VIP and PACAP as Regulators of Immunity: New Perspectives from A Receptor Point of View
Received: 11-Feb-2016 / Accepted Date: 12-Mar-2016 / Published Date: 15-Mar-2016
Abstract
Vasoactive Intestinal Peptide (VIP) and Pituitary Adenylate Cyclase-activating Polypeptide (PACAP) are two neuropeptides acting through three common G-protein coupled receptors (VPAC1, VPAC2 and PAC1). Among their pleiotropic actions within the organism, VIP and PACAP are known to exhibit immunomodulatory properties in both the innate and adaptive immune axes. The fact that they inhibit inflammation in murine models of disease has brought these peptides into the spotlight within the field of therapeutic discovery for autoimmune/inflammatory diseases. Pharmacological tools and transgenic mice have been useful in order to investigate the involvement of each of their three receptors in these actions. This review focuses on the relevance of the VPAC2 receptor on VIP and PACAP modulation of immune responses, and discusses its potential as a target for the treatment of Th1-driven inflammatory disorders.
Keywords: Vasoactive intestinal peptide (VIP); Inflammatory diseases; Pituitary Adenylate Cyclase-activating Polypeptide (PACAP)
5377VIP And PACAP
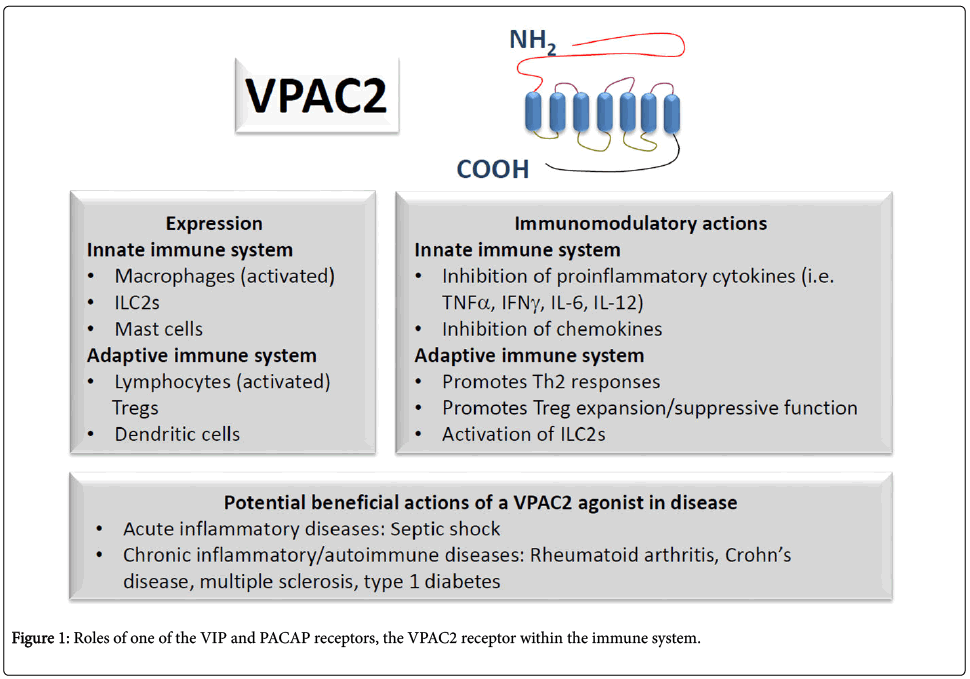
VIP (vasoactive intestinal peptide) and PACAP (pituitary adenylate cyclase-activating polypeptide) belong to a superfamily of structurally related peptides including secretin and glucagon. Whereas VIP is a 28 aminoacid peptide, PACAP can be found in two amidated forms of 27 (PACAP27) or 38 (PACAP38) aminoacids. Because these peptides exhibit a high sequence and structural homology (i.e. 68% identity between VIP and PACAP27), it has been proposed that their genes derived from a common ancestral gene subjected to duplication and divergence during the course of evolution [1-3]. VIP and PACAP were originally isolated from the small intestine and the pituitary, respectively, although it has been later demonstrated that they are widely distributed in the organism [4,5]. The fact that their primary structures have been well conserved in vertebrates suggest that they play important physiological actions. In fact, they modulate multiple processes of the digestive, respiratory, reproductive and cardiovascular systems among others. VIP and PACAP act through three G-protein coupled receptors (GPCRs) named VPAC1, VPAC2 and PAC1 [6]. Whereas VPAC1 and VPAC2 bind both VIP and PACAP with equal high affinity, PAC1 exhibits 100 to 1000 times higher affinity for PACAP than for VIP [1]. Their main signaling pathway involves adenylate cyclase activation through accessory G-proteins and cyclic AMP (cAMP) synthesis. Nevertheless, activation of other signaling pathways involving phospholipase C (PLC) or phospholipase D (PLD) or intracellular calcium increases has been also reported [7]. An overview of the roles of one of the VIP and PACAP receptors, the VPAC2 receptor within the immune system compartment is discussed in the present review and is illustrated in the figure 1.
The VPAC2 Receptor
VPAC2 was first cloned by Lutz et al. [8] from rat olfactory bulb. Mouse and human VPAC2 were subsequently cloned from insulinsecreting beta-cell line MIN6 and SUP-T1 lymphoblast libraries, respectively [9]. Its gene Vipr2 maps to the rat chromosome 4, the mouse F2 region of chromosome 12 and human chromosomal region 7q36.3 [10,11]. Regarding its distribution, in the central nervous system, its highest expression is found in the suprachiasmatic nuclei, where it modulates circadian rhythms [12], but it is also present in the thalamus, hypothalamus, midbrain and brainstem. In the periphery, a systematic study in mice revealed its expression in the smooth muscle of blood vessels and gastrointestinal and reproductive systems, lung, colon, kidney, adrenal medulla, retina and pancreas [13].
Based on its protein structure, VPAC2 belongs to the class B GPCR family receptors, which exhibit seven transmembrane domains and a series of common features such as a large N-terminal (Nter) ectodomain containing several N-glycosylation sites and six highly conserved cysteine residues forming three disulfide bridges, and a signal peptide for addressing the receptor towards the plasma membrane. Multiple structure-activity relationship studies from several research groups have shown that in all class B GPCRs, the large Nter domain is critical for ligand recognition; mostly due to the presence of a Sushi domain which is characterized by two antiparallel β sheets and stabilized by three disulfide bonds and by a salt bridge between acidic and basic residues.
Based on (1) photoaffinity experiments which identified four physical interaction sites between VIP and the Nter domain of VPAC1 receptor, (2) the NMR structure of the ligand VIP which revealed mostly an alpha helical structure, and (3) the 3D model of the VPAC1 N terminal domain comprising a Sushi domain [14,15], an accurate 3D model illustrating the interaction between the VIP molecule and the Nter domain of the VPAC1 receptor has been created. This model suggested that the C-terminal and central α-helical parts of the VIP peptide interact with the VPAC1 Sushi domain [14-17]. Consistent with a two-site binding model, it has been speculated that this may bring the N-terminus of the peptide into the appropriate position to contact the transmembrane region of the receptor leading to its subsequent activation.
Several VIP analogs with highly specific binding to VPAC2 have been generated. Among them, Ro 25-1392 and Ro 25-1553, are two cyclic derivatives of VIP [18-20]. Although these have been useful to identify VIP-VPAC2 mediated actions in various experimental settings, the presence of N-terminal acylation, cyclization from Lys-21 to Asp-25, C-terminal amidation, and O-Me-Tyr-10 or Nle-17 on these analogs imposes a big challenge for their synthesis, which may hamper their potential use as therapeutic drugs. These difficulties have been bypassed by the generation of a peptidic VPAC2 specific agonist with a simpler structure, BAY 55-9837, which was developed by Tsutsumi et al. through site-directed mutagenesis based on sequence alignments of PACAP, VIP, and related analogs [21]. Substitution of a valine in the aminoacid position 5 by a Cα-methylated valine in Ro 25-1553, and substitution of asparagines in positions 9 and 28 followed by sitespecific cysteine conjugation with a 22- or 43-kDa polyethylene glycol (PEG) for BAY 55-9837, have improved the stability of these agonists [22,23]. Recently, the use of chitosan-decorated selenium nanoparticles (CS-SeNPs) as protein carriers of BAY 55-9837 prolonged its half-life in vivo [24]. Due to a potential use of VPAC2 activation for the treatment of diabetes and asthma, most of the efforts have focused on developing VPAC2 agonists. However, a highly specific antagonist for this receptor was generated by myristoylation of the amino-terminus of (K(12)) VIP(1-26) extended carboxylterminally with a five aminoacid sequence of Ro 25-1553 [25]. This may be a useful tool in studies to dissect specific roles played by VPAC2.
VPAC2 Expression By Immune Cells
The expression of VIP and PACAP receptors in immune cell types has been described with different patterns. In resting lymphocytes, VPAC1 has been found to be constitutively expressed, and VPAC2 is absent or expressed at very low levels [26-28]. Nevertheless, upon in vitro activation of the CD3/TCR complex, VPAC1 is downregulated, at least transiently, and VPAC2 upregulated [26-28]. This has led to the hypothesis that VPAC2 may be the main receptor modulating the functionality of activated T cells, and it may become more relevant than other VIP and PACAP receptors in pathogenic situations that lead to T cell activation. Interestingly, VPAC2 has been reported to be upregulated in T CD4+ cells from HIV patients, which was not associated to the viral load but was suggested to reflect a repetitive exposure to antigens [29]. In another study, a higher VPAC2/VPAC1 ratio was found in memory T helper (Th) cells from early rheumatoid patients compared to healthy subjects, although in this case, this was mostly due to a strong decrease in VPAC1 expression, rather than a strong up regulation of VPAC2 [30].
In addition to wild type (WT) full length VPAC2, splice variants of VPAC2 have been identified by PCR in murine and human lymphocytes [31,32]. In mice lymphocytes, a variant with a 14 aminoacid deletion in the carboxyl-terminal end of the seventh transmembrane domain has been identified, which has the same affinity for VIP as the WT receptor, but does not induce cAMP upon binding [31,32]. In human lymphocytes, a variant with a 114 aminoacid deletion beginning with the carboxyl-terminal end of the third cytoplasmic loop variant was found, with reduced affinity for VIP and multiple functional differences with the WT receptor [32]. Nevertheless, the significance of these variants in vivo has not been further investigated.
Some studies have investigated the expression of VPAC2 expression in thymocytes. It has been reported that VPAC2 is the main VIPPACAP receptor expressed in human thymocytes [33]. However, different results were published in two studies in mice. Delgado et al. found a constitutive expression of VPAC1 and lack of VPAC2, which was induced by PMA and an anti CD3 antibody [27]. On the other hand, Vomhof-DeKrey et al. reported the presence of both VPAC1 and VPAC2 receptors in thymocytes, with a relative expression that varies along maturation: a predominant expression of VPAC1 in earliest thymic progenitor (ETP) and CD4 and CD8 double negative (DN) 1 cells, a switch to VPAC2 in DN2 and DN3 stages, and then back to VPAC1 in DN4 and subsequent double positive (DP) and single positive (SP) stages [34]. Species-specific differences in gene expression may explain the discrepancy between the profile of expression in human and mice thymocytes. In any case, because VPAC2 deficient mice do not exhibit alterations in the frequency of different thymocyte populations, the relevance of VPAC2 expression during thymocyte maturation remains to be elucidated.
In addition to lymphocytes, other immune cell populations have been reported to express VPAC2. For example, it is expressed in peritoneal macrophages and the macrophage cell line Raw 264.7 with a similar pattern to that in lymphocytes: VPAC2 has been reported to be absent or expressed at low levels in unstimulated cells, but is induced upon activation in vitro with gram-positive (toll like receptor (TLR)2 ligands) and gram-negative bacteria wall constituents (TLR4 ligands) [35-37]. Moreover, the TLR7 synthetic ligand imiquimod induced VPAC2 mRNA expression. The expression of VPAC2 has been also reported to be low in monocytes from healthy human subjects, but to be elevated in monocytes from patients with Sjogren’s syndrome [38]. Other studies, suggest that the inducible nature of VPAC2 expression on macrophages may be tissue specific. For example, a study reported a constitutive expression of VPAC2 in human lung macrophages [39]. Moreover, it has been shown that murine primary microglial cells, which are considered as the resident macrophages of the brain, do not express VPAC2, even after exposure to lipopolysaccharide (LPS), a TLR4 ligand [40]. Other cells, such as murine bone marrow-derived dendritic cells, murine Langerhans cells and human plasmacytoid dendritic cells have been found to express VPAC2 constitutively [41-43]. VPAC2 has been also reported to be expressed on human skin mast cells and in the human mast cell lines HMC1 and LAD2 [44,45]. In LAD2 cells, VPAC2 became upregulated through IgE/anti-IgE activation.
VIP And PACAP Immunomodulatory Roles
Multiple actions of VIP and PACAP in the immune system have been described [46]. One of the most relevant from a therapeutic standpoint is their ability to inhibit at multiple levels innate and adaptive inflammatory responses [47]. Regarding the innate immune axis, the VIP and PACAP inhibition of chemokine and proinflammatory cytokine production by macrophages has a central role [48,49]. Contributing to its ability to abrogate inflammation, VIP has been shown to down regulate the expression of the pathogen associated molecular pattern receptors of innate immunity toll-like receptors 2 and 4 (TLR2 and TLR4), which was found in vivo in tissues undergoing inflammation, but also in vitro in isolated peritoneal macrophages and the macrophage cell line Raw 264.7 [50-53]. In addition, VIP and PACAP induce anti-inflammatory mediators such as IL-10 [54]. The actions on a wide variety of inflammatory mediators are possible because downstream of their receptors they modulate several key transduction pathways and factors controlling the expression of a wide range of target genes with immunoregulatory roles (thoroughly reviewed in [55]). This includes the inhibition of one of the most important pathways involved in inflammation, the NF-kB pathway, through inhibition of IkB phosphorylation and subsequent degradation. The involvement of cAMP in this effect is not clear, and seems to vary in different myeloid cell types studied (i.e. peritoneal macrophages, macrophage and monocyte cell lines and microglia), and has been postulated to depend on the cellular differentiation state. Moreover, VIP and PACAP receptors lead to CREB phosphorylation implicated in the synthesis of IL-10 and inhibition of the MAP kinase pathways MEKK1/MEK3/MEK6/p38 and MEKK1/MEK4/JNK, involved in the expression of proinflammatory cytokines. The effects on these pathways contribute to VIP and PACAP down regulation of genes downstream of LPS-TLR4. On the other hand, it was shown that in both macrophages and microglia, VIP blocks IFNγ signal transduction by suppressing Jak1 and Jak2 phosphorylation and therefore STAT1 phosphorylation. This is particularly relevant in chronic inflammation driven by Th1 cells, of which IFNγ, a potent activator of macrophages, is a hallmark cytokine.
Besides their effects on innate immunity, VIP and PACAP modulate adaptive immune responses. In this sense, a well-recognized action for these neuropeptides is their ability to promote Th2 cell differentiation, critically involved in type-2 cell responses. These have a protective role against helminth infection, although in certain circumstances they can be deleterious and lead to chronic allergic diseases. Eosinophils, basophils and mast cells are the ultimate effector cells in these responses, driven by IL-4, IL-5, IL-9 and IL-13 cytokines, all of which are known to be produced by Th2 cells. It has been shown that VIP promotes Th2 polarization in vitro and in vivo through multiple nonexclusive mechanisms. In this sense, a series of data has shown that VIP modifies the co-stimulatory molecule expression profile and cytokine and chemokine secretion profile in macrophage and dendritic cells, in a manner that promotes Th2 responses [36,56,57]. Moreover, in vivo , VIP or PACAP administrations protected certain CD4 Th2 cells from apoptosis and allow the survival of Th2 effectors and the generation of long-lived memory cells [58,59].
The anti-inflammatory properties of VIP and PACAP led to promising therapeutic activities in murine models of acute inflammatory disorders such as septic shock and chronic inflammatory autoimmune diseases such as rheumatoid arthritis, Crohn’s disease and multiple sclerosis [49,60-63]. The latter belong to a group of diseases with common pathogenic traits: local inflammation (in different target tissues, such as joints, colon and central nervous system, respectively), driven by autoreactive Th1 cells. Therefore, the fact that VIP and PACAP favor Th2 at expense of Th1 responses played a critical role in their therapeutic effects. In these studies, the peptides were administered intraperitoneally, and the treatments, at doses between 1 to 10 n moles per injection, were most efficient when started at early stages of the disease. VIP and PACAP abrogated the inflammatory response, and switched the T cell phenotype from Th1 to Th2, leading to an amelioration of the clinical symptoms of these diseases.
VPAC2-mediated Actions In The Immune System
Pharmacological studies with specific receptor agonists have demonstrated that both VPAC1 is the main receptor involved on VIP and PACAP anti-inflammatory actions in vitro and in vivo , and VPAC2 has a partial role [49,60-62,64]. Likewise, VIP and PACAP effects on Th2 polarization through accessory cells have been suggested to be largely dependent on VPAC1. Nevertheless, other studies have involved VPAC2 in the ability of VIP to induce Th2 responses directly on T cells. In this sense, lymphocytes from mice that were genetically modified to express human VPAC2 constitutively in T CD4+ cells (VPAC2 TG) exhibit a Th2 phenotype with production of IL-4 and IL-5 in response to TCR stimulation [65]. These mice were found to be naturally in an allergic state with elevated IgE, IgG1 and eosinophils in blood. Moreover, these mice exhibited reduced hapten-induced delayed-type hyper sensibility (DTH), due to a higher Th1/Th2 ratio. In a complementary study, the same team showed that mice with a global deletion of VPAC2 (knock-out mice (KO)) [12], developed the opposite phenotype. Although different from the VPAC2 TG mice, VPAC2 KO seem normal in a resting state, as expected, these mice exhibited enhanced DTH in response to hapten [66]. Conversely, these mice developed reduced immediate-type hyper sensibility allergic responses to hapten, with diminished blood IgE levels and cutaneous anaphilactic responses. Regarding the mechanisms of action by which VIP/VPAC2 induce the cells to become Th2, it was found that this is mediated by an up regulation of certain Th2-related transcription factors (i.e. c-Maf and JunB, but not GATA-3), which consequently enhanced IL-4 and IL-5 production [67].
The fact that DTH is exacerbated in VPAC2 KO mice, with enhanced Th1 vs. Th2 cytokines, suggest that this receptor may play a protective role against Th1-driven diseases. Recently, we subjected VPAC2 KO mice to experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS) [68]. MS is a chronic inflammatory disease of the CNS thought to be driven by auto reactive T cells against myelin peptides. A role of Th1 and Th17 cells in the pathogenesis of the disease has been implied by multiple studies. We found that VPAC2 KO mice exhibited exacerbated clinical EAE, with increased immune cell infiltration and demyelination compared to WT mice. This phenotype was associated to elevated Th1 and Th7 cell but reduced Th2 responses. In addition, these mice exhibited a striking deficiency in the number of regulatory T cells (Tregs) (identified as CD4+CD25+Foxp3+) in the CNS, lymph nodes and thymus. The latter has been suggested to be the main source for Tregs during EAE [69]. This is in agreement with multiple publications supporting that VIP promotes Treg generation and functionality (reviewed in [70]). A similar response to EAE induction was found in PACAP KO mice [71,72], implying that VPAC2 mediates the anti-inflammatory actions of PACAP. PACAP/VPAC2 signaling could contribute to the maintenance and expansion of Tregs directly, as we found that VPAC2 is expressed in these cells. Supporting this possibility, we found that the expansion of Tregs isolated from VPAC2 KO mice with anti-CD3/CD28 beads and IL-2 in vitro was diminished compared to that of WT Tregs. Moreover, the in vitro suggests that PACAP/VPAC2 pathway is critical to maintain normal Treg expansion and activity.
In another study, Yadav et al. [73] demonstrated that VPAC2 KO mice exhibited greater weight loss and intestinal histopathology than WT mice in the model of dextran sodium sulfate (DSS)-induced colitis. Probably contributing to this exacerbated response, the levels of certain proinflammatory mediators (IL-6, IL-1β and MMP-9) were higher in VPAC2 KO vs. WT mice. This phenotype could be related to an immunosuppressive role for this receptor. Nevertheless, the authors suggested that it might reflect proinflammatory actions of a VIP/ VPAC1 signaling axis, which could be responsible for the reduced DSS-pathology they observed in VPAC1 KO mice. In fact, the same team previously demonstrated that VIP through VPAC1 lead to the differentiation of T lymphocytes into Th17 cells [74]. The fact that VIP could promote immune responses is also supported by previous data reporting stimulatory actions on IL-6 secretion and a role in chemo taxis. Nevertheless, further investigations are required to dissect the dual roles of VIP in immunity.
In addition to the cell types mentioned above, group 2 innate lymphoid cells (ILC2) have been more recently identified as effectors of type 2 responses [75]. ILCs are a population of lineage-negative (Lin−, i.e. lacking surface markers for T, B, NK and monocytes/macrophage lineages) lymphocyte-like cells which offer critical first-line immune responses against pathogens. Despite their lack of T cell and B cell antigen receptors (TCRs and BCRs), they can produce effector cytokines comparable to those produced by CD4+ Th cell subsets. Among all ILCs, ILC2 cells were initially identified in mice as MHC class IIhigh, CD11null and Lin− cells, which amplified type 2 immune responses upon treatment with IL-25, a Th2-produced cytokine [76]. In mice, the existence of two ILC2 populations, natural (nILC2) vs. inflammatory (iILC2), has been proposed [77]. These are different in that whereas the former can be found in homeostatic conditions and respond to IL-33, the latter rapidly expands in response to N. brasiliensis infection or IL-25 but not IL-33 administration. ILC2 have been later identified in several locations in humans, including lung, intestine and skin [78-81]. Recently, VIP has been suggested to modulate the activity of ILC2s through its VPAC2 receptor [82]. In this study, it was found that VPAC1 and VPAC2 are expressed in both gut and lung ILC2s. In addition, it was shown that VIP, as well as a VPAC2 agonist stimulated at similar levels the production of IL-5 by isolated intestinal Lin−CD45+KLRG1+ ILC2 cells in the presence of IL-7. Because VIP-innervation is rich in the intestine and lung, where these cells are abundant, this could be a natural regulator factor of ILC2s. In addition, it has been shown by using TCR transgenic mice that Th2 cells produce VIP, suggesting that in fact this neuropeptide could act as a Th2 cytokine, and suggesting a potential new mechanism by which Th2 cells may modulate the activity of ILC2s [83].
VPAC2 Perspectives As A Therapeutic Target
Although there are very few studies investigating the effects of VIP or PACAP administration to patients with inflammatory pathologies, these have provided encouraging results. For example, the effect of synthetic VIP (aviptadil) administration by inhalation to patients with sarcoidosis, an inflammatory Th1-driven systemic disease characterized by granuloma formation mainly in the lung, has been investigated. In this study, the patients exhibited an amelioration of the symptoms, with no adverse side effects [84]. This effect was associated with a reduction in the levels of TNFα but an increase of Tregs in bronchoalveolar lavage. In another study, inhaled aviptadil ameliorated pulmonary hypertension due to its action as a vasodilator [85]. Promising preclinical studies by the team of Pr. Gomariz have tested the actions of VIP in cultures of synovial fibroblasts and peripheral T cells from rheumatoid arthritis patients, and support the antiinflammatory potential of this peptide [30,53,86-89]. In vitro studies using samples from patients suffering inflammatory human diseases (as it has been done for arthritis), or animal models of disease, seem to be the most suitable approaches to dissect which VIP/PACAP receptor mediates their beneficial actions, and should precede clinical trials.
Studies comparing the outcomes of an administration of VIP and PACAP receptor agonists in the murine models of LPS-induced endotoxemia, collagen-induced arthritis and multiple sclerosis have shown a superior efficacy of VPAC1 vs. VPAC2 and PAC1 agonists. Nevertheless, the fact that VPAC2 KO mice exhibited exacerbated EAE, strongly support the antiinflammatory potential of this receptor. Thus, perhaps the idea of targeting VPAC2 for the treatment of those diseases in humans should not be completely abandoned. In fact, different factors such as the time (i.e. of highest upregulation in immune cells), route or length of administration of a VPAC2 agonist may improve its beneficial effects. Moreover, targeting VPAC2 may contribute to reduce potential VIP or PACAP secondary effects driven by VPAC1. In this sense, in a study testing the efficiency of a VPAC2 agonist for the treatment for asthma based on the bronchodilatory effects of VIP, Ro 25-1553 given by inhalation did not cause adverse effects, at least at short term [90]. Finally, although the studies in mice support the use of a VPAC1 vs. VPAC2 agonist for inflammatory disorders, this remains to be proven in humans, where due to differences in receptor expression among species or in the mechanisms implicated in human disorders vs. their animal models, this may not be true. Future studies will be required to further approach the use of VPAC2 in the clinic.
Acknowledgements
The authors are funded by the NMSS (Grant TA 3048-A-1) and ARSEP.
References
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, et al. (2000) Pituitary adenylatecyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52: 269-324.
- Holland PW, Garcia-Fernà ndez J, Williams NA, Sidow A (1994) Gene duplications and the origins of vertebrate development. DevSuppl 125-33.
- Ng SY, Chow BK, Kasamatsu J, Kasahara M, Lee LT (2012) Agnathan VIP, PACAP and their receptors: ancestral origins of today's highly diversified forms. PLoS One 7: e44691.
- Said SI, Mutt V (1970) Polypeptide with broad biological activity: isolation from small intestine. Science 169: 1217-1218.
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, et al. (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylatecyclase in pituitary cells. BiochemBiophys Res Commun 164:567-574.
- Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, et al. (2012) Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylatecyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 166: 4-17.
- Dickson L, Finlayson K (2009) VPAC and PAC receptors: From ligands to function. PharmacolTher 121: 294-316.
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, et al. (1993) The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett 334: 3-8.
- Svoboda M, Tastenoy M, Van Rampelbergh J, Goossens JF, De Neef P, et al. (1994) Molecular cloning and functional characterization of a human VIP receptor from SUP-T1 lymphoblasts. BiochemBiophys Res Commun 205: 1617-1624.
- Mackay M, Fantes J, Scherer S, Boyle S, West K, et al. (1996) Chromosomal localization in mouse and human of the vasoactive intestinal peptide receptor type 2 gene: a possible contributor to the holoprosencephaly 3 phenotype. Genomics 37: 345-353.
- Cai Y, Xin X, Yamada T, Muramatsu Y, Szpirer C, et al. (1995) Assignments of the genes for rat pituitary adenylatecyclase activating polypeptide (Adcyap1) and its receptor subtypes (Adcyap1r1, Adcyap1r2, and Adcyap1r3). Cytogenet Cell Genet 71: 193-196.
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, et al. (2002) The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109: 497-508.
- Harmar AJ, Sheward WJ, Morrison CF, Waser B, Gugger M, et al. (2004) Distribution of the VPAC2 receptor in peripheral tissues of the mouse. Endocrinology 145: 1203-1210.
- Tan YV, Couvineau A, Murail S, Ceraudo E, Neumann JM, et al. (2006) Peptide agonist docking in the N-terminal ectodomain of a class II G protein-coupled receptor, the VPAC1 receptor. Photoaffinity, NMR, and molecular modeling. J BiolChem 281: 12792-12798.
- Tan YV, Couvineau A, Van Rampelbergh J, Laburthe M (2003) Photoaffinity labeling demonstrates physical contact between vasoactive intestinal peptide and the N- terminal ectodomain of the human VPAC1 receptor. J BiolChem 278: 36531-36536.
- Tan YV, Couvineau A, Laburthe M (2004) Diffuse pharmacophoric domains of vasoactive intestinal peptide (VIP) and further insights into the interaction of VIP with the N-terminal ectodomain of human VPAC1 receptor by photoaffinity labeling with [Bpa6]-VIP. J BiolChem 279: 38889-38894.
- Tan YV, Couvineau A, Lacapere JJ, Laburthe M (2006) Characterization of the new photoaffinity probe (Bz2-K24)-VIP. Ann N Y AcadSci 1070: 575-580.
- Xia M, Sreedharan SP, Bolin DR, Gaufo GO, Goetzl EJ (1997) Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. J PharmacolExpTher 281: 629-633.
- O'Donnell M, Garippa RJ, Rinaldi N, Selig WM, Simko B, et al. (1994) Ro 25-1553: a novel, long-acting vasoactive intestinal peptide agonist. Part I: In vitro and in vivo bronchodilator studies. J PharmacolExpTher 270: 1282-1288.
- Gourlet P, Vertongen P, Vandermeers A, Vandermeers-Piret MC, Rathe J, et al. (1997) The long-acting vasoactive intestinal polypeptide agonist RO 25-1553 is highly selective of the VIP2 receptor subclass. Peptides 18: 403-408.
- Tsutsumi M, Claus TH, Liang Y, Li Y, Yang L, et al. (2002) A potent and highly selective VPAC2 agonist enhances glucose-induced insulin release and glucose disposal: a potential therapy for type 2 diabetes. Diabetes 51: 1453-1460
- Tannu SA, Renzetti LM, Tare N, Ventre JD, Lavelle D, et al. (2010) Dual bronchodilatory and pulmonary anti-inflammatory activity of RO5024118, a novel agonist at vasoactive intestinal peptide VPAC2 receptors. Br J Pharmacol, 161: 1329-1342.
- Pan CQ, Li F, Tom I, Wang W, Dumas M, et al. (2007) Engineering novel VPAC2-selective agonists with improved stability and glucose-lowering activity in vivo. J PharmacolExpTher 320: 900-906.
- Rao L, Ma Y, Zhuang M, Luo T, Wang Y, et al. (2014) Chitosan-decorated selenium nanoparticles as protein carriers to improve the in vivo half-life of the peptide therapeutic BAY 55-9837 for type 2 diabetes mellitus. Int J Nanomedicine 9: 4819-4828.
- Moreno D, Gourlet P, De Neef P, Cnudde J, Waelbroeck M, et al. (2000) Development of selective agonists and antagonists for the human vasoactive intestinal polypeptide VPAC(2) receptor. Peptides 21: 1543-1549.
- Lara-Marquez M, O'Dorisio M, O'Dorisio T, Shah M, Karacay B (2001) Selective gene expression and activation-dependent regulation of vasoactive intestinal peptide receptor type 1 and type 2 in human T cells. J Immunol 166: 2522-2530.
- Delgado M, Martinez C, Johnson MC, Gomariz RP, Ganea D (1996) Differential expression of vasoactive intestinal peptide receptors 1 and 2 (VIP-R1 and VIP-R2) mRNA in murine lymphocytes. J Neuroimmunol 68: 27-38.
- Vomhof-DeKrey EE, Hermann RJ, Palmer MF, Benton KD, Sandy AR, et al. (2008) TCR signaling and environment affect vasoactive intestinal peptide receptor-1 (VPAC-1) expression in primary mouse CD4 T cells. Brain BehavImmun 22: 1032-1040.
- Ipp H, Nkambule BB, Reid TD, de Swardt D, Bekker LG, et al. (2014) CD4+ T cells in HIV infection show increased levels of expression of a receptor for vasoactive intestinal peptide, VPAC2. Immunol Res 60: 11-15.
- Jimeno R, Gomariz RP, Garin M, Gutierrez-Canas I, Gonzalez-Alvaro I, et al. (2015) The pathogenic Th profile of human activated memory Th cells in early rheumatoid arthritis can be modulated by VIP. J Mol Med (Berl) 93: 457-467.
- Grinninger C, Wang W, Oskoui KB, Voice JK, Goetzl EJ (2004) A natural variant type II G protein-coupled receptor for vasoactive intestinal peptide with altered function. J BiolChem 279: 40259-40262.
- Miller AL, Verma D, Grinninger C, Huang MC, Goetzl EJ (2006) Functional splice variants of the type II G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide in mouse and human lymphocytes. Ann N Y AcadSci 1070: 422-426.
- Lara-Marquez ML, O'Dorisio MS, Karacay B (2000) Vasoactive intestinal peptide (VIP) receptor type 2 (VPAC2) is the predominant receptor expressed in human thymocytes. Ann N Y AcadSci 921: 45-54.
- Vomhof-DeKrey EE, Sandy AR, Failing JJ, Hermann RJ, Hoselton SA, et al. (2011) Radical reversal of vasoactive intestinal peptide (VIP) receptors during early lymphopoiesis. Peptides 32: 2058-2066.
- Delgado M, Munoz-Elias EJ, Kan Y, Gozes I, Fridkin M, et al. (1998) Vasoactive intestinal peptide and pituitary adenylatecyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-kB and cAMP response element-binding protein/c-Jun. J BiolChem 273: 31427-31436.
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D (1999) VIP and PACAP inhibit IL-12 production in LPS-stimulated macrophages. Subsequent effect on IFNgamma synthesis by T cells. J Neuroimmunol 96: 167-181.
- Herrera JL, Gonzalez-Rey E, Fernandez-Montesinos R, Quintana FJ, Najmanovich R, et al. (2009) Toll-like receptor stimulation differentially regulates vasoactive intestinal peptide type 2 receptor in macrophages. J Cell Mol Med 13: 3209-3217.
- Hauk V, Fraccaroli L, Grasso E, Eimon A, Ramhorst R, et al. (2014) Monocytes from Sjogren's syndrome patients display increased vasoactive intestinal peptide receptor 2 expression and impaired apoptotic cell phagocytosis. ClinExpImmunol 177: 662-670.
- Groneberg DA, Hartmann P, Dinh QT, Fischer A (2001) Expression and distribution of vasoactive intestinal polypeptide receptor VPAC(2) mRNA in human airways. Lab Invest 81: 749-755.
- Delgado M, Jonakait GM, Ganea D (2002) Vasoactive intestinal peptide and pituitary adenylatecyclase-activating polypeptide inhibit chemokine production in activated microglia. Glia 39: 148-161.
- Fabricius D, O'Dorisio MS, Blackwell S, Jahrsdorfer B (2006) Human plasmacytoid dendritic cell function: inhibition of IFN-alpha secretion and modulation of immune phenotype by vasoactive intestinal peptide. J Immunol 177: 5920-5927.
- Delgado M, Reduta A, Sharma V, Ganea D (2004) VIP/PACAP oppositely affects immature and mature dendritic cell expression of CD80/CD86 and the stimulatory activity for CD4(+) T cells. J LeukocBiol 75: 1122-1130.
- Torii H, Yan Z, Hosoi J, Granstein RD (1997) Expression of neurotrophic factors and neuropeptide receptors by Langerhans cells and the Langerhans cell-like cell line XS52: further support for a functional relationship between Langerhans cells and epidermal nerves. J Invest Dermatol 109: 586-591.
- Groneberg DA, Welker P, Fischer TC, Dinh QT, Grützkau A, et al. (2003) Down-regulation of vasoactive intestinal polypeptide receptor expression in atopic dermatitis. J Allergy ClinImmunol 111: 1099-1105.
- Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP (2008) Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 123: 398-410.
- Delgado M, Pozo D, Ganea D (2004) The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev 56: 249-290.
- Delgado M, Ganea D (2008) Anti-inflammatory neuropeptides: a new class of endogenous immunoregulatory agents. Brain BehavImmun 22: 1146-1151.
- Delgado M, Ganea D (2001) Inhibition of endotoxin-induced macrophage chemokine production by VIP and PACAP in vitro and in vivo. Arch PhysiolBiochem 109: 377-382.
- Delgado M, Gomariz RP, Martinez C, Abad C, Leceta J (2000) Anti-inflammatory properties of the type 1 and type 2 vasoactive intestinal peptide receptors: role in lethal endotoxic shock. Eur J Immunol 30: 3236-3246.
- Arranz A, Abad C, Juarranz Y, Torroba M, Rosignoli F, et al. (2006) Effect of VIP on TLR2 and TLR4 expression in lymph node immune cells during TNBS-induced colitis. Ann N Y AcadSci 1070: 129-134.
- Arranz A, Androulidaki A, Zacharioudaki V, Martinez C, Margioris AN, et al. (2008) Vasoactive intestinal peptide suppresses toll-like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. MolImmunol 45: 2970-2980.
- Gomariz RP, Arranz A, Abad C, Torroba M, Martinez C, et al. (2005) Time-course expression of Toll-like receptors 2 and 4 in inflammatory bowel disease and homeostatic effect of VIP. J LeukocBiol 78: 491-502.
- Juarranz Y, Gutiérrez-Cañas I, Arranz A, MartÃnez C, Abad C, et al. (2006) VIP decreases TLR4 expression induced by LPS and TNF-alpha treatment in human synovial fibroblasts. Ann N Y AcadSci 1070: 359-364.
- Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D (1999) Vasoactive intestinal peptide and pituitary adenylatecyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J Immunol 162: 1707-1716.
- Chorny A, Gonzalez-Rey E, Varela N, Robledo G, Delgado M (2006) Signaling mechanisms of vasoactive intestinal peptide in inflammatory conditions. RegulPept 137: 67-74.
- Delgado M, Leceta J, Sun W, Gomariz RP, Ganea D (2000) VIP and PACAP induce shift to a Th2 response by upregulating B7.2 expression. Ann N Y AcadSci 921: 68-78.
- Delgado M, Gonzalez-Rey E, Ganea D (2004) VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. FASEB J 18: 1453-1455.
- Delgado M, Ganea D (2001) VIP and PACAP enhance the in vivo generation of memory TH2 cells by inhibiting peripheral deletion of antigen-specific effectors. Arch PhysiolBiochem 109: 372-376.
- Delgado M, Leceta J, Ganea D (2002) Vasoactive intestinal peptide and pituitary adenylatecyclase-activating polypeptide promote in vivo generation of memory Th2 cells. FASEB J 16: 1844-1846.
- Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP(2001) Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med 7: 563-568.
- Abad C, Martinez C, Juarranz MG, Arranz A, Leceta J, et al. (2003) Therapeutic effects of vasoactive intestinal peptide in the trinitrobenzene sulfonic acid mice model of Crohn's disease. Gastroenterology 124: 961-971.
- Abad C, Martinez C, Leceta J, Gomariz RP, Delgado M (2001) Pituitary adenylatecyclase-activating polypeptide inhibits collagen-induced arthritis: an experimental immunomodulatory therapy. J Immunol 167: 3182-3189.
- Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, et al. (2004) Pituitary adenylatecyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. MultScler 10: 651-659.
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, et al. (2006) Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol 168: 1179-1188.
- Voice JK, Dorsam G, Lee H, Kong Y, Goetzl EJ (2001) Allergic diathesis in transgenic mice with constitutive T cell expression of inducible vasoactive intestinal peptide receptor. FASEB J 15: 2489-2496.
- Goetzl EJ, Voice JK, Shen S, Dorsam G, Kong Y, et al. (2001) Enhanced delayed-type hypersensitivity and diminished immediate-type hypersensitivity in mice lacking the inducible VPAC(2) receptor for vasoactive intestinal peptide. ProcNatlAcadSci U S A 98: 13854-13859.
- Voice J, Donnelly S, Dorsam G, Dolganov G, Paul S, et al. (2004) c-Maf and JunB mediation of Th2 differentiation induced by the type 2 G protein-coupled receptor (VPAC2) for vasoactive intestinal peptide. J Immunol 172: 7289-7296.
- Abad C, Cheung-Lau G, Coute-Monvoisin AC, Waschek JA (2015) Vasoactive intestinal peptide-deficient mice exhibit reduced pathology in trinitrobenzene sulfonic acid-induced colitis. Neuroimmunomodulation 22: 203-212.
- Chen X, Fang L, Song S, Guo TB, Liu A, et al. (2009) Thymic regulation of autoimmune disease by accelerated differentiation of Foxp3+ regulatory T cells through IL-7 signaling pathway. J Immunol 183: 6135-6144.
- Gonzalez-Rey E, Delgado M (2007) Vasoactive intestinal peptide and regulatory T-cell induction: a new mechanism and therapeutic potential for immune homeostasis. Trends Mol Med 13: 241-251.
- Tan YV, Abad C, Lopez R, Dong H, Liu S, et al. (2009) Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. ProcNatlAcadSci U S A 106: 2012-2017.
- Tan YV, Abad C, Wang Y, Lopez R, Waschek JA (2013) Pituitary adenylatecyclase activating peptide deficient mice exhibit impaired thymic and extrathymic regulatory T cell proliferation during EAE. PLoS One 8: e61200.
- Yadav M, Huang MC, Goetzl EJ (2011) VPAC1 (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis. Cell Immunol 267: 124-132.
- Yadav M, Rosenbaum J, Goetzl EJ (2008) Cutting edge: vasoactive intestinal peptide (VIP) induces differentiation of Th17 cells with a distinctive cytokine profile. J Immunol 180: 2772-2776.
- Huang Y, Paul WE (2016) Inflammatory group 2 innate lymphoid cells. IntImmunol 28: 23-28.
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, et al. (2001) IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15: 985-995.
- Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, et al. (2015) IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential 'inflammatory' type 2 innate lymphoid cells. Nat Immunol 16: 161-169.
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, et al. (2011) Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 12: 1055-1062.
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, et al. (2011) Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045-1054.
- Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, et al. (2013) A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med 210: 2939-2950.
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA et al. (2013) TSLP elicits IL-33- independent innate lymphoid cell responses to promote skin inflammation. SciTransl Med 5: 170ra116.
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, et al. (2013) Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502: 245-248.
- Delgado M, Ganea D (2001) Cutting edge: is vasoactive intestinal peptide a type 2 cytokine? J Immunol 166: 2907-2912.
- Prasse A, Zissel G, Lutzen N, Schupp J, Schmiedlin R, et al. (2010) Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J RespirCrit Care Med 182: 540-548.
- Leuchte HH, Baezner C, Baumgartner RA, Bevec D, Bacher G, et al. (2008) Inhalation of vasoactive intestinal peptide in pulmonary hypertension. EurRespir J 32: 1289-1294.
- Carrion M, Perez-Garcia S, Jimeno R, Juarranz Y, Gonzalez-Alvaro I, et al. (2013) Inflammatory mediators alter interleukin-17 receptor, interleukin-12 and -23 expression in human osteoarthritic and rheumatoid arthritis synovial fibroblasts: immunomodulation by vasoactive intestinal Peptide. Neuroimmunomodulation 20: 274-284.
- Arranz A, Gutierrez-Canas I, Carrion M, Juarranz Y, Pablos JL, et al. (2008) VIP reverses the expression profiling of TLR4-stimulated signaling pathway in rheumatoid arthritis synovial fibroblasts. MolImmunol 45: 3065-3073.
- Gutierrez-Canas I, Juarranz Y, Santiago B, Arranz A, Martinez C, et al. (2006) VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology (Oxford) 45: 527-532.
- Perez-Garcia S, Carrion M, Gutierrez-Canas I, Gonzalez-Alvaro I, Gomariz RP, et al. (2016) VIP and CRF reduce ADAMTS expression and function in osteoarthritis synovial fibroblasts. J Cell Mol Med
- Linden A, Hansson L, Andersson A, Palmqvist M, Arvidsson P, et al. (2003) Bronchodilation by an inhaled VPAC(2) receptor agonist in patients with stable asthma. Thorax 58: 217-221.
Citation: Abad C, Var Tan Y (2016) VIP and PACAP as Regulators of Immunity: New Perspectives from A Receptor Point of View. J Clin Exp Neuroimmunol 1:104.
Copyright: © 2016 Abad C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 12830
- [From(publication date): 3-2016 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 11871
- PDF downloads: 959