Short Communication Open Access
Vigilance to Limit the Bidirectional Introduction and Co-circulation of Virus Serotypes Aims to Reduce Risk of Severe Dengue Disease in North East India
Leonie J Barnett1 and Andrew W Taylor-Robinson2*1School of Access Education, Central Queensland University, Bruce Highway, North Rockhampton, QLD 4702, Australia
2School of Medical & Applied Sciences, Central Queensland University, 160 Ann Street, Brisbane, QLD 4000, Australia
- *Corresponding Author:
- Andrew W Taylor-Robinson
Infectious Diseases Research Group, School of Medical & Applied Sciences
Central Queensland University, 160 Ann Street, Brisbane, QLD 4000, Australia
Tel: +61 7 3295 1185
E-mail: a.taylor-robinson@cqu.edu.au
Received date: October 13, 2016; Accepted date: November 09, 2016; Published date: November 20, 2016
Citation: Barnett LJ, Taylor-Robinson AW (2016) Vigilance to Limit the Bidirectional Introduction and Co-circulation of Virus Serotypes Aims to Reduce Risk of Severe Dengue Disease in North East India. J Emerg Infect Dis 1:115. doi: 10.4172/2472-4998.1000115
Copyright: © 2016 Barnett LJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Disease and Pathology
Abstract
Dengue is a serious re-emerging arboviral disease that presents a global human health challenge. Infection is caused by one of several recognised serotypes of dengue virus, a member of the mosquito-transmitted Flaviviridae family of human pathogens, which also includes yellow fever, Zika and West Nile viruses. The greatest burden of dengue is borne by countries in the Asia Pacific region, in particular South East Asia and the Indian subcontinent where infection breaks out with increasing frequency and intensity. The World Health Organization South East Asia Region has a population of 1.3 billion people and includes 11 countries, of which Thailand, Myanmar and India have the highest reported incidence of dengue. Infection outcomes vary from the commonly asymptomatic or a self-limiting fever to more severe manifestations such as dengue haemorrhagic fever and dengue shock syndrome. Although primary infection with one serotype elicits lifelong immunity against homologous reinfection, severe dengue is linked to an antibody-related immunopathological response that is triggered by secondary infection with a heterologous serotype. The principal vectors of transmission are the day-biting mosquitoes Aedes aegypti and Ae. albopictus, the respective preference of which for urban and rural habitats has facilitated the rapid and continued spread of dengue across tropical and sub-tropical climatic zones. The narrow elevated corridor of North East India was considered historically to be non-endemic for dengue and as such separated areas of high endemicity in the Bengal Delta and the other zones of India from those in Thailand and Myanmar. In these regions the predominant serotype is usually distinct, so a breach of this geographical bottleneck would potentially enable the co-circulation of virus serotypes, thus posing an increased risk of severe disease upon secondary infection. This alarming scenario may now be realized following the notification of clinical cases in the states of Arunachal Pradesh, Assam and Manipur in recent years. While incidence is still sporadic in North East India limitation of local outbreaks is required through vigilant adherence to the integrated implementation of clinical management, diagnosis, surveillance and vector control in order to prevent the transcontinental spread of dengue.
Keywords
Dengue; Virus; Mosquito; Aedes; Vector; Disease; Serotype; India
Introduction
Dengue is considered as one of the most significant arboviral diseases of humans worldwide, with a distribution predominantly in tropical and subtropical regions that are the habitat of its vector of transmission, female mosquitoes of the genus Aedes [1,2]. At present, there is a risk of infection for over 2.5 billion people in about 125 countries worldwide [1]. The most reliable recent estimate of the burden of disease imposed by dengue is that globally each year it causes an estimated 390 million infections, with 96 million symptomatic cases [1]. Among at least 500,000 dengue-related hospitalisations annually [2], an estimated 22,000 are fatal [3]. These statistics indicate that most people remain unaffected by the virus, while a smaller proportion exhibit extreme symptoms, with a low chance of survival without supportive tertiary care. Currently, there is neither a specific anti-dengue therapy nor a commercially available preventive vaccine [4,5].
Dengue Virus, Serotypes and Transmission
The aetiological agent is dengue virus (DENV), an enveloped, single-stranded, positive sense RNA virion of the Flavivirus genus, which belongs to the Flaviviridae. This family is closely related to viruses that cause other notable infectious diseases in humans including yellow fever, Zika, West Nile encephalitis, Japanese encephalitis and hepatitis C infection [6]. There are four established serotypes, DENV 1-4, characterized by neutralization assays [6]. The existence of a fifth serotype was advanced recently [7]. However, definitive demonstration of this serotype awaits the recovery and characterization of an isolate so as to confirm, or conversely to refute, its singularity [8].
While humans are the principal host some DENV serotypes also infect non-human primates such as macaques. Although many potential DENV vectors have been considered, evidence points to Ae. aegypti, the yellow fever mosquito, and Ae. albopictus, the Asian tiger mosquito, both day-biting, as the major vectors for dengue transmission around the world [9].
Clinical Manifestations and Immunopathology
The possible outcomes of dengue virus infection in humans occur in three traditionally recognized clinical forms. These range vastly from asymptomatic cases or those causing mild influenza-like symptoms known as dengue fever (DF), lasting between 2-10 days, through to severe disease, notably dengue haemorrhagic fever (DHF) and the rarer dengue shock syndrome (DSS) [6]. These manifestations lead to metabolically dangerous conditions including increased vascular permeability, hypovolaemia and circulatory failure [10]. DF is due to primary infection with any serotype, is generally mild and self-limiting, and from which recovery is generally completed by lifelong antibody-dependent homotypic immunity [11]. In 2009, the World Health Organization proposed a revised, broader-based clinical classification which is gradually being adopted: dengue; dengue with warning signs; and severe dengue [10].
Secondary infection with a heterotypic serotype generates crossreactive antibodies, which, when present in the peripheral circulation, increase the potential risk of antibody-dependent enhancement of disease, a form of immunopathology. Hence, recurrent infection is the major risk factor for the serious, often fatal, complications of DHF and DSS. Infants who are immunized passively through receiving maternal antibodies from a dengue pre-immune mother are at high risk of severe dengue infection [12,13]. Different dengue serotypes vary in their capacity to cause severe illness, but there is no expert consensus as to the extent of a causal association [14]. In addition, it cannot be predicted as to which individuals will progress from experiencing only DF to suffering life-threatening DHF or DSS. This places a substantial burden on public health service providers, especially referral hospitals in developing countries where dengue is particularly prevalent.
Recent History of Dengue in Asia
Disruption of ecosystems, increased troop movement, and rapid urbanization after World War II has each promoted the wide dissemination of the dengue vector and virus across Asia [15]. By the end of the war, countries (notably Cambodia, Philippines, Thailand, Vietnam) susceptible to dengue epidemics became hyperendemic for DENV [16]. DENV 1-4 serotypes were first isolated during the 1940s and 1950s in this area although they were probably present earlier [15,17]. DHF emerged in Manila (Philippines) in 1954 [18]; thereafter, it appeared in Thailand in 1958, while Cambodia, Malaysia, Singapore and Vietnam all reported the presence of DHF cases in the 1960s [19]. In India, the first virologically proven epidemic took place in Calcutta and on the Eastern Coast Plains in 1963-1964 [20]. DHF began occurring in various parts of India in 1988 [21]. Pakistan reported cases of DHF in Karachi in 1994 [22].
Current Epidemiology in Asia
In the World Health Organization South East Asia Region now only the Democratic People’s Republic of Korea has yet to report any outbreak [23]. Dengue is hyperendemic in South East Asia and in all Indian zones other than North East (i.e. North West, North Central, West Central and Peninsular), where the predominant circulating serotypes vary on a cyclical basis. In Thailand, it was noted that the predominant serotype cycled annually during 2004-10, a phenomenon which was presumed to be due to herd immunity among the human population and to competition between serotypes [24]. The existence of multiple DENV serotypes in the same locality is a major threat to resident communities [10,25]. In the monsoonal countries of Myanmar and Thailand multiple serotypes of dengue virus are in circulation and high rates of hospitalization and death from severe dengue are reported [23,24]. Regions of Myanmar that record the highest number of cases include many in the west of the country, bordering North East India [10].
There are also multiple serotypes in circulation in India and Bangladesh, from where dengue has spread into Nepal and Bhutan in just over a decade [10]. In Bangladesh, DF was documented from the mid-1960s to the mid-1990s, but re-emergence of DHF was reported in 2000 [26]. Epidemics in Bhutan and Nepal were reported only in 2004 [27,28], but these small nations transitioned from non-endemic to hyperendemic status rapidly [29,30]. Bhutan shares borders to the east, south and west with the North East Indian states of Arunachal Pradesh, Assam and Sikkim, respectively (Fig. 1). The primary vector Ae. aegypti and secondary vector Ae. albopictus were reported to have expanded into the middle mountain ranges of Nepal and were commonly found up to 1,350 m altitude [31].
The Case of North East India
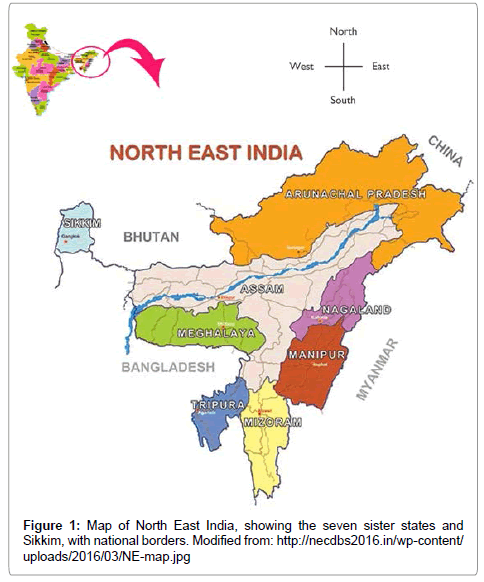
Historically, infections in the Bengal Delta and four of the five geographical zones of India were separated physically from those in Thailand and Myanmar in South East Asia by the land mass of North East India, which is a narrow corridor situated between Bangladesh (to the west), Bhutan (to the north-west), China (Tibet) (to the north) and Myanmar (to the east) [32]. North East India comprises the contiguous so-called ‘seven sister states’ of Arunachal Pradesh, Assam, Manipur, Mizoram, Meghalaya, Nagaland and Tripura, as well as the Himalayan state of Sikkim (Figure 1), which borders Nepal to its west. This physiographical region was hypoendemic for dengue, despite being surrounded by dengue-endemic countries and so represented a bottleneck to the spread of dengue across the Asian continent [33]. However, of public health concern North East India is now reporting outbreaks of dengue [34-36]. This is occurring in a region in which the incidence of disease was hitherto sufficiently low that the population has limited or no immunity to any DENV serotype. The situation is exacerbated by both an expansion of vector ranges and increased urbanization.
Figure 1: Map of North East India, showing the seven sister states and Sikkim, with national borders. Modified from: http://necdbs2016.in/wp-content/ uploads/2016/03/NE-map.jpg
Vector Distribution
Initially considered to be an urban and peri-urban disease, dengue has extended into rural areas in India [32]. In North East states, Ae. aegypti displays a peridomestic habit and is the dominant vector mosquito species in urban areas, whereas Ae. albopictus is found more in semi-urban and rural environments [37]. The distribution of Ae. albopictus has expanded rapidly [38], and this species is a potential vector in areas where Ae. aegypti is not established. In the late 1990s both vectors were identified in waste tyre dumps in townships along the main trunk roads of North East India, up to an altitude of 1,500 m above sea level [39]. Tyre dumps were noted as a preferred habitat of Ae. aegypti and a major contributor to its presence, for which community action was advised in order to reduce standing water collection [37]. Both mosquito species were also detected in the hills of Nagaland [40]. Surveillance of vectors for viral infection is considered a critical measure to provide early warning of a possible dengue outbreak [41].
Incidence of Infection
The first major outbreak of dengue in North East India was in Manipur in 2007-08, in a town bordering Myanmar [34]. This incidence was due to a Cambodian strain of dengue and was linked to the increased local presence of Ae. aegypti and Ae. albopictus. Although Manipur was noted to have a porous border with Myanmar, there was no suggestion that the outbreak originated in Myanmar, so it is probable that it was introduced there by an infectious traveller from Cambodia or elsewhere [34]. Of 275 suspected cases in Manipur, 42 from 2007 and 16 from 2008 were tested using immunoglobulin (Ig)M anti-dengue antibodies (20 and 12, respectively, were also tested by RT-PCR). There were 25/42 and 2/16 positive by IgM, and 17/20 and 10/12 by RT-PCR for DENV-2, respectively [34].
Further outbreaks followed in Assam in 2010, 2012 and 2013 [35], and in Arunachal Pradesh in 2012 [36]. The number of confirmed dengue cases in Assam increased markedly from 237 in 2010 to 1058 in 2012 and then to 4526 in 2013 [35]. The circulating serotype was not confirmed from clinical samples in Assam; however, a study of wildcaught Ae. aegypti during the 2013 outbreak identified the presence of serotype DENV-2 [41]. Of 164 suspected cases in Arunachal Pradesh, 107 were confirmed by serology [36]. Of these, 89 were tested by RTPCR and dengue was detected in 35: 27 instances of single serotype infection of DENV-3; 7 dual infections of DENV-1 and DENV-3; and one case of DENV-2 and DENV-3 mixed infection [36]. A retrospective surveillance study of 430 suspected dengue cases during 2009-2011 found 143 to be dengue positive (92 by IgM-capture ELISA and 51 by RT-PCR); these were collected from Assam (82), Meghalaya (35), Nagaland (15), Manipur (8) and Arunachal Pradesh (3) [42]. All serotypes were identified in the RT-PCR samples and DENV-1 was predominant (45.1%) [42]. Dual infections were also detected: DENV- 1 and DENV-2 (3 cases); DENV-1 and DENV-3 (1 case); DENV-1 and DENV-4 (1 case); and DENV-2 and DENV-3 (3 cases) [42].
Current issue and Future Perspective
The region comprising Bhutan, Bangladesh, North East India and Myanmar provides a natural connection between India and South East Asia. Both these latter regions have been hyperendemic for dengue for a long time but the predominant circulating serotypes are usually distinct [33]. While Myanmar is also an established hyperendemic nation, with infections reported to be related to those circulating in Thailand [10], the resident populations of North East India, Bangladesh and Bhutan are at risk of suffering the more severe disease manifestations of dengue infection. Expansion of the distribution of virus serotypes from both east and west directions will lead to co-circulation of dominant serotypes. In a dengue-naive population this will bring about an increased incidence of mixed infections and the potential for increased virulence.
Conclusions
In North East India there is currently a real possibility of a rise in number of both people infected with dengue and, consequently, of clinical cases that necessitate specialist treatment. In light of this threat to public health, increased vigilance in diagnosis and therapy, as well as with respect to mosquito surveillance and control, is required in order to combat infection at a state level. Virus identification, disease notification and vector management will also have the substantial benefit of reducing an individual’s risk of exposure to different virus serotypes that is a major predisposing factor for severe disease manifestations that are consequent to secondary infection. Raising the level of public awareness through community education and engagement in vector control is also central to preventing the urban spread of Ae. aegypti and Ae. albopictus mosquitoes. Importantly, local implementation of such an integrated program will act to preserve for as long as possible the apparent bottleneck of this most eastern region of India to the otherwise continued spread of dengue across Asia.
Conflict of Interest
The authors declare that they have no competing issues of interest.
Acknowledgements
The authors’ research is supported by Central Queensland University and the Australian Government’s Collaborative Research Networks Program.
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504-507.
- World Health Organization (2016) Dengue and severe dengue..
- Centers for Disease Control and Prevention (2014) Dengue: Epidemiology.
- Sanyal S, Taylor-Robinson AW (2013) Host-virus interactions in dengue infection indicate targets for detection and therapeutic interventions. Immun Dis 1: 1-4.
- Ansari S, Taylor-Robinson AW (2014) Strategic approaches to multivalent vaccine development against dengue virus infection. Ann Vaccines Immunization 1: 1005.
- Halstead SB (2007) Dengue. Lancet 370: 1644-1652.
- Mustafa MS, Rasotgi V, Jain S, Gupta V (2015) Discovery of fifth serotype of dengue virus (DENV-5): A new public health dilemma in dengue control. Med J Armed Forces India 71: 67-70.
- Taylor-Robinson AW (2016) A putative fifth serotype of dengue–potential implications for diagnosis, therapy and vaccine design. Int J Clin Med Micro 1: 101-102.
- Burke DS, Monath TP (2001) Flavivirus. In: Knipe DM, Howley PM, eds. Fields Virology, 4th edn, Philadelphia, 1043-1126.
- World Health Organization (2009) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. WHO, Geneva,147.
- Rothman A L (2004) Dengue: defining protective versus pathologic immunity. J Clin Invest 113: 946-951.
- Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, et al. (2010) The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8: 271-283.
- Wahala WMPB, de Silva AM (2011) The human antibody response to dengue virus infection. Viruses 3: 2374-2395.
- Endy TP (2002) Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol 156: 40-51.
- Sabin AB (1952) Research on dengue during World War II. Am J Trop Med Hyg 1: 30-50.
- Halstead SB (2006) Dengue in the Americas and Southeast Asia: do they differ? Rev Panam Salud Publica 20: 407-415.
- Gubler DJ (1997) Dengue and dengue hemorrhagic fever: its history and resurgence as a global public health problem. In: Gubler DJ, Kuno G, eds. Dengue and dengue hemorrhagic fever. CAB International, London, 1-22.
- Hammon WM, Rudnick A, Sather GE (1960) Viruses associated with epidemic hemorrhagic fevers of the Philippines and Thailand. Science 131: 1102-1103.
- Gubler DJ (2006) Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp 277: 3-16.
- Sarkar JK, Chatterjee SN, Chakravarty SK (1964) Haemorrhagic fever in Calcutta: some epidemiological observations. Indian J Med Res 52: 651-659.
- Gupta N, Srivastav S, Jain A, Chaturvedi UC (2012) Dengue in India. Indian J Med Res 136: 337-390.
- Chan YC, Salahuddin NI, Khan J, Tan HC, Seah CL, et al. (1995) Dengue haemorrhagic fever outbreak in Karachi, Pakistan, 1994. Trans R Soc Trop Med Hyg 89: 619-620.
- World Health Organization (2011) Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Hemorrhagic Fever. WHO Regional Office for South-East Asia 196.
- Pongsiri P, Themboonlers A, Poovorawan Y (2012) Changing pattern of dengue virus serotypes in Thailand between 2004 and 2010. J Health Popul Nutr 30: 366-370.
- Halstead SB, Heinz FX, Barrett AD, Roehrig JT (2005) Dengue virus: molecular basis of cell entry and pathogenesis, 25-27 June 2003, Vienna, Austria. Vaccine 23: 849-856.
- Rahman M, Rahman K, Siddque AK, Shoma S, Kamal AH, et al. (2002) First outbreak of dengue hemorrhagic fever, Bangladesh. Emerg Infect Dis 8: 738-740.
- Dorji T, Yoon IK, Holmes EC, Wangchuk S, Tobgay T, et al. (2009) Diversity and origin of dengue virus serotypes 1, 2, and 3, Bhutan. Emerg Infect Dis 15: 1630-1632.
- Pandey BD, Rai SK, Morita K, Kurane I (2004) First case of dengue in Nepal. Nepal Med Coll J 6: 157-159.
- Malla S, Thakur GD, Shrestha SK, Banjeree MK, Thapa LB, et al. (2008) Identification of all dengue serotypes in Nepal. Emerg Infect Dis 14: 1669-1670.
- Subedi D, Taylor-Robinson AW (2016) Epidemiology of dengue in Nepal: history of incidence, current prevalence and strategies for future control. J Vector Borne Dis 53: 1-7.
- Dhimal M, Gautam I, Joshi HD, O'Hara RB, Ahrens B, et al. (2015) Risk factors for the presence of chikungunya and denge vectors (Aedes aegypti and Aedes albopictus), their altitudinal distribution and climatic determinants of their abundance in central Nepal. PLoS Negl Trop Dis 9: e0003545.
- Gupta B, Reddy BPN (2013) Fight against dengue in India: progresses and challenges. Parasitol Res 112: 1367-1378.
- Islam R, Salahuddin M, Ayubi MS, Hossain T, Majumder A, et al. (2015) Dengue epidemiology and pathogenesis: images of the future viewed through a mirror of the past. Virol Sin 30: 326-343.
- Sankari T, Hoti SL, Singh TB, Shanmugavel J (2012) Outbreak of dengue virus serotype-2 (DENV-2) of Cambodian origin in Manipur, India–association with meteorolical factors. Indian J Med Res 136: 649-655.
- Dev V, Mahanta N, Baruah B ( 2015) Dengue, an emerging arboviral infection in Assam, northeast India. Trop Biomed 32: 796-799.
- Khan SA, Dutta P, Topno R, Soni M, Mahanta J (2014) Dengue outbreak in a hilly state of Arunachal Pradesh in northeast India. Scientific World J 2014: 584093.
- Dutta P, Mahanta J (2006) Potential vectors of dengue and the profile of dengue in the north-eastern region of India: an epidemiological perspective. Dengue Bull 30: 234-242.
- Lambrechts L, Scott TW, Gubler DJ (2010) Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis 4: e646.
- Dutta P, Khan SA, Sharma CK, Doloi P, Hazarika NC, et al. (1998) Distribution of potential dengue vectors in major townships along the national highways and trunk roads of northeast India. Southeast Asian J Trop Med Public Health 29: 173-176.
- Dutta P, Khan SA, Sharma CK, Mahanta J (2010) Survey of mosquito species in Nagaland, a hilly state of north east region of India. J Environ Biol 31: 781-785.
- Dutta P, Khan SA, Chetry S, Dev V, Sarmah CK, et al. (2015) First evidence of dengue virus infection in wild caught mosquitoes during an outbreak in Assam, Northeast India. J Vector Borne Dis 52: 293-298.
- Dutta P, Khan SA, Borah J, Mahanta J (2012) Demographic and clinical features of patients with dengue in Northeastern region of India: a retrospective cross-sectional study during 2009-2011. J Virol Microbiol 2012: 786298.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 12637
- [From(publication date):
December-2016 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 11713
- PDF downloads : 924