional finding in Group 1 patients. Examination of Group 2 patients revealed that the predominant etiological agents of post-transplant viral complications were CMV (24.4%), EBV (18.8%), and BKV (17.6%).
The current study is the first complex investigation into the etiological structure and activation kinetics of viral infections in renal transplant recipients in Belarus. The results have major implications for early prevention of potential viral complications and adjustment of the therapy regimens for kidney transplant recipients currently in use.
| Keywords Renal transplantation; Viral infection; Viraemia; Viruria |
| Introduction Approximately 200 successful kidney allograft transplantations are carried out annually in Belarus. Complications of infectious origin remain a serious issue impacting the ultimate outcome and general health status of a recipient in the post-transplant period. |
| Patients who have undergone organ transplantation can face both a new post-transplant viral infection and reactivation of a pre-existing latent infection. The etiological agents include cytomegalovirus (CMV), herpes simplex virus 1 and 2 (HSV-1, 2), Epstein-Barr virus (EBV), varicella zoster virus (VZV), human herpes virus 6 (HHV-6), parvovirus B19 (PVB19), BK virus (BKV), adenovirus (AdV), hepatitis B virus and hepatitis C virus (HBV and HCV), and human immunodeficiency virus (HIV) [1-16]. The available data suggest that CMV and BKV are the predominant agents of the most severe and hard to manage post-transplant complications which, in the setting of immunosuppressive therapy and lack of antiviral treatment, not infrequently lead to nephropathy and hemorrhagic cystitis in renal transplant recipients [3,4,8]. Currently, etiotropic therapy against CMV infection is successfully used for treatment of such patients as well as for prevention of these complications after organ transplantation [17]. There are also a number of therapeutic agents effective against BK virus infection [18,19]. Another well-established approach to reducing the BK virus load in immunodeficient patients such as kidney transplant recipients involves adjustment of the immunosuppressive therapy regimens (dose reduction, immunosuppressant repertoire alteration, etc.). In this context, specific differential diagnostics of viral infections, aimed at ascertainment of the etiology in order to prescribe an adequate therapy which would include antiviral agents, is of significant practical importance. |
| This study is dedicated to the analysis of the results of serological and genetic diagnostics of viral infections associated with renal transplantation. Presented are the data on the prevalence and etiological structure of post-transplant viral complications as well as the assessment of infection development kinetics. |
| Materials and Methods In total 591 blood samples and 446 urine samples from 196 patients (20 donors and 176 recipients) were tested. The samples were received from the Republican Research and Practical Center of Organ and Tissue Transplantation at the 9th Minsk City Hospital. Two groups of patients were formed: Group 1 comprised patients without any clinical signs of infection (n=84) monitored over a year after organ transplantation; Group 2 included recipients with apparent clinical manifestations of infection (n=92), these were examined only when clinically indicated. |
| Whole blood samples were incubated at 37°C for an hour and centrifuged at 239 × g for 10 minutes to separate the serum. Urine samples were diluted 5 times with transport medium (AmpliSens, Russia) prior to nucleic acid isolation. |
| Viral nucleic acids were isolated with RNA-SORB (serum samples), DNA-SORB B (whole blood samples) and RIBO-PREP kits (AmpliSens, Russia). |
| For detection of CMV, EBV, HSV-1, 2, HHV-6, VZV, PVB19, and AdV DNA real-time PCR kits (AmpliSens, Russia) and RotorGene 3000 and 6000 thermocyclers (Corbett Life Sciences, Australia) were used. |
| BKV DNA detection in real-time PCR was carried out with Taq-polymerase, 10� reaction buffer and MgCl2 solution (PrimeTech, Belarus), dNTP mix (Fermentas, Lithuania), and a set of primers and probes [14] synthesized by PrimeTech (Belarus). |
| Results Donors and recipients serostatus prior to organ transplantation One of the approaches to early identification of recipients with high risk of viral complications consists in determining the serostatus (presence/absence of antiviral IgG) in donor/recipient (D/R) pairs with regard to the most relevant viral pathogens – CMV, EBV, and HSV-1, 2. |
| In the present study the overall rate of antiviral IgG detection in donors (n=20) was 100% for HSV-1, 2, 85.0% for EBV, and 95.0% for CMV. In recipients (n=36) the respective values amounted to 97.2% for HSV-1, 2, 97.2% for EBV, and 94.4% for CMV. |
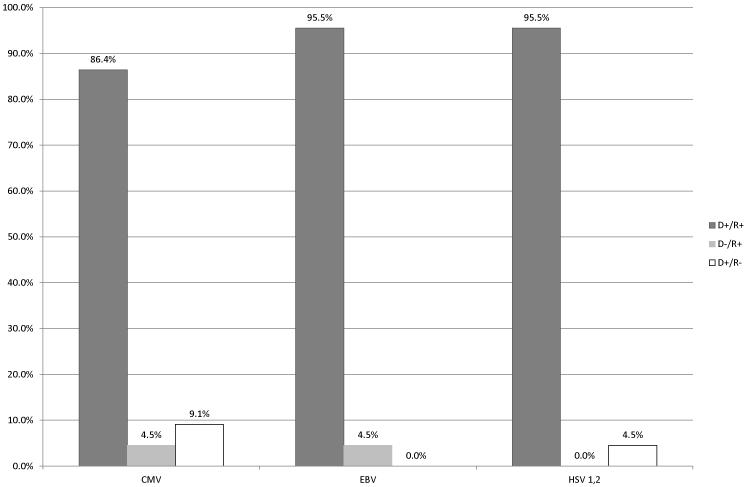
| Analysis of serostatus in D/R pairs (n=22) for each of the viruses demonstrated that the vast majority of recipients belonged to the moderate risk group in regard to post-transplant viral complications (Figure 1). In particular, this group included 90.9% of recipients (86.4% [D+/R+], 4.5% [D–/R+]) in respect to CMV, 100% of recipients (95.5% [D+/R+], 4.5% [D–/R+]) in respect to EBV, and 95.5% of recipients (all of them [D+/R+]) in respect to HSV-1, 2. Only a small proportion of patients belonged to the high-risk group [D+/R–]: 9.1% of recipients in respect to CMV and 4.5% of recipients in respect to HSV-1, 2. Patients with low-risk of viral complications [D–/R–] were not observed. |
| Virological monitoring of renal transplant recipients Eighty four patients (Group 1) were monitored over a year after kidney allograft transplantation for potential reactivation of latent viral infections (CMV, EBV, HSV-1, 2, BKV, HHV-6, VZV, AdV, and PVB19). Prior to organ transplantation, single blood and urine samples were taken from each patient. Viral DNA detection in serum and urine samples collected prior to transplantation revealed active CMV, EBV, AdV, and BKV infection in 5.0%, 6.3%, 5.6%, and 1.8% of patients, respectively; HSV-1, 2, VZV, and PVB19 were not detected. |
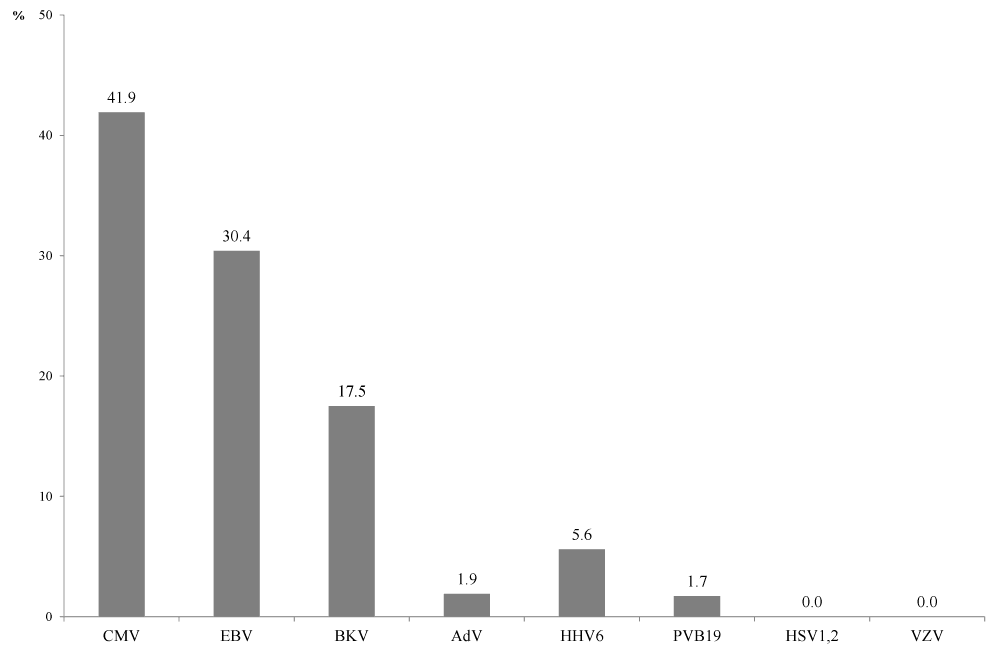
| In general, the proportion of recipients with reactivated viral infection (viraemia confirmed at least once over the period of observation) was 41.9 % (n=74, 31 positive findings) for CMV, 30.4% (n=79, 24 positive findings) for EBV, 17.4% (n=63, 11 positive findings), 5.6% (n=71, 4 positive findings) for HHV-6, 1.9% (n=54, 1 positive finding) for AdV, and 1.7% (n=60, 1 positive finding) for PVB19 (Figure 2). No recipient tested positive for HSV-1, 2 and VZV. |
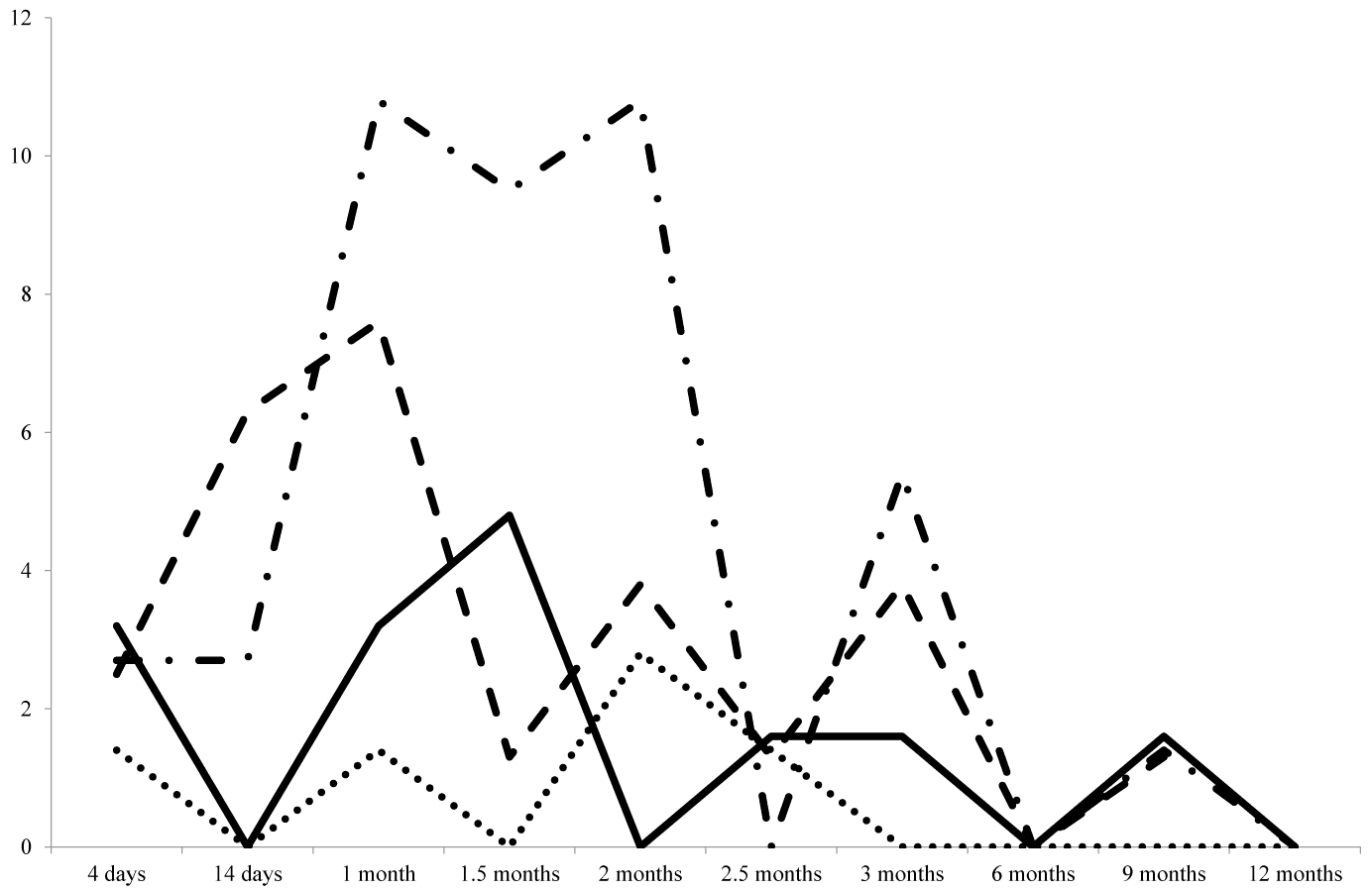
| After transplantation, the following sample collection scheme was used: within first 3 months – day 4 and every 2 weeks afterwards (14 days, 1 month, 1.5 months, 2 months, 2.5 months, and 3 months), after 3 months and up to 1 year – every 3 months (6, 9 and 12 months). The percentage of renal allograft recipients with viral infections reactivation (the viraemic patients in whom virus reactivation had been previously confirmed were not taken into account) was estimated for each of the time points. Figure 3 demonstrates the kinetics of the predominant infections (CMV, EBV, BKV, and HHV-6) reactivation over a year after organ transplantation. The first occurrences of viraemia and viruria were registered as early as 4 days after surgery (2.7%, 2.5%, 1.4%, and 3.2% of patients in regard to CMV, EBV, HHV-6, and BKV, respectively). Follow-up observation showed that viral reactivation was most frequent in the first 2 months after transplantation; however, various agents were characterized by slightly different patterns of reactivation kinetics. Thus, virus reactivation rate peaked between 14 days and 1 month after transplantation for EBV (6.3–7.6% of patients), between 1 and 2 months after transplantation for CMV (10.8% of patients), between 1 and 1.5 months after transplantation for BKV (4.8% of patients), and in 2 months after transplantation for HHV-6 (2.8% of patients). HHV-6 infection reactivation was only transient, subsequent testing did not show the presence of the virus in blood. After the virus reactivation, CMV viraemia in 48.4% of patients was transient, in 9.6% of patient persisted for 2 weeks and in 35.0% persisted for over a month, 6.4% of patients remained viraemic for 2–4.5 months. |
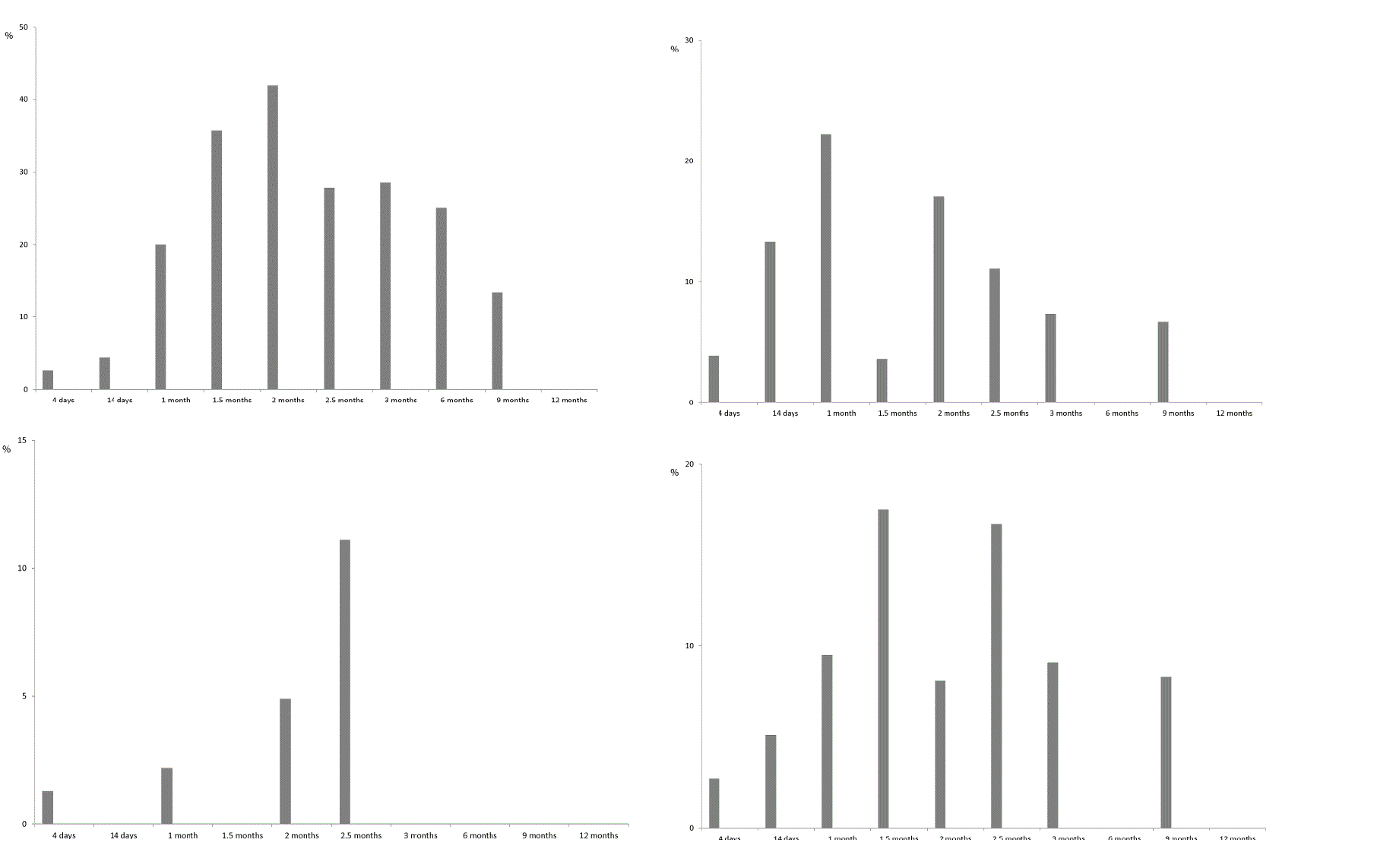
| EBV viraemia was transient in 65.2% of patients and persisted for 2 weeks or more than a month in 8.7% and 26.0% of patients, respectively. Viruria, which indicates active BKV infection, in 54.0% of patients was transient and in 46% of patients persisted for 1–3 months. All the patients with Ð�Ð�V viruria were tested for BKV viraemia, but there were no positive findings in blood samples. Figure 4 show the results of renal allograft recipients monitoring for the signs of viraemia or viruria. As can be seen from the figure, the proportion of patients with active viral infections gradually increases with the build-up of immunosupression. The maximum percentage of recipients with markers of CMV infection in blood was registered in 2 months after transplantation (41.9% of recipients), with EBV viraemia or BKV viruria-in a month after transplantation (22.2% and 17.4% of recipients, respectively), and with HHV-6 viraemia-in 2.5 months after transplantation. There were only single instances of PVB19 and AdV DNA detection – 14 days and 2 months after transplantation. |
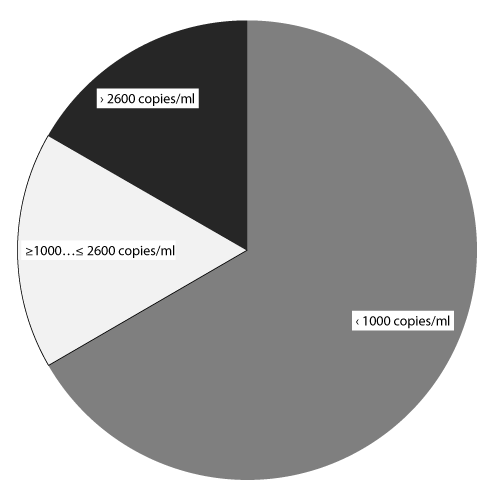
| Since CMV reactivation and persistence were seen in patients after transplantation most often, patients with active CMV infection were quantitatively assessed for viral load in blood. For the 24 recipients with established CMV viraemia viral load levels were estimated in quantitative real-time PCR (copies/ml), which varied from tens of copies per ml to 133 000 copies/ml (Figure 5). In the majority of recipients (66.7%, 16/24 patients) viral load values didn’t exceed 1000 copies/ml, 4 patients (16.7%) had viral load in the range of 1000–2600 copies/ml, and in another 4 patients (16.7%) viral load levels exceeded 2600 copies/ml. |
| Genetic diagnostics of viral complications in renal transplant recipients Virological examination of the Group 2 renal transplant recipients (n=92) with specific clinical manifestations of infection (different flu-like conditions, fever, myalgia, arthralgia, anorexia, asthenia, leucopenia, thrombocytopenia) as well as with signs of transplant dysfunction, or relapses of autoimmune hepatitis or hepatitis B virus reactivation demonstrated presence of viral genetic markers in almost half of them (47.8%). The predominant agents were CMV (24.4%, 22/90 patients), EBV (18.8%, 16/85 patients), and BKV (17.6%, 6/34 patients). The proportion of recipients with HHV-6 infection was 7.9% (5/63), and PVB19 was detected in 6.7% of patients (2/30). There were no positive findings for HSV-1, 2, VZV, or AdV (number of patients tested for each of the viruses was 31, 7, and 6, respectively). |
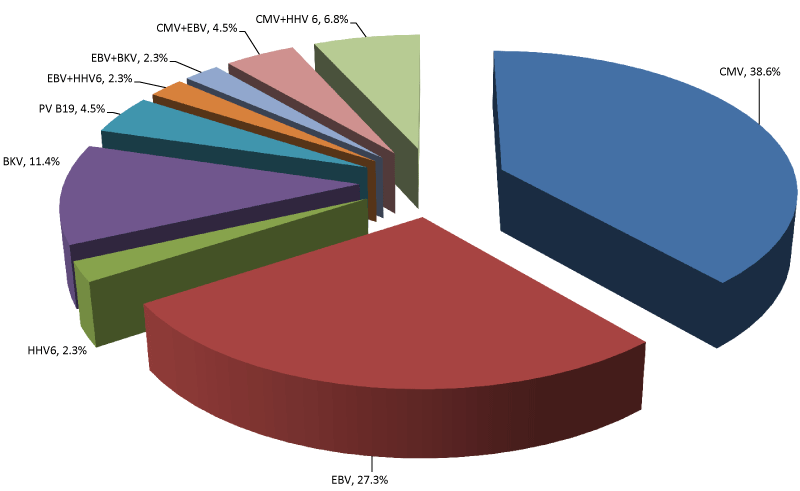
| The majority of virus-positive recipients (84.1%, 37/44 patients) had monoinfection caused by CMV (n=17, 38.6%), EBV (n=12, 27.3%), BKV (n=5, 11.4%), PVB19 (n=2, 4.5%), or HHV-6 (n=1, 2.3%). Seven of 44 positive recipients (15.9%) experienced a co-infection of 2 agents in different combinations: CMV+HHV-6 (6.8%), CMV+EBV (4.5%), EBV+BKV (2.3%), EBV+HHV-6 (2.3%) (Figure 6). |
| Discussion |
| It is currently believed that up to a half of post-transplant complications can be of viral origin. Various studies showed that the proportion of renal transplant recipients with viral infection was 8–32% for CMV, up to 53% for HSV-1, 2, and 4–12% for VZV [1]. Viral complications are often associated not with primary infection but rather with reactivation of previously latent viruses. In view of the recent advance of solid organ transplantation in Belarus, research into the risk factors of post-transplant complications is of current concern for our country. In the present study we investigated the spectrum of latent viral infections which most often reactivate in the first year after renal allograft transplantation, the kinetics of their reactivation, the impact of a donor/recipient pair serostatus on the frequency of latent infections reactivation, as well as the proportion of post-transplant complications likely associated with reactivation of latent infections. In total 176 renal allograft recipients were tested, including 84 patients without clinical signs of post-transplant complications in Group 1 (this group was monitored for viral infections reactivation in the post-transplant period) and 92 patients with clinical symptoms potentially associated with latent viral infections reactivation (fever, myalgia, arthralgia, anorexia, asthenia, leucopenia, thrombocytopenia, signs of transplant dysfunction) in Group 2. |
| Diagnostic testing was performed in accordance with a special scheme specifying the list of viral agents typically associated with post-transplant complications to be detected in clinical samples (blood, urine). These included CMV, BKV, EBV, HSV-1, 2, HHV-6, VZV, PVB19, HBV, HCV, and HIV. The scheme comprised 2 stages: 1) establishing the serological and infection status in donors and recipients prior to renal transplantation; and 2) viral infection screening with subsequent monitoring for viral complications in post-transplant period. Prior to renal transplantation, screening for viral infections was mandatory both for potential donors and recipients. At that time, patients were tested for herpetic viruses serostatus (CMV, EBV, and HSV-1, 2), active BK virus infection (detection of BKV DNA in urine) as well as chronic viral hepatitis Ð� and hepatitis С, and HIV infection. In post-transplant period, laboratory monitoring consisted in PCR screening of blood and urine samples for DNA of HSV-1, 2, CMV, BKV, EBV, HHV-6, VZV, PVB19 and AdV as most likely agents of viral complications. In the event of CMV detection, viral load was determined in quantitative real-time PCR (DNA level, copies/ml). Reaching of a certain viral load threshold value triggered initiating of a specific etiotropic treatment and/or adjustment of the immunosuppressive therapy regimen. Testing of the recipients for the other viral infections from the pre-specified list was performed only when clinically indicated. |
| The primary objective of serological examination of D/R pairs was to prevent organ transplantation from a seropositive donor to a seronegative recipient, and also to perform targeted prophylaxis of potential post-transplant viral complications by prescribing a patient-specific antiviral treatment and adjusting the regimen of immunosuppressive therapy. The results of serological diagnostics indicated which group in regard to post-transplant viral complications risk a given D/R pair belonged to: [D−/R−] serostatus (seronegative donor and recipient) – low-risk group, [D+/R+] serostatus (seropositive donor and recipient) and [D−/R+] serostatus (seronegative donor and seropositive recipient) – moderate risk group, [D+/R−] (seropositive donor and seronegative recipient) – high-risk group. Our results demonstrated that the vast majority of the tested recipients (90.9% â�� 100.0%) belonged to the moderate risk group ([D+/R+] and [D−/R+]) in regard of complications caused by CMV, EBV, and HSV-1, 2. No low-risk recipients were found in regard of any of the infections under study, and among the high-risk recipients most were at risk of complications caused by CMV (9.1%, 2/22 patients). |
| The assessment of reactivation kinetics for the most prevalent viral agents (CMV, EBV, BKV, and HHV-6) revealed that the time of virus reactivation in post-transplant period varied. CMV was first detected 45.4±4.05 days after transplantation, EBV – 31.8±5.06 days after transplantation, and BKV – 38.1±8.67 days after transplantation. |
| Cytomegalovirus infection is one of the most widespread post-transplant complications. CMV disease is a common cause of acute graft rejection and loss [20]. In our studies CMV was the predominant viral agent found both in asymptomatic patients (Group 1) and in recipients with clinical signs of post-transplant complications (Group 2). Analysis of the frequency and time of viraemia appearance showed that 42.1% of patients in the moderate risk group in respect to CMV infection became viraemic (CMV DNA detection in the serum) within 3 months after transplantation. The first occurrences of CMV viraemia were registered as early as 4 days after surgery. Later on, the rate of virus detection markedly increased and peaked by the 2nd month (41.9% of patients). Thereafter, the proportion of patients with active CMV infection (i.e. with viraemia) decreased. There was a plateau between 2.5 and 3 months when the rate of the virus genetic markers detection was almost constant (27.8% to 28.6% of patients, respectively). Three to 6 months after transplantation, detection rate declined to 25.0%, and afterwards CMV infection was registered only occasionally or not at all. In the majority of the patients (66.7%) the viral load levels didn’t reach the threshold values (the conventional threshold value for viral load in the case of CMV infection was 2600 or 1000 copies/ml, depending on the administration of prophylactic therapy or lack thereof, respectively). All of the recipients with subthreshold CMV load levels had been seropositive prior to transplantation and received kidney transplant form a seropositive donor (CMV serostatus [D+/R+]). Eight patients (33.3%) had significantly higher levels of CMV replication: 2000 to 2600 copies/ml in 4 patients, 7000 to 21 000 copies/ml in 3 patients and 133 000 copies/ml in one patient. Further investigation revealed development of the CMV syndrome in these patients (CMV DNA load over 2000 copies/ml in combination with generalized symptoms of viral infection: fever over 38°Ð¡ without apparent cause for 2 days or more, myalgia, arthralgia, anorexia, asthenia, leucopenia, thrombocytopenia, and other flu-like conditions). Time of CMV syndrome occurrence differed: 5 recipients developed it within 3 months after transplantation, 3 patients reported it between 3 and 9 months after surgery. |
| Latent EBV infection reactivation can lead to post-transplant lymphoproliferative disorder [21]. The risk of post-transplant lymphoproliferative disorder after renal transplantation is lower than after transplantation of other organs (1–3%). Besides, the main risk group for the development of this condition consists of recipients from donor/recipient pairs with [D+/R–] serostatus [22]. As has been previously mentioned, all the recipients in our studies belonged to the moderate risk group ([D+/R+] and [D−/R+]) in regard to EBV-associated complications. Peak of EBV infection reactivation was registered 14 days after transplantation. EBV viraemia was characterized by marked increase in percentage of positive patients in the period from 14 days and up to 2 months after transplantation. Peak of viraemia rate was registered at the timepoint of 1 month after transplantation (22.2% of patients), subsequently the frequency of EBV detection declined (down to 7.3%of patients by the 3rd month). From the 3rd to the 12th months this infection was diagnosed only occasionally. Our data on the EBV reactivation timeframe are consistent with the results obtained by other researchers which suggested that in most patients EBV viraemia occurred in the first month after transplantation [23]. Most often the viraemia was only transient, but in 26% of patients the infection remained active for a month and longer. Bamoulid et al. have shown that persistent EBV viraemia increased the risk for emergence of other opportunistic infections [23]. However, in our experience patients with enduring EBV infection didn’t have any opportunistic infections. |
| Since the end 1990s, BKV has been considered as one of the dominant etiological agents of infectious complications after renal transplantation [24]. Given the fact that viraemia and viruria precede the development of BKV-associated nephropathy, monitoring of BKV activation is an important part of today’s routine examination of the recipients in post-transplant period. Although BK-viraemia is a more reliable predictor of BKV-associated nephropathy than BK-viruria [25], to assess the kinetics of reactivation we monitored the presence of the virus in patients’ urine. Subsequently, all the patients with BK-viruria were screened for BK-viraemia. None of the patients of Group 1 had Ð�Ð�-viraemia during the period of observation. One of the patients of Group 2 with clinical signs of transplant dysfunction was confirmed to have Ð�Ð�-viraemia later after detection of the virus in urine, however, during repeated testing the viraemia was absent. In general, the proportion of patients with active BKV-infection in our studies amounted to 17.4%, which is consistent with the results of other authors [26]. BKV infection reactivation rate reached its maximum 1.5 months after transplantation with subsequent decline down to 9.1% by the 3rd month. Afterwards, this virus was identified only incidentally. |
| Although HHV-6 is considered by some authors as an emerging virus causing post-transplant complications in transplant recipients [27], and HHV-6 viraemia has been reported for a large proportion of patients [27–29], in our experience markers of HHV-6 infection were much rarer findings in the recipients’ blood samples; maximum detection rate was registered 2–2.5 months after transplantation (4.88% and 11.11% of patients, respectively) and later on (up to 12 months of follow-up) this agent was not detected. Our data suggest that HHV-6 reactivation occurs later than in the case of other opportunistic infections (EBV, CMV, BKV); while it has been shown elsewhere that HHV-6 viraemia develops earlier than EBV, or CMV viraemia [27]. Also, several authors described HHV-6 interaction with other viruses such as CMV [28, 30]. In the present study 80% of patients with HHV-6 viraemia had concurrent active CMV infection, which was not transient (CMV viraemia persisted for 1–2 months). |
| In summary, the results of the study have demonstrated presence of a wide range of viral complications in renal transplant recipients, with CMV, EBV, and BKV being the predominant etiological causes. |
| The presented data have apparent practical relevance highlighting the need for mandatory testing of recipients serostatus with regard to certain viral infections (CMV, EBV, and HSV-1, 2) in order to assess the risk of post-transplant viral complications and to elaborate adequate antiviral prophylaxis and treatment strategy. Subsequent virological monitoring of the recipients, even before they develop any clinical signs of infection, along with early differential genetic diagnostics of viral complications constitute obligatory elements of laboratory examination in post-transplant period. The resulting data on the infection status of recipients have significant importance for the opportune adjustment of the therapy regimens, including addition of etiotropic antivirals at a certain level of viral load and/or immunosuppressant dose reduction. |
| The proposed approach to the management of patients undergoing renal transplantation has been successfully introduced into practice of specialized medical institutions in Belarus. |
References
- Muñoz P, Fernández NS, Fariñas MC (2012) Epidemiology and risk factors of infections after solid organ transplantation. EnfermInfeccMicrobiolClin 30 Suppl 2: 10-18.
- Fishman JA (2007) Infection in solid-organ transplant recipients. N Engl J Med 357: 2601-2614.
- Rowshani AT, Bemelman FJ, van Leeuwen EM, van Lier RA, ten Berge IJ (2005) Clinical and immunologic aspects of cytomegalovirus infection in solid organ transplant recipients. Transplantation 79: 381-386.
- Razonable RR, Rivero A, Rodriguez A, Wilson J, Daniels J, et al. (2001) Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxis with oral ganciclovir. J Inf Dis 184: 1461-1464.
- Kotton CN, Fishman JA (2005) Viral infection in the renal transplant recipient. J Am SocNephrol 16: 1758-1774.
- Weikert BC, Blumberg EA (2008) Viral infection after renal transplantation: surveillance and management. Clin J Am SocNephrol 3 Suppl 2: S76-86.
- Zuckerman R, Wald A; AST Infectious Diseases Community of Practice (2009) Herpes simplex virus infections in solid organ transplant recipients. Am J Transplant 9 Suppl 4: S104-107.
- Mylonakis E, Goes N, Rubin RH, Cosimi AB, Colvin RB, et al. (2001) BK virus in solid organ transplant recipients: an emerging syndrome. Transplantation 72: 1587-1592.
- Waldman M, Kopp JB (2007) Parvovirus-B19-associated complications in renal transplant recipients. Nat ClinPractNephrol 3: 540-550.
- Waldman M, Kopp JB (2007) Parvovirus B19 and the kidney. Clin J Am SocNephrol 2 Suppl 1: S47-56.
- Morton LM, Gibson, Clarke, Lynch CF, Weisenburger DD, et al. (2007) Hepatitis C virus infection and risk of posttransplantationlymphoproliferative disorder among solid organ transplant recipients. Blood 110: 4599-4605.
- Jenkins FJ, Rowe DT, Rinaldo CR Jr (2003) Herpesvirus infections in organ transplant recipients. ClinDiagn Lab Immunol 10: 1-7.
- Ison MG (2006) Adenovirus infections in transplant recipients. Clin Infect Dis 43: 331-339.
- Hoffman NG, Cook L, Atienza EE, Limaye AP, Jerome KR (2008) Marked variability of BK virus load measurement using quantitative real-time PCR among commonly used assays. J ClinMicrobiol 46: 2671-2680.
- Frassetto LA, Tan-Tam C, Stock PG (2009) Renal transplantation in patients with HIV. Nat Rev Nephrol 5: 582-589.
- Dall A, Hariharan S (2008) BK virus nephritis after renal transplantation. Clin J Am SocNephrol 3 Suppl 2: S68-75.
- Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, et al. (2006) Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant 6: 2134-2143.
- Held TK, Biel SS, Nitsche A, Kurth A, Chen S, et al. (2000) Treatment of BK virus-associated hemorrhagic cystitis and simultaneous CMV reactivation with cidofovir. Bone Marrow Transplant 26: 347-350.
- Williams JW, Javaid B, Kadambi PV, Gillen D, Harland R, et al. (2005) Leflunomide for polyomavirus type BK nephropathy. N Engl J Med 352: 1157-1158.
- Baldanti F, Lilleri D, Gerna G (2008) Monitoring human cytomegalovirus infection in transplant recipients. J ClinVirol 41: 237-241.
- Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, et al. (2009) Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia.Bone Marrow Transplant 43: 757-770.
- Weikert BC, Blumberg EA (2008) Viral infection after renal transplantation: surveillance and management. Clin J Am SocNephrol 3 Suppl 2: S76-86.
- Bamoulid J, Courivaud C, Coaquette A, Chalopin JM, Gaiffe E, et al. (2013) Subclinical Epstein-Barr virus viremia among adult renal transplant recipients: incidence and consequences. Am J Transplant 13: 656-662.
- Hirsch HH, Steiger J (2003) Polyomavirus BK. Lancet Infect Dis 3: 611-623.
- Hirsch HH, Brennan DC, Drachenberg CB, Ginevri F, Gordon J, et al. (2005) Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 79: 1277-1286.
- Schiavelli R, Bonaventura R, Rial MC, Petrone H, Pujol GS, et al. (2014) First epidemiologic study in Argentina of the prevalence of BK viruria in kidney transplant patients. Transplant Proc 46: 3010-3014.
- Smith JM, McDonald RA (2006) Emerging viral infections in transplantation. Pediatr Transplant 10: 838-843.
- Griffiths PD, Ait-Khaled M, Bearcroft CP, Clark DA, Quaglia A, et al. (1999) Human herpesviruses 6 and 7 as potential pathogens after liver transplant: prospective comparison with the effect of cytomegalovirus. J Med Virol 59: 496-501.
- Costa FA, Soki MN, Andrade PD, Bonon SH, Thomasini RL, et al. (2011) Simultaneous monitoring of CMV and human herpesvirus 6 infections and diseases in liver transplant patients: one-year follow-up. Clinics (Sao Paulo) 66: 949-953.
- Humar A, Kumar D, Caliendo AM, Moussa G, Ashi-Sulaiman A, et al. (2002) Clinical impact of human herpesvirus 6 infection after liver transplantation. Transplantation 73: 599-604.
Figures at a glance
 |
 |
 |
 |
 |
 |
|||||
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14363
- [From(publication date):
April-2015 - Jul 12, 2025] - Breakdown by view type
- HTML page views : 9704
- PDF downloads : 4659
