Research Article Open Access
Vibrational and NMR Investigation on Pharmaceutical Activity of 2,5- Dimethoxy-4-Ethylamphetamine by Theoretical and Experimental Support
A Madanagopal1, S Periandy2, P Gayathri1, S Ramalingam3* and S Xavier41Department of Physics, Periyar Maniammai University, Thanjavur, Tamilnadu, India
2Department of Physics, Kanchi Mamunivar Centre for PG studies, Puducherry, India
3PG and Research Department of Physics, A.V.C. College, Mayiladuthurai, Tamilnadu, India
4Department of Physics, St. Joseph College of Arts and Science, Cuddalore, Tamil Nadu, India
- Corresponding Author:
- S Ramalingam
PG and Research Department of Physics
A.V.C. College, Mayiladuthurai, Tamilnadu, India
Tel: 04364 222264
Fax: 04364 222264
E-mail: ramalingam.physics@gmail.com
Received Date: January 31, 2017; Accepted Date: February 28, 2017; Published Date: March 07, 2017
Citation: Madanagopal A, Periandy S, Gayathri P, Ramalingam S, Xavier S (2017) Vibrational and NMR Investigation on Pharmaceutical Activity of 2,5-Dimethoxy-4-Ethylamphetamine by Theoretical and Experimental Support. J Mol Pharm Org Process Res 5:135. doi: 10.4172/2329-9053.1000135
Copyright: © 2017 Madanagopal A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Molecular Pharmaceutics & Organic Process Research
Abstract
The Detailed physical, chemical, thermal and circular vibrational investigations have been made on FT-IR, FTRaman, NMR and UV-Visible spectra of 2,5-Dimethoxy-4-ethylamphetamine. The modification of the basic property (deficit hyperactivity disorder) of the base compound (Amphetamine) is favoured by the insertion of two methoxy and ethyl-methyl groups have been discussed in detail. The transitional pattern among NBO emphasized the inducement of the psychedelic activity in the compound. The strong interpretation made on the physical and chemical properties by intense observation using excitations between the electronic energy levels within the molecule have been carried out. The arrangement of the dipole moment of the bonds and the change of resultant magnetic moment were observed from the average Polarizability first order diagonal hyperpolarizability. The receptor and inhibition property of the molecule were interpreted by the identification of reactive sites from molecular electrostatic potential contour map. The chemical reaction continuity is keenly observed from thermodynamical analysis.
Keywords
2,5-Dimethoxy-4-Ethylamphetamine; Amphetamine; Transitional Pattern; Hyperactivity Disorder; Amphetamine; Chemical Reaction Continuity
Introduction
The 2,5-Dimethoxy-4-ethylamphetamine is commonly known as substituted amphetamines and is a psychedelic(also known as psychotogenic) drug [1,2]. It has an active stereocenter which is more active enantiomer and it is a potent and long-acting psychedelic [3,4].
The compound is composed systematically and heavily by methoxy, methyl, ethyl and amino substitutions. Two methoxy groups are loaded symmetrically at ortho and meta positions of left and right moiety respectively of the benzene ring. Similarly, the chain of ethyl and methyl groups substituted at ortho in right moiety whereas the chain of ethyl and methyl groups along with amino ligand present at meta position of left moiety.
The benzene ring with chain of CH, CH2, CH3, and NH2 groups forms alpha-methylphenethylamine called as Amphetamine. It is a potent drug which stimulant central nervous system (CNS) and is used for the treatment of attention deficit hyperactivity disorder, narcolepsy and obesity [5,6]. The compound; Amphetamine drug existed in two enantiomer forms, such as levoamphetamine and dextroamphetamine.
Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. Hence, when the Amphetamine is substituted by symmetrical insertion of two methoxy and asymmetrical addition of ethyl-methyl groups, the composite compound is changed as psychotogenic drug.
In spite of its important pharmaceutical applications thereof; 2,5- Dimethoxy-4-ethylamphetamine has not been subjected to systematic investigation on the structure activity related to its pharmaceutical potential. Therefore, the present investigation is made for the strong interpretation on the structure activity associated with active drug property of the compound using FT-IR, FT-Raman, NMR and UVVisible spectroscopical data and computational results.
Experimental Profile
Physical state
The compound has been taken in solid form which is pure and spectroscopic grade.
Recording profile
The FT-IR and FT-Raman spectra of the compound were recorded using a Bruker IFS 66V spectrometer and the instrument adopted with an FRA 106 Raman module equipped with aNd:YAG laser source operating at 1.064 μm line widths with 200 mW power [7].
The high resolution 1HNMR and 13CNMR spectra were recorded using 300 MHz and 75 MHz FT-NMR spectrometer [8].
The UV-Vis spectrum was recorded in the range of 200 nm to 800 nm, with the scanning interval of 0.2 nm, using the UV-1700 series instrument [9].
Computational Profile
In order to design the structure precisely, calculate geometrical parameters, display the Mulliken charge levels, study the vibrational spectral properties, observe the molecular orbital interactions, examine the frontier molecular transitions on the electronic structure, the entire quantum chemical computations were performed using the Gaussian 09 D. 01. version software program in core i7 computer [10].
The computational calculations were performed over entire geometrical parameters, vibrational frequencies, simulation of molecular structure and spectra using B3LYP and B3PW91 methods adopted with 6-31++G(d, p) and 6-311++G(d,p) basis sets (Table 1). The energy absorbance by the present compound related with electronic spectra, the NBO and HOMO-LUMO energies were calculated using time-dependent SCF method with best fit basis set. In the same way, the 1H and 13C NMR chemical shifts with respect to TMS were calculated by GIAO method using I-PCM model in combination with B3LYP/6-311++G(2d,p). The Mullikan charge assignment on different parts of the compound was calculated and was purposely elucidated for the determination of key factor for pharmaceutical activity of the compound. The dipole moment, linear polarizability and the first order hyper polarizability in different coordinates of the compound were computed using B3LYP method with the 6-311++G(d,p) basis set. The ECD and VCD spectra were simulated from available frequencies and the optical chirality was studied and the mechanism for masking the toxicity was interpreted.
| Geometrical Parameters | Methods | |||
|---|---|---|---|---|
| HF/6-311++G(d,p) | B3LYP/6-311++G(d,p) | CAM-B3LYP/6-311++G(d,p) | B3PW91/6-311++G(d,p) | |
| Bond length(Å) | ||||
| C1-C2 | 1.391 | 1.404 | 1.402 | 1.4 |
| C1-C6 | 1.386 | 1.397 | 1.396 | 1.394 |
| C1-O12 | 1.381 | 1.403 | 1.374 | 1.367 |
| C2-C3 | 1.39 | 1.401 | 1.397 | 1.395 |
| C2-C13 | 1.511 | 1.511 | 1.51 | 1.505 |
| C3-C4 | 1.386 | 1.397 | 1.396 | 1.394 |
| C3-H7 | 1.071 | 1.08 | 1.082 | 1.083 |
| C4-C5 | 1.39 | 1.404 | 1.402 | 1.4 |
| C4-O10 | 1.381 | 1.403 | 1.375 | 1.368 |
| C5C-6 | 1.389 | 1.4 | 1.396 | 1.394 |
| C5-C9 | 1.511 | 1.513 | 1.511 | 1.506 |
| C6-H8 | 1.071 | 1.08 | 1.082 | 1.084 |
| H7-H26 | 3.229 | 3.24 | 1.093 | 1.094 |
| C9-H14 | 1.082 | 1.091 | 1.094 | 1.095 |
| C9-H15 | 1.085 | 1.093 | 1.539 | 1.532 |
| C9-C34 | 1.536 | 1.543 | 1.418 | 1.41 |
| O10-C11 | 1.426 | 1.449 | 1.096 | 1.097 |
| C11-H16 | 1.082 | 1.092 | 1.096 | 1.096 |
| C11-H17 | 1.082 | 1.092 | 1.089 | 1.09 |
| C11-H18 | 1.076 | 1.085 | 1.418 | 1.41 |
| O12-C30 | 1.426 | 1.449 | 1.092 | 1.093 |
| C13-H19 | 1.08 | 1.089 | 1.097 | 1.097 |
| C13-H20 | 1.087 | 1.096 | 1.544 | 1.538 |
| C13-C21 | 1.541 | 1.549 | 1.093 | 1.095 |
| C21-H22 | 1.081 | 1.091 | 1.535 | 1.53 |
| C21-C23 | 1.535 | 1.54 | 1.473 | 1.466 |
| C21-N27 | 1.461 | 1.475 | 1.094 | 1.094 |
| C23-H24 | 1.085 | 1.092 | 1.096 | 1.097 |
| C23-H25 | 1.087 | 1.095 | 1.093 | 1.094 |
| C23-H26 | 1.083 | 1.091 | 1.015 | 1.014 |
| N27H28 | 0.995 | 1.012 | 1.017 | 1.016 |
| N27H-29 | 0.997 | 1.014 | 1.096 | 1.097 |
| C30-H31 | 1.082 | 1.092 | 1.095 | 1.096 |
| C30-H32 | 1.082 | 1.092 | 1.089 | 1.09 |
| C30-H33 | 1.076 | 1.085 | 1.092 | 1.092 |
| C34-H35 | 1.082 | 1.089 | 1.094 | 1.094 |
| C34-H36 | 1.085 | 1.092 | 1.093 | 1.094 |
| C34-H37 | 1.084 | 1.092 | 1.093 | 1.089 |
| Bond angle(Ë?) | ||||
| C2-C1-C6 | 120.784 | 120.931 | 120.213 | 120.137 |
| C2-C1-O12 | 116.171 | 115.552 | 116.033 | 116.079 |
| C6-C1-O12 | 123.044 | 123.515 | 123.752 | 123.781 |
| C1-C2-C3 | 117.628 | 117.577 | 117.892 | 117.935 |
| C1-C2-C13 | 121.133 | 120.971 | 120.945 | 120.851 |
| C3-C2-C13 | 121.23 | 121.448 | 121.159 | 121.211 |
| C2-C3-C4 | 121.599 | 121.493 | 121.888 | 121.917 |
| C2-C3-H7 | 118.212 | 118.041 | 117.895 | 117.846 |
| C4-C3-H7 | 120.187 | 120.464 | 120.215 | 120.234 |
| C3-C4-C5 | 120.706 | 120.874 | 120.181 | 120.107 |
| C3-C4-O10 | 123.173 | 123.619 | 123.791 | 123.808 |
| C5-C4-O10 | 116.119 | 115.505 | 116.026 | 116.083 |
| C4-C5-C6 | 117.77 | 117.697 | 117.981 | 118.026 |
| C4-C5-C9 | 121.15 | 120.971 | 121.092 | 120.994 |
| C6-C5-C9 | 121.076 | 121.325 | 120.92 | 120.972 |
| C1-C6-C5 | 121.509 | 121.423 | 121.841 | 121.872 |
| C1-C6-H8 | 120.213 | 120.461 | 120.226 | 120.232 |
| C5-C6-H8 | 118.277 | 118.114 | 117.931 | 117.894 |
| C3-H7-H26 | 73.274 | 72.766 | 109.291 | 109.233 |
| C5-C9-H14 | 109.292 | 109.165 | 108.782 | 108.799 |
| C5-C9-H15 | 108.884 | 108.96 | 113.286 | 113.092 |
| C5-C9-C34 | 112.966 | 113.257 | 106.967 | 106.921 |
| H14-C9-H15 | 107.182 | 107.132 | 108.959 | 109.087 |
| H14-C9-C34 | 109.046 | 108.777 | 109.359 | 109.519 |
| H15-C9-C34 | 109.303 | 109.365 | 109.78 | 118.315 |
| C4-O10-C11 | 121.649 | 119.028 | 118.681 | 111.675 |
| O10-C11-H16 | 111.196 | 111.416 | 111.595 | 111.657 |
| O10-C11-H17 | 111.179 | 111.386 | 111.582 | 106.057 |
| O10-C11-H18 | 105.645 | 105.184 | 105.919 | 109.207 |
| H16-C11-H17 | 109.613 | 109.63 | 109.27 | 109.051 |
| H16-C11-H18 | 109.538 | 109.535 | 109.169 | 109.095 |
| H17-C11-H18 | 109.585 | 109.586 | 109.21 | 118.4 |
| C1-O12-C30 | 121.75 | 119.091 | 118.753 | 109.896 |
| C2-C13-H19 | 109.812 | 110.1 | 109.821 | 108.935 |
| C2-C13-H20 | 108.896 | 109.047 | 108.884 | 115.006 |
| C2-C13-C21 | 115.273 | 115.216 | 115.268 | 107.143 |
| H19-C13-H20 | 107.267 | 107.323 | 107.149 | 107.027 |
| H19-C13-C21 | 106.86 | 106.443 | 106.958 | 108.54 |
| H20-C13-C21 | 108.433 | 108.408 | 108.453 | 107.237 |
| C13-C21-H22 | 107.515 | 107.157 | 107.377 | 112.35 |
| C13-C21-C23 | 112.788 | 112.6 | 112.532 | 107.752 |
| C13-C21-N27 | 107.909 | 107.887 | 107.719 | 108.774 |
| H22-C21-C23 | 108.943 | 108.989 | 108.756 | 106.51 |
| H22-C21-N27 | 106.788 | 106.619 | 106.375 | 113.86 |
| C23-C21-N27 | 112.604 | 113.247 | 113.723 | 110.672 |
| C21-C23-H24 | 110.247 | 110.3 | 110.576 | 110.654 |
| C21-C23-H25 | 110.619 | 110.442 | 110.679 | 111.737 |
| C21-C23-H26 | 111.933 | 111.948 | 111.753 | 107.726 |
| H24-C23-H25 | 107.745 | 107.806 | 107.751 | 107.949 |
| H24-C23-H26 | 108.063 | 108.153 | 107.948 | 107.952 |
| H25-C23-H26 | 108.091 | 108.054 | 107.984 | 110.432 |
| H7-H26-C23 | 97.0435 | 98.176 | 110.619 | 110.191 |
| C21-N27-H28 | 115.959 | 113.805 | 110.455 | 106.554 |
| C21-N27-H29 | 115.758 | 113.459 | 106.733 | 111.696 |
| H28-N27-H29 | 112.717 | 110.925 | 111.613 | 111.605 |
| H12-C30-H31 | 111.215 | 111.432 | 111.525 | 106.019 |
| O12-C30-H32 | 111.147 | 111.33 | 105.885 | 109.222 |
| O12-C30-H33 | 105.607 | 105.154 | 109.285 | 109.071 |
| H31-C30H-32 | 109.617 | 109.639 | 109.189 | 109.129 |
| H31-C30-H33 | 109.542 | 109.56 | 109.25 | 110.75 |
| H32-C30-H33 | 109.627 | 109.623 | 110.766 | 110.851 |
| C9-C34-H35 | 110.532 | 110.424 | 110.743 | 111.029 |
| C9-C34-H36 | 110.638 | 110.714 | 111.009 | 108.042 |
| C9-C34-H37 | 110.987 | 111.017 | 108.082 | 108.021 |
| H35-C34-H36 | 108.195 | 108.201 | 108.091 | 108.024 |
| C35-C34H-37 | 108.339 | 108.303 | 108.032 | 108.068 |
| H36-C34-H37 | 108.048 | 108.08 | 108.11 | 108.126 |
| Dihedral angle(º) | ||||
| C6-C1-C2-C3 | 0.2271 | 0.2242 | -0.324 | -0.3673 |
| C6-C1-C2-C13 | 179.2725 | 179.7039 | -179.731 | -179.94 |
| O12-C1-C2-C3 | 179.6798 | 79.6527 | 179.2584 | 179.155 |
| O12-C1-C2-C13 | 0.6344 | 0.173 | -0.1483 | -0.4175 |
| C2-C1-C6-C5 | -0.0646 | 0.0888 | -0.0672 | -0.0459 |
| C2-C1-C6-H8 | 79.6903 | 79.6153 | 179.5592 | 179.6015 |
| O12-C1-C6-C5 | 179.9649 | 179.9555 | -179.616 | -179.53 |
| O12-C1-C6-H8 | -0.21 | -0.2515 | 0.0105 | 0.1171 |
| C2-C1-O12-C30 | -174.47 | 176.1274 | -176.683 | -176.968 |
| C6-C1-O12-C30 | 5.4349 | 3.7459 | 2.8833 | 2.5358 |
| C1-C2-C3-C4 | 0.4012 | 0.4049 | 0.4779 | 0.5127 |
| C1-C2-C3-H7 | 179.3774 | 179.394 | -179.226 | -179.193 |
| C13-C2-C3-C4 | 179.4455 | 179.882 | 179.8833 | -179.916 |
| C13-C2-C3-H7 | -0.333 | 0.0831 | 0.1797 | 0.379 |
| C1-C2-C13-H19 | 46.169 | 46.8291 | 46.0494 | 45.665 |
| C1-C2-C13-H20 | 163.3504 | 164.332 | 163.0863 | 162.7675 |
| C1-C2-C13-C21 | -74.5629 | -73.5367 | -74.8138 | -75.1622 |
| C3-C2-C13-H19 | -132.842 | -132.63 | -133.338 | -133.894 |
| C3-C2-C13-O20 | -15.6605 | -15.1274 | -16.3009 | -16.7915 |
| C3-C2-C13-C21 | 106.4263 | 107.0039 | 105.7989 | 105.2788 |
| C2-C3-C4-C5 | -0.2852 | -0.2765 | -0.2377 | -0.2405 |
| C2-C3-C4-O10 | 179.8851 | 179.9045 | -179.927 | -179.901 |
| H7-C3-C4-C5 | 179.489 | 79.5175 | 179.4591 | 179.4578 |
| H7-C3-C4-O10 | -0.3407 | -0.3014 | -0.2304 | -0.2022 |
| C2-C3-H7-H26 | -59.4852 | -59.615 | -0.1607 | -0.1807 |
| C4-C3-H7-H26 | 120.733 | 120.584 | 178.9917 | 178.8832 |
| C3-C4-C5-C6 | -0.0153 | -0.0438 | 179.5522 | 179.5047 |
| C3-C4-C5-C9 | 179.3232 | 79.1222 | -1.2955 | -1.4314 |
| O10-C4-C5-C6 | 179.8259 | 179.7891 | 1.6901 | 1.638 |
| O10-C4-C5-C9 | -0.8355 | -1.0448 | -178.011 | -178.035 |
| C3-C4-O10-C11 | 2.3475 | 2.2294 | 0.3115 | 0.3225 |
| C5-C4-O10-C11 | -177.489 | -177.598 | -179.323 | -179.333 |
| C4-C5-C6-C1 | 0.1873 | 0.2234 | -178.842 | -178.742 |
| C4-C5-C6-H8 | 179.5722 | 179.4874 | 1.5231 | 1.6031 |
| C9-C5-C6-C1 | 179.1517 | 178.9396 | 42.3411 | 41.8907 |
| C9-C5-C6-H8 | 1.0887 | 1.3497 | 158.8045 | 158.2769 |
| C4-C5-C9-H4 | 42.0464 | 43.2342 | -79.3545 | -79.7956 |
| C4-C5-C9-H15 | 158.8247 | 159.9241 | -138.532 | -139.073 |
| C4-C5-C9-C34 | -79.5454 | -78.1186 | -22.0681 | -22.6869 |
| C6-C5-C9-H14 | -138.637 | 137.6302 | 99.7729 | 99.2406 |
| C6-C5-C9-H15 | -21.8586 | -20.9403 | 60.2666 | 60.2085 |
| C6-C5-C9-C34 | 99.7713 | 101.017 | -179.827 | -179.875 |
| C3-H7-C6-C23 | 125.5512 | 125.2534 | -59.8252 | -59.7981 |
| C5-C9-C34-H35 | 60.7348 | 60.6445 | -61.615 | -61.5602 |
| C5-C9-C34-H36 | -179.438 | -179.543 | 58.2913 | 58.3561 |
| C5-C9-C34-H37 | -59.4996 | -59.4932 | 178.2932 | 178.4331 |
| H14-C9-C34-H35 | -60.9959 | -60.9262 | -178.216 | -178.268 |
| H14-C9-C34-H36 | 58.8312 | 58.8861 | -58.3095 | -58.3518 |
| H14-C9-C34-H37 | 178.7697 | 178.9361 | 61.6924 | 61.7253 |
| H15-C9-C34-H35 | -177.872 | -177.625 | -62.1885 | -62.2328 |
| H15-C9-C34-H36 | -58.045 | -57.8131 | 60.3786 | 60.3644 |
| H15-C9-C34-H37 | 61.8935 | 62.237 | 179.1162 | 179.088 |
| C4-O10-C11-H16 | -62.5093 | -62.4929 | -63.0582 | -63.0069 |
| C4-O10-C11-H17 | 59.927 | 60.2695 | 59.4997 | 59.5879 |
| C4-O10-C11-H18 | 178.7322 | 178.9106 | 178.2336 | 178.3013 |
| C1-O12-C30-H31 | -64.418 | -63.4613 | 59.2628 | 59.2119 |
| C1-O12-C30-H32 | 58.015 | 59.2837 | -60.3804 | -60.2541 |
| C1-O12-C30-H33 | 176.8317 | 177.9217 | 173.4756 | 173.5269 |
| C2-C13-C21-H22 | 61.3757 | 60.8094 | -63.1458 | -63.1723 |
| C2-C13-C21-C23 | -58.7462 | -59.019 | 177.211 | 177.3617 |
| C2-C13-C21-N27 | 176.2293 | 175.2768 | 51.067 | 51.1427 |
| H19-C13-C21-H22 | -60.951 | -61.5383 | -178.406 | -178.505 |
| H19-C13-C21-C23 | 178.927 | 178.6333 | 61.9505 | 62.029 |
| H19-C13-C21-N27 | 53.9026 | 52.9291 | -64.1935 | -64.19 |
| H20-C13-C21-H22 | -176.289 | -176.717 | 179.7739 | 179.4964 |
| H20-C13-C21-C23 | 63.5891 | 63.4547 | -60.8983 | -61.1621 |
| H20-C13-C21-N27 | -61.4353 | -62.2495 | 59.4959 | 59.1624 |
| C13-C21-C23-H24 | -179.219 | 179.7443 | 60.9338 | 60.9309 |
| C13-C21-C23-H25 | -60.1479 | -61.1871 | -179.738 | -179.728 |
| C13-C21-C23-H26 | 60.4624 | 59.2642 | -59.3443 | -59.4031 |
| H22-C21-C23-H24 | 61.4838 | 60.9816 | -57.39 | -57.6603 |
| H22-C21-C23-H25 | -179.446 | -179.95 | 61.9378 | 61.6812 |
| H22-C21-C23-H26 | -58.8352 | -59.4985 | -177.668 | -177.994 |
| N27-C21-C23-H24 | -56.791 | -57.5096 | -175.142 | -175.66 |
| N27-C21-C23-C5 | 62.2798 | 61.559 | 66.9091 | 66.8902 |
| N27-C21-C23-H26 | -177.11 | -177.99 | -60.2637 | -60.8623 |
| C13-C21-N27-H28 | -158.498 | -165.162 | -178.212 | -178.312 |
| C13-C21-N27-H29 | 66.1179 | 66.742 | 59.4193 | 59.0155 |
| H22-C21-N27-H28 | -43.1647 | -50.3384 | -58.5294 | -58.4343 |
| H22-C21-N27-H29 | -178.549 | -178.434 | -178.9 | -178.879 |
| C23-C21-N27-H28 | 76.3685 | 69.5166 | 68.974 | 68.982 |
| C23-C21-N27-H29 | -59.0159 | -58.5792 | 58.398 | 58.391 |
| C21-C23-H26-H7 | -71.0767 | -70.3717 | 70.692 | 70.684 |
| 24H-C23-H26-H7 | 167.3395 | 167.9067 | 167.256 | 167.281 |
| H25-C23-H26-H7 | 50.9918 | 51.46 | 51.745 | 51.698 |
Table 1: Optimized geometrical parameters for 2,5-Dimethoxy-4-ethylamphetamine computed at HF and DFT [B3LYP] methods with 6-311+ +G(d,p) basis sets.
Results and Discussion
Structural deformation analysis
The Molecular Weight of the compound and the Monoisotopic Mass are found to be 223.31 g/mol and 223.15 g/mol respectively. The present compound acting as good inhibitor since the Hydrogen Bond Acceptor Count was 3 and Hydrogen Bond donor was 1. Due to 2 Rotatable Bond Count of the present compound, the molecule possesses five stable conformers with mirror symmetry. Since the Defined and undefined Atom Stereocenter count of the compound were found to be zero and one, the resultant dipole moment was so high. Since the covalently-bonded unit count was unity, the entire bonds were saturated. The rough Complexity of 4-Methoxy-3- methylbenzaldehyde was observed to be 198 which are very high enough to make multi dynamic functions.
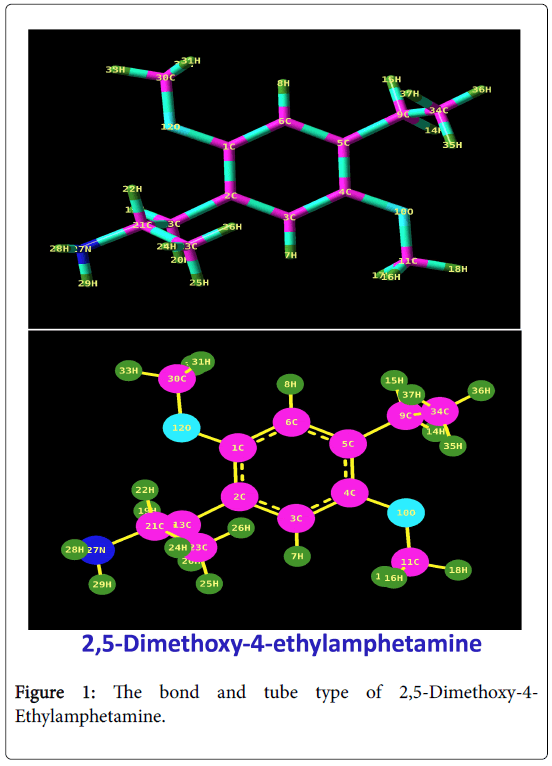
The bond and tube type of present compound was displayed in the Figure 1 and the corresponding (111) plane crystal view of thereof shown in same. The compound under study was basically the derivative of Amphetamine which was composed with couple of methoxy group and ethyl-methyl groups. According to the previous work [11], the bond length between CC of the benzene ring was ranging from 1.392-1.397 Å. In this case, the substituted benzene ring was found to be multi dimensionally broken by the ligand and was evident by the stretching bond length of CC in the range of 1.397-1.404 Å. The entire CC bond length of the ring stretched out and the hexagonal pattern of the ring expanded. The bond length C2-C13 (bond between ring C and amino with ethyl-methyl group chain) was 0.002 Å lesser than C5-C9 (bond between ring C and ethyl-methyl group chain). The bond length limitation was mainly due to the placement of different groups in different dimensions. The ethylmethyl chain was moved apart from the chain due to the electrochemical polar forces. The symmetrical substitutions of methoxy groups proved their symmetry by the constant bond length; C1-O12=C4=O10=1.403Å.
The bond lengths of C-H of the methyl groups were 1.092, 1.092 and 1.085 respectively which are same for that entire methyl group’s present compound. This view showed the consistency of methyl groups. The bond angles C1-O12-C30 and C4-O10-C11 were found to same and were equal to 119Ë? and making the R enantiomer which has four times potency in terms of psychedelic activity. The multiple injections of substitutional groups in the base ring showed the resultant molecule in mighty form and renovate the important pharmaceutical phase.
Mulliken charge analysis
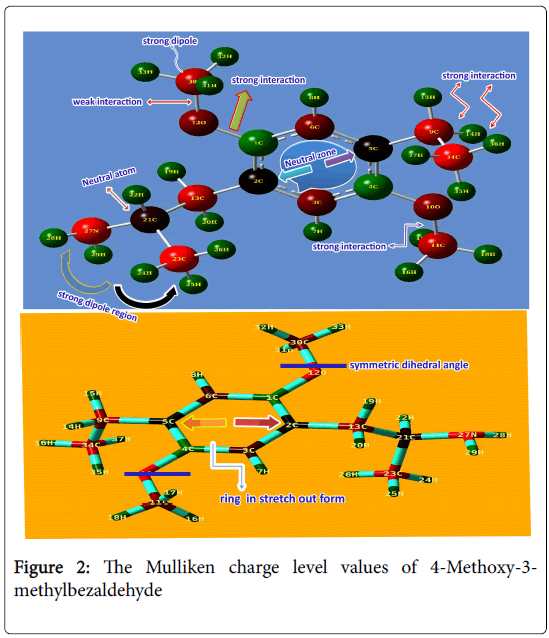
The Mulliken charge level values of 4-Methoxy-3- methylbezaldehyde were displayed in the Table 2 and its diagram was shown in Figure 2. Generally, the charge levels are oriented in carbons (negatively charged) and hydrogen’s (positively charged) of the benzene ring without substitutions. When it is substituted, the charges are depleted with respect to the production of the polar and non-polar bonds among the atoms. Thus the charges are reoriented and dynamic chemical potential are generated for inducing the meticulous property. Here, the carbons C2 and C5 in the ring were found to be neutral where the important substitutions were injected whereas at the point of methoxy substitutions, the carbons C1 and C4 are appeared as positive due the sucking of negative charges by O in order to make polar dipoles in methyl group. Rest of two carbons were happened to be negative since there was no ligand. The benzene ring was stretched parallel to the long chain of methyl-ethyl groups.
| Atom Position | Charge level |
|---|---|
| C1 | 0.25 |
| C2 | 0.021 |
| C3 | -0.144 |
| C4 | 0.247 |
| C5 | 0.033 |
| C6 | -0.149 |
| C9 | 0.413 |
| C11 | -0.288 |
| C13 | -0.375 |
| C21 | -0.073 |
| C23 | -0.485 |
| C34 | -0.504 |
| C30 | -0.289 |
| H7 | 0.168 |
| H8 | 0.166 |
| H14 | 0.195 |
| H16 | 0.18 |
| H17 | 0.181 |
| H18 | 0.196 |
| H19 | 0.205 |
| H20 | 0.149 |
| H22 | 0.2 |
| H24 | 0.157 |
| H25 | 0.146 |
| H26 | 0.185 |
| N27 | -0.684 |
| H28 | 0.28 |
| H29 | 0.272 |
| H31 | 0.178 |
| H32 | 0.181 |
| H33 | 0.198 |
| H35 | 0.195 |
| H36 | 0.167 |
| H37 | 0.167 |
| O12 | -0.538 |
| O10 | -0.541 |
Table 2: Mulliken Charges of 2,5-Dimethoxy-4-ethylamphetamine
Dynamic state of charges generate strong dipole moments between the atoms and the substitutions in ortho and meta positions of the universal hexagonal pattern induced special pharmaceutical properties particularly antifungal and anti-biotic properties [12].
Here, two same substituent (methoxy group) were penetrated in ortho and meta positions and made strong dipole moments in the ring which was the main cause of the inducement of the psychedelic activity. There was neutral atom found at midpoint of the CH2-CH3- NH2 chain on meta position of left moiety of ring.
Usually, when the charges are abruptly depleted at a point of atom, a neutral region is formed due to asymmetrical suction of electron cloud. Here, C of CH group was changed as neutral for the creation of strong dipole moment which was also the reason of the incentive of the drug property.
Vibrational analysis
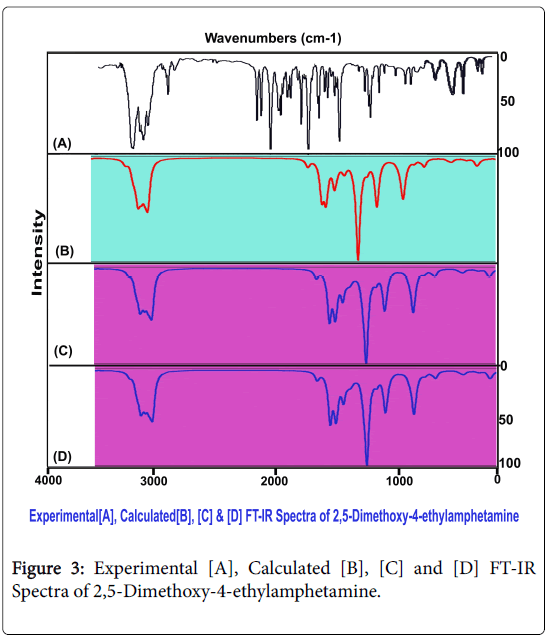
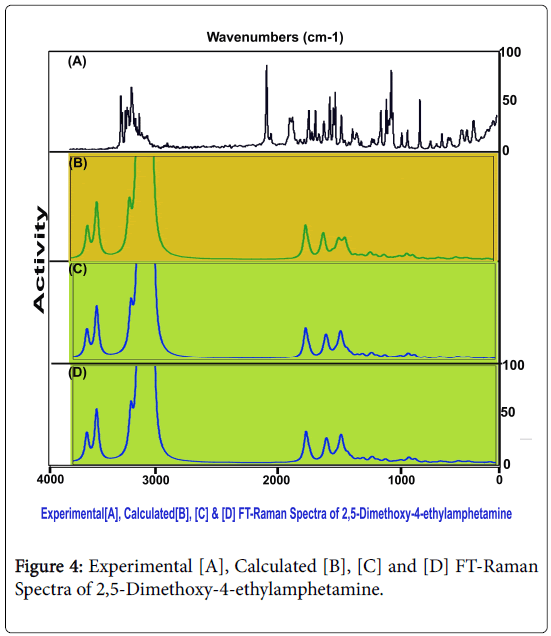
The distinct vibrational fundamental pattern of 2,5-Dimethoxy-4- ethylamphetamine was presented in Table. 3. The scanned FT-IR and FT-Raman vibrational frequencies of observed and simulated spectra by HF and DFT were matched and exhibited in the Figures 3 and 4 respectively. The present novel composite was assembled by two methoxy, two ethyl-methyl group and amino groups with benzene ring. The resultant compound consists of 37 atoms and the structure belongs to CS point group. The 105 fundamental modes of vibrations were dispersed as Γvib = 71A′ + 34 A″.
| S. No. | Symmetry Species CS | Observed frequency(cm-1) | Calculated frequency | Vibrational Assignments | |||
|---|---|---|---|---|---|---|---|
| HF | B3LYP | B3PW91 | |||||
| FT-IR | FT-Raman | 6-311++G(d,p) | 6-311++G(d,p) | 6-311++G(d,p) | |||
| 1 | A′ | 3250s | - | 3279 | 3298 | 3298 | (N-H) υ |
| 2 | A′ | 3220s | - | 3226 | 3230 | 3232 | (N-H) υ |
| 3 | A′ | - | 3080s | 3064 | 3098 | 3089 | (C-H) υ |
| 4 | A′ | - | 3050s | 3042 | 3035 | 3032 | (C-H) υ |
| 5 | A′ | - | 3030s | 3028 | 3018 | 3024 | (C-H) υ |
| 6 | A′ | - | 3010s | 3008 | 3010 | 3019 | (C-H) υ |
| 7 | A′ | 2970s | - | 2984 | 2988 | 2992 | (C-H) υ |
| 8 | A′ | 2950m | 2950s | 2948 | 2987 | 2995 | (C-H) υ |
| 9 | A′ | 2940w | 2940s | 2938 | 2962 | 2942 | (C-H) υ |
| 10 | A′ | 2930w | - | 2938 | 2946 | 2912 | (C-H) υ |
| 11 | A′ | 2910m | - | 2901 | 2906 | 2896 | (C-H) υ |
| 12 | A′ | 2900m | - | 2892 | 2894 | 2888 | (C-H) υ |
| 13 | A′ | - | 2890m | 2858 | 2865 | 2873 | (C-H) υ |
| 14 | A′ | 2870w | 2870w | 2835 | 2842 | 2838 | (C-H) υ |
| 15 | A′ | 2850w | - | 2824 | 2836 | 2816 | (C-H) υ |
| 16 | A′ | - | 2840s | 2818 | 2812 | 2807 | (C-H) υ |
| 17 | A′ | - | 2835s | 2808 | 2798 | 2791 | (C-H) υ |
| 18 | A′ | 2830m | - | 2802 | 2788 | 2789 | (C-H) υ |
| 19 | A′ | 2790w | - | 2789 | 2776 | 2765 | (C-H) υ |
| 20 | A′ | 2770w | 2770vw | 2735 | 2729 | 2754 | (C-H) υ |
| 21 | A′ | 2740w | 2740w | 2728 | 2713 | 2731 | (C-H) υ |
| 22 | A′ | - | 1620s | 1632 | 1625 | 1618 | (N-H) δ |
| 23 | A′ | - | 1600s | 1627 | 1614 | 1610 | ( N-H) δ |
| 24 | A′ | 1590s | - | 1608 | 1603 | 1598 | (C=C) υ |
| 25 | A′ | 1560s | - | 1580 | 1569 | 1562 | (C=C) υ |
| 26 | A′ | 1510m | - | 1528 | 1526 | 1511 | (C=C) υ |
| 27 | A′ | 1460m | - | 1475 | 1471 | 1460 | (C-C)υ |
| 28 | A′ | - | 1440s | 1466 | 1498 | 1482 | (C-C)υ |
| 29 | A′ | 1410s | 1410s | 1421 | 1426 | 1421 | (C-C)υ |
| 30 | A′ | 1380m | - | 1397 | 1375 | 1392 | (N-H)γ |
| 31 | A′ | 1370m | 1370w | 1387 | 1362 | 1374 | (N-H)γ |
| 32 | A′ | - | 1345s | 1361 | 1328 | 1321 | (C-O) υ |
| 33 | A′ | - | 1340s | 1338 | 1315 | 1312 | (C-O) υ |
| 34 | A′ | - | 1305s | 1325 | 1305 | 1309 | ( C-H) δ |
| 35 | A′ | - | 1300s | 1312 | 1298 | 1291 | ( C-H) δ |
| 36 | A′ | 1250m | 1250s | 1268 | 1251 | 1243 | ( C-H) δ |
| 37 | A′ | 1240m | - | 1269 | 1243 | 1221 | ( C-H) δ |
| 38 | A′ | 1225m | 1225s | 1236 | 1212 | 1209 | ( C-H) δ |
| 39 | A′ | - | 1220s | 1225 | 1208 | 1203 | ( C-H) δ |
| 40 | A′ | - | 1185s | 1198 | 1191 | 1172 | ( C-H) δ |
| 41 | A′ | - | 1180s | 1187 | 1174 | 1158 | ( C-H) δ |
| 42 | A′ | 1170s | 1170vs | 1168 | 1151 | 1146 | ( C-H) δ |
| 43 | A′ | - | 1150s | 1154 | 1138 | 1131 | ( C-H) δ |
| 44 | A′ | - | 1140s | 1135 | 1123 | 1118 | ( C-H) δ |
| 45 | A′ | 1070m | 1089 | 1079 | 1074 | ( C-H) δ | |
| 46 | A′ | 1040s | - | 1069 | 1059 | 1035 | ( C-H) δ |
| 47 | A′ | 990m | - | 1003 | 996 | 988 | ( C-H) δ |
| 48 | A′ | - | 980w | 993 | 974 | 966 | ( C-H) δ |
| 49 | A′ | - | 970m | 987 | 961 | 947 | ( C-H) δ |
| 50 | A′ | - | 960m | 978 | 949 | 928 | ( C-H) δ |
| 51 | A′ | - | 940w | 958 | 922 | 916 | ( C-H) δ |
| 52 | A′ | - | 920w | 933 | 906 | 902 | ( C-H) δ |
| 53 | A′ | 880m | - | 901 | 892 | 867 | ( C-N)υ |
| 54 | A′ | 870m | 870w | 897 | 872 | 848 | ( C-H)υ |
| 55 | A′ | 850m | - | 872 | 848 | 824 | ( O-C) υ |
| 56 | A′ | 840m | 840w | 864 | 814 | 807 | ( O-C) υ |
| 57 | A′ | - | 835s | 842 | 803 | 799 | ( C-C) υ |
| 58 | A′ | 830w | 830s | 838 | 801 | 797 | ( C-C) υ |
| 59 | A′ | - | 825s | 832 | 821 | 781 | ( C-C) υ |
| 60 | A′ | - | 820s | 825 | 805 | 778 | ( C-C)υ |
| 61 | A′ | 810w | - | 822 | 792 | 773 | ( C-C) υ |
| 62 | A′ | 800w | - | 787 | 787 | 768 | ( C-H) γ |
| 63 | A′ | 795w | - | 781 | 781 | 760 | ( C-H) γ |
| 64 | A″ | 790w | - | 753 | 763 | 758 | ( C-H) γ |
| 65 | A″ | 780m | - | 824 | 824 | 746 | ( C-H) γ |
| 66 | A″ | - | 760m | 726 | 726 | 724 | ( C-H) γ |
| 67 | A″ | 750w | - | 780 | 780 | 718 | ( C-H) γ |
| 68 | A″ | 740w | - | 766 | 766 | 710 | ( C-H) γ |
| 69 | A″ | - | 730m | 755 | 755 | 705 | ( C-H) γ |
| 70 | A″ | - | 720m | 711 | 711 | 698 | ( C-H) γ |
| 71 | A″ | 688s | - | 702 | 702 | 694 | ( C-H) γ |
| 72 | A″ | 680s | - | 686 | 686 | 671 | ( C-H) γ |
| 73 | A″ | - | 645s | 646 | 646 | 639 | ( C-H) γ |
| 74 | A″ | - | 640s | 634 | 634 | 618 | ( C-H) γ |
| 75 | A″ | 600m | - | 605 | 605 | 606 | ( C-H) γ |
| 76 | A″ | 595w | - | 578 | 578 | 568 | ( C-H) γ |
| 77 | A″ | 590w | - | 567 | 567 | 566 | ( C-H) γ |
| 78 | A″ | 580w | - | 582 | 582 | 657 | ( C-H) γ |
| 79 | A″ | 570m | 570w | 566 | 556 | 592 | ( C-H) γ |
| 80 | A″ | 530w | - | 546 | 586 | 556 | ( C-H) γ |
| 81 | A″ | 560w | - | 537 | 577 | 536 | ( C-O) δ |
| 82 | A″ | 555w | - | 528 | 519 | 521 | ( C-O) δ |
| 83 | A′ | 530w | 530w | 517 | 507 | 513 | ( C-C-C) δ |
| 84 | A′ | - | 500m | 525 | 498 | 506 | ( C-C-C) δ |
| 85 | A′ | - | 460w | 470 | 470 | 472 | ( C-C-C) δ |
| 86 | A′ | - | 450w | 411 | 411 | 443 | ( C-C-C) γ |
| 87 | A′ | - | 370m | 388 | 388 | 378 | ( C-C-C) γ |
| 88 | A″ | - | 360m | 376 | 376 | 365 | ( C-C-C) γ |
| 89 | A″ | 340w | 340m | 351 | 351 | 348 | ( C-O) γ |
| 90 | A″ | 310 | - | 323 | 323 | 314 | ( C-O) γ |
| 91 | A′ | 300 | 300m | 234 | 302 | 302 | ( O-C)δ |
| 92 | A′ | - | 290m | 219 | 288 | 288 | ( O-C)δ |
| 93 | A″ | - | 250w | 213 | 276 | 276 | ( C-N) δ |
| 94 | A′ | 240w | 240vw | 202 | 258 | 258 | ( C-C) δ |
| 95 | A′ | 230w | - | 188 | 238 | 238 | ( C-C) δ |
| 96 | A′ | 210w | - | 179 | 229 | 229 | ( C-C) δ |
| 97 | A′ | 170w | 170vw | 166 | 170 | 170 | ( C-C) δ |
| 98 | A′ | 160w | 160vw | 149 | 148 | 148 | ( C-C) γ |
| 99 | A″ | 150w | - | 123 | 134 | 124 | ( C-C) γ |
| 100 | A″ | 110w | - | 73 | 117 | 110 | ( C-C) γ |
| 101 | A″ | 100w | 100w | 68 | 101 | 91 | ( C-C) γ |
| 102 | A″ | 90w | - | 56 | 58 | 68 | ( C-C) γ |
| 103 | A″ | 80w | - | 51 | 54 | 48 | ( O-C) τ |
| 104 | A″ | 70w | - | 42 | 44 | 41 | ( O-C) τ |
| 105 | A″ | 50w | - | 38 | 37 | 37 | (C-N) τ |
Table 3: Observed and HF and DFT (B3LYP & B3PW91) with 6-31++G(d,p) & 6-311++G(d,p) level Calculated vibrational frequencies of 2,5- Dimethoxy-4-ethylamphetamine.
To get a good correlation with the experimental vibrational modes, it is essential to correct the calculated fundamental frequencies. For this reason, one possible approach involves the rescaling of the force constant matrix, as proposed by Meyer and Pulay [13,14]. The improved procedure has been adopted certainly to improve the agreement between computed and experimental frequencies. However, it was preferable to introduce necessary scaling factors for the fundamental modes was the circuitous approach of scaling the force constants [15]. The HF calculated wave numbers were scaled by the factor 0.910, 0.857 and 0.808, 0.903. The method of B3LYP calculated wavenumbers were scaled by 0.874, 0.933, 0.910 and 0.852 and in the same way B3PW91were scaled by the factors 0.908, 0.914, 0.852 and 0.879 respectively.
Base ring C–H vibrations: Regularly, the ring and chain complex compound is linked with sustainable ligand tailored fascinated compound for the desired chemical properties. By injecting ligand groups with the base molecule, the vibrational fundamentals might be affected. The impression of interference of ligand group over the base can be measured from the rate of appearance of fundamental pattern of the frequencies and consequently pioneer property of the base compound is altered accordingly. Here, three dissimilar ligand groups were linked with the base compound and by studying the suppression of vibrational pattern of thereof, it can be concluded that, whether the property of the base is changed or not. Accordingly, in general, the CH stretching vibrations are observed in the region 3000-3100 cm1 for benzene derivatives [16-18]. In this case, the C-H stretching bands have been found with medium intensity at 3080 and 3050 cm1 in Raman spectrum only. Two vibrations were found within the expected region. This native attitude showed the less influence of ligand on the ring. Here, the C-H in plane and out of plane bending modes were found at 1305 and 1300 cm1 and 800 and 790 cm1 respectively. Usually, those vibrational two different bending bands identified in the region 1300-1000 cm1 and 1000-750 cm1 respectively [19-21]. The in plane bending were pushed well above the expected region whereas out of plane vibrations were pulled down to the lower end of the expected region. Unlike stretching, the bending modes have rather influenced since their strong dipole character of C-H. The entire ring C-H vibrations have not suffered much. This view cleared that, the ring C-H bonds took part in the inducement of new property of the compound.
CC vibrations: Generally, the CC (C=C and C-C) stretching vibrations for phenyl ring are observed in the region 1600 - 1400 cm1 [22-24], in which the wavenumbers in the region 1600 - 1500 cm1 are fundamentally assigned to C=C stretching and the rest to C-C stretching conventionally. In such a case, since C=C and C-C bonds are uncertainty in the ring, three bonds of each to be appeared. Accordingly, the C=C and C-C stretching bands were found at 1590, 1560 & 1510 cm1 and 1460, 1440 & 1410 cm-1 respectively. Though the substitutions strongly bonded with the ring and stretched diagonally, the bands related to C=C and C-C stretching were substantially found with strong and medium intensity within the expected region of the spectrum. This appearance depicted the ring enhancement for the compound being with spectacular property. The ring CCC in plane and out of plane breathing have been found at 460, 450 and 370 cm1 and 360, 340 and 310 cm1 respectively. Even a single ring breathing mode was not been identified within the limit of the observed region. From this condition, it was well known that, due to the loading of different ligand group with huge mass, the ring could not be breathed well.
Methyl groups vibrations: The substitution of methyl group with the aromatic ring expressed their vibrational frequencies for three; stretching, in plane and out of plane bending vibrations normally taking place in the region of 3000- 2750 cm1, 1250-950 cm1 and 950- 720 cm1[23,24] respectively. Accordingly, the stretching vibrational peaks have been identified at 3030, 3010, 2970, 2950, 2940 and 2930 cm-1, in plane bending vibrational peaks were found at 1250, 1240, 1225, 1220, 1185 and1180 cm-1 and out of plane bending signals were found at 780, 760, 750, 740, 730 and 720 cm-1.
All the CH3 stretching vibrations were located in asymmetric region of methyl group vibrations which represent the enhancement of CH3 group in the present molecule. Similarly, the bending group of bands; in plane vibrations was observed within the expected region whereas some of out of plane bending modes have appeared below the expected level. Hopefully, such the vibrational impression in the spectrum, explored the certainty that, the methyl group actively participate in the pharmaceutical reactivity.
The methyl group deformation vibrations are very rare to observe and if they are present, the methyl group will be making strong impact on the base structure [25]. Usually, the CH3 deformation vibrations are expected in the region 1460-1430 cm1 for methyl derivative compounds. But unfortunately, there was no deformation found in the vibrational sequence which was due to the existence of strong dipole moment between C and H.
OCH3 vibrations: The methoxy group is compiled with base ring at para position with respect to ethyl-methyl groups which plays the important role in the property of the product. In this case, the electron clouds on O are significantly high and created very weak interaction with C of methyl group. But it forms strong dipole moment with C of ring. Usually, in this condition, strong absorption taking place in IR spectrum. Here, the C-H stretching vibrations appeared with weak intensity at 2910, 2900, 2890, 2870, 2850 and 2840 cm1. Actually these vibrational region for C-H asymmetric and symmetric stretching is sectioned in the region 2860-2935 cm1 and 2825-2870 cm1 respectively [26,27]. But, here, most of the stretching belongs to asymmetric and rest of some located in symmetric. Therefore such consistent hike observed in the stretching limit and the above said effect was observed in this case. The in plane and out of plane bending modes were found at 1170, 1150, 1140, 1040, 990 and 980 cm1 and 688, 680, 645, 640, 600 and 595 cm1 respectively. The methoxy derivative compounds have multiple peaks by the absorptions related to C-H in plane and out of plane bending vibrations in the region 1250- 875 cm1 and 850-710 cm1 respectively. In this observation, the considerable impact was found in the out of plane bending absorption bands and this was surely by the asymmetric charge orientation on O.
The C-O and O-CH3 stretching mode is normally assigned in the region 1350-1300 [28] cm-1 and 1100-1000 cm1 respectively for anisole compounds. In this case, the C-O and O-CH3 stretching vibrations were happened at 1345 & 1340 cm1 and 850 & 840 cm1 respectively. Obviously, the C-O vibrational bands occupied at the top position of well above the expected region whereas the O-CH3 stretching moved down well below the expected region. This explicit that, the first part have participated in the product property which was found being active. The C-O in plane and out of plane deformations observed at 560 & 555 cm1 and 340 & 310 cm1 respectively. Similarly, the O-C in and out of plane bending modes was found at 300 & 290 cm1 and 80 & 70 cm1. These bending modes were found at far infrared region and such that the frequencies were also downward due to the rotational effect.
Ethyl group vibrations: The aliphatic C-H stretching bands are expected in the region 3000 - 2900 cm-1 [29,30]. In the present compound the vibrations of the ethyl group are observed at 2830, 2790, 2770 and 2740 cm-1. Similarly, the in-plane and out of-plane deformations of such C-H bond are expected in the regions 1200–1100 cm-1 and 900–700 cm-1 respectively. Four bands due to in-plane and out-of-plane bending are observed at 970, 960, 940 cm1 and 920 cm-1 and 590, 580, 570 and 530 cm1 respectively. These observations indicate that, the energy of stretching modes was consumed for the inducement of the new property. Similarly, the in-plane and out-ofplane bending vibrations are moved down from the expected region, because ethylene group acts as bridge between methyl and phenyl ring and it is always affected by either sides of the groups vibrations.
Amino group and C-N vibrations: Generally, the NH group vibrations are very dominative and no way have their vibrational bands not affected. Here the mono amine group was substituted along with the chain of ethyl-methyl group. When the NH group placed between chain and aromatic ring, the secondary N-H stretching vibrational frequencies are observed in the region 3360-3310 cm1 [31,32]. In this case, the N-H stretching bands were observed at 3250 and 3220 cm-1. The in plane and out of plane bending signals have appeared at 1620 & 1600 cm1 and 1380 & 1370 cm1 respectively. The N-H in plane and out of plane bending are expected in the range 1490-1580 cm-1 and 900-700 cm1 [33,34] respectively. In this case, the stretching vibrations were moved down well below the expected region where as in plane and out of plane bending bands moved up extremely well above the expected region. Due to the favouring of charge levels in amino group, the bending mode only were active. The C-N stretching vibrations, in plane bending and out of plane bending vibrations are generally observed in the region 1155-1130 cm1, 550-400 cm1 and 400-360 cm1 respectively [35,36]. In this title compound, the C-N stretching, in plane and out of plane bending bands were observed at 880, 250 and 50 cm1 respectively. These vibrations were affected much due to the lees energy availability and moved in far infrared region.
NMR Analysis
The paramagnetic shield of group of atoms is broken by the attainment of bonding. The chemical properties are alternatively changed with respected to the dynamic character of the electron cloud. Thus the chemical property is exchanged and modified according to the electronic charge transformation. Similarly, the molecule is formed by making bonds with substitutional groups. Therefore corresponding chemical property of the product-compound is complicated and which depends upon their asymmetrical displacement of electron clouds [11]. The change of chemical property is scaled by the chemical shift of associated atoms.
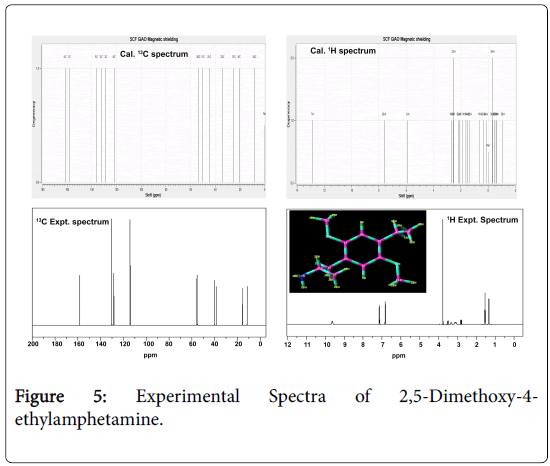
The computed values in gas and solvent phase, along with the experimental values are presented in the Table 4 and the experimental spectra are presented in Figure 5. The aromatic carbon atoms generally [37] have shifts in the range of 120-130 ppm. In the present compound the chemical shifts of the aliphatic carbon atoms C9, C11, C13, C21, C23, C30 and C34 were ranging from 11-55 ppm. But, the carbons of the aromatic ring; C1-C6 were lie in the range of 115-159 ppm experimentally and between 121-161 ppm theoretically. In the case of C3 and C6, there was no substitutional group found, the chemical shift was found to be 115 and 128 ppm respectively. But, the rest of others have large shift which was purely due to the asymmetrical breaking of the paramagnetic shield of the particular carbon. The chemical shift of C1 and C4 was so high which was mainly due to the energy transformation from methoxy group via ring. The transferred energy was exchanged between ring and ethyl-methyl groups. Due to this transformation, the particular carbon in the ring appeared to be neutral. Such a condition shows that, the inherent change of property of the benzene ring in this compound. This trend is in accordance with the charge predicted by Mullikan analysis.
| Atom position | Chemical Shift - TMS-B3LYP/6-311G(2d,p) (ppm) | Experimental shift (ppm) | ||
|---|---|---|---|---|
| Gas | Solvent phase | |||
| DMSO | Chloroform | |||
| C1 | 158.9 | 157.99 | 158.28 | 159.5 |
| C2 | 131.742 | 132.49 | 132.31 | 128.5 |
| C3 | 127.77 | 129.28 | 128.75 | 129 |
| C4 | 161.67 | 161.67 | 161.69 | 159.5 |
| C5 | 136.46 | 136.11 | 136.2 | 130 |
| C6 | 122.33 | 121.73 | 121.91 | 115 |
| C9 | 20.77 | 20.46 | 20.55 | 15 |
| C11 | 49.67 | 50.42 | 50.18 | 40 |
| C13 | 25.3 | 24.81 | 24.96 | 15 |
| C21 | 45.21 | 44.77 | 44.89 | 54.5 |
| C23 | 34.69 | 34.01 | 53.67 | 38 |
| C30 | 53.32 | 53.81 | 53.67 | 55 |
| C34 | 8.35 | 8.2 | 8.24 | 11 |
| H7 | 12.6 | 12.98 | 12.87 | 9.6 |
| H8 | 5.8 | 5.92 | 5.88 | 7.2 |
| H14 | 1.59 | 1.46 | 1.51 | 1.5 |
| H15 | 0.57 | 0.65 | 0.61 | - |
| H16 | 2.5 | 2.75 | 2.68 | - |
| H17 | 1.7 | 1.93 | 1.89 | 1.2 |
| H18 | 2.42 | 2.59 | 2.56 | 2.7 |
| H19 | 1.74 | 1.53 | 1.61 | - |
| H20 | 0.26 | 0.36 | 0.33 | - |
| H22 | 1.27 | 1.34 | 1.32 | - |
| H24 | 0 | 0.21 | 0.14 | - |
| H25 | 0.64 | 0.4 | 0.48 | - |
| H26 | 7.8 | 7.5 | 7.61 | 7.2 |
| H28 | 0.84 | 0.55 | 0.64 | - |
| H29 | 0.74 | 1 | 1.08 | - |
| H31 | 2 | 2.21 | 2.18 | - |
| H32 | 2 | 2.16 | 2.11 | - |
| H33 | 2.4 | 2.54 | 2.56 | - |
| H35 | 0.25 | 0.39 | 0.3 | - |
| H36 | 0.25 | 0.27 | 0.487 | - |
| H37 | 0.63 | 0.53 | 0.57 | - |
Table 4: Experimental and calculated 1H and 13C NMR chemical shift in 2,5-Dimethoxy-4-ethylamphetamine.
The chemical shift of carbon atoms C13 and C34 in the ethyl and methyl groups has of 15 and 11 ppm experimentally and 24 and 8 ppm theoretically, these were lower than the expected values. When the negative charge domain is dislocated towards the CH and NH2 groups, the negative charges were exchanged via C13 and made as virtually shielded and neutral.
So, the chemical shift of such carbon becomes very low and below 50 ppm. In the case of C34, the charges were moved asymmetrically to the ethyl group and making strong dipole. The chemical shift of C21 was found to be 54.5 ppm and made as neutral which was due to the absorption of charges by the amino group.
In this case, the chemical shift value was rather increased which was clearly due to the presence of four σ bond character.
The chemical shifts of the hydrogen atoms in benzene ring as well as methyl group are expected between 7-8 ppm. In this case, ring related hydrogen’s H7 and H8, the chemical shift was found to be within the limit at both experimentally and theoretically.
The entire H of alkyl, ethyl and methyl groups were found to be very low and some of the chemical shift was not observed. This view showed the charge prediction by Mullikan analysis for hydrogen atoms are correct. Except H7 and H8, the entire theoretical shift was found to be 0.5 - 2.0 ppm and this trend is in tune with the above literature.
There was no appreciable difference observed in the chemical shifts in different solvents phases. Hence the impact of the solvents on the chemical shifts of the compound for various atoms is negligibly small.
Frontier molecular interaction profile
After the assembly of molecular orbitals in the compound, the charge depletion region is formed generally between two elevated orbitals with different characteristics called HOMO and LUMO.
Such these orbitals are arranged with respect to the energy of bonded molecules and some the orbitals with same energy are usually overlapped with one another and intersected. The overlapped orbitals are shared by the electrons and they spent most of the time on blended orbitals separately in HOMO and LUMO.
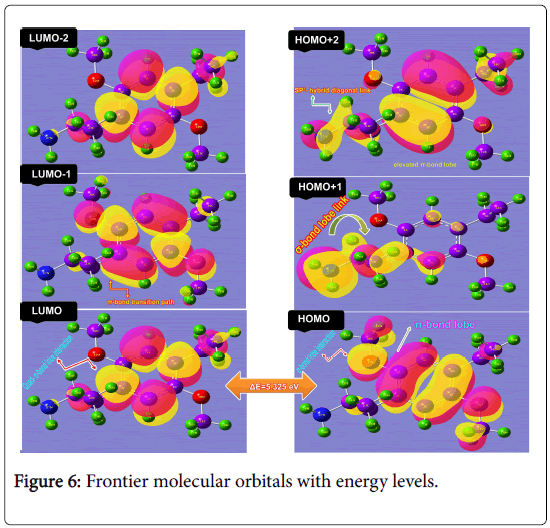
The transitions taking place between those orbitals strongly set the chemical character of the compound and thus, the new physicochemical property was induced in the compound. The energy of Frontier molecular structure was depicted in the Table 5 and the diagram was displayed in Figure 6.
| Energy levels | IR region | UV-Visible region |
|---|---|---|
| B3LYP/ 6311G Energy (eV) | B3LYP/ 6311G Energy (eV) | |
| H+10 | -9.8918 | -9.599 |
| H+9 | -9.6056 | -9.469 |
| H+8 | -9.4709 | -9.469 |
| H+7 | -9.1615 | -9.1503 |
| H+6 | -9.0983 | -9.0298 |
| H+5 | -9.0586 | -8.2932 |
| H+4 | -8.799 | -7.9593 |
| H+3 | -8.43 | -7.3073 |
| H+2 | -7.731 | -6.7925 |
| H+1 | -6.6733 | -6.2474 |
| H | -5.8675 | -6.0597 |
| L | -0.117 | -0.3333 |
| L-1 | 0.4764 | 0.0712 |
| L-2 | 1.203 | 0.9455 |
| L-3 | 1.3344 | 1.3698 |
| L-4 | 1.6593 | 1.4645 |
| L-5 | 1.9243 | 1.7567 |
| L-6 | 2.2389 | 1.8569 |
| L-7 | 2.382 | 1.9763 |
| L-8 | 2.2389 | 2.415 |
| L-9 | 2.382 | 2.5959 |
| L-10 | 2.4558 | 2.6332 |
Table 5: Frontier molecular orbitals with energy levels.
Here, in HOMO, the right and left moiety (CCC semi-circle) of the ring system occupied by π- bond overlapping whereas methoxy group making δ-bond overlapping with O. There was no electron occupied orbitals found on ethyl and methyl groups’ and also there was no orbital interaction lobes were found on same system. In the case of LUMO, σ-bonding overlapping was appeared on the C-C and C-H of the ring and another σ-bond overlapping lobes were occupied over ring ethyl and methyl group whereas the methoxy groups were abandoned. From this view, it was clear that, the electron density were reoriented asymmetrically and they were prepared to provide the charges to the LUMO to induce chemical energy for generating psychotogenic character. In addition to that, HOMO+1, σ-bond lobes in cascade form were found at amino-ethyl-methyl chain group and some of the orbital interaction residue was observed over the ring carbons and methoxy groups. From this view, the chemical energy was started from this group and transferred via C of the ring. In the case of HOMO+2, there were strong π and δ-bond overlapping lobes identified over the ring carbons and two ethyl-methyl chains. From this view, it was observed that, the energy was exchanged between two chains via ring. There were no other lobes over rest of the atoms. In LUMO-1, two π-bond and three σ-bond overlapping of orbitals were to be appeared in ring and methoxy group while in the case of LUMO-2, only σ-bonded lobes were found in the ring. Form this display of orbital lobes; it was obvious that, in HOMO spatial quantization, the aggressive δ-bonding donor orbitals were available for supplying the chemical energy over the empty orbitals whereas in LUMO sequence, σ and π bonding lobes appeared on ring and ligand groups. This arrangement was suitable for creating the drug for treating hyperactivity disorder. For forming potential drug, the chemical energy transition was restricted among the orbitals by 5.325 eV which was very high and enough to sustain the property. The energy values of frontier molecular levels were presented in the Table 5.
UV-visible absorption analysis
The confinement of vibrational energy states depends on the impact of the ligand groups on the base molecule. The energy was supposed to be within the transition among the energy states which shift the vibrational pattern (wavenumber region) of the resultant compound from lower to higher or vice versa. Thus the electronic shift also is observed in the electronic energy states pattern. A charge transfer complex or electron donor-acceptor complex is associated with different energy domain of the molecule, in which electronic charges are transferred between the two entities of molecule. The resulting electrostatic attraction provides a stabilizing force for the molecular complex. The charge transfer is taking place anywhere in the molecular complex and usually, the electronic transition is occur into an excited electronic states of the substitutional group to base, among electronic states of the substitutional group and among different parts of the base molecule. These electronic transitions into the coordinated excited electronic states of different entities of the compound frequently occur in UV-Visible region which characterize the physical and chemical property.
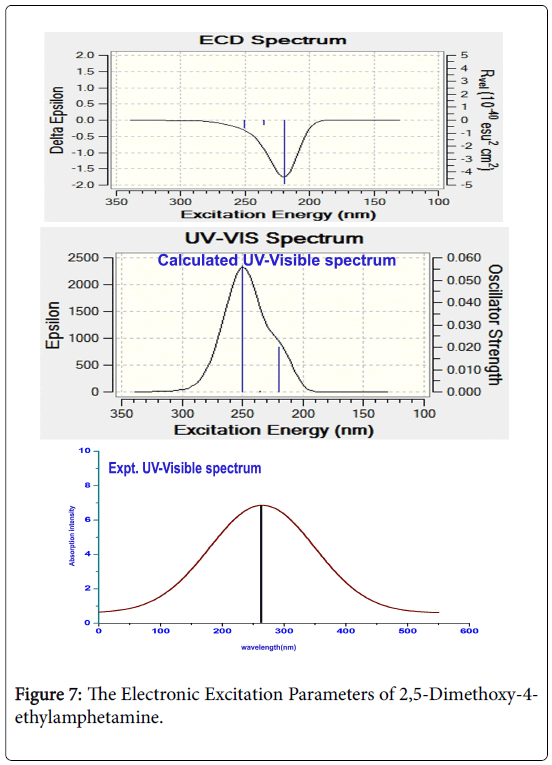
In this case, the electronic excitation absorption CT band was found at 250 nm of oscillator strength 0.05 on the energy gap of 4.95 eV and was assigned to n→ π* in gas phase. The energy of CT complex was found to be 4.95 eV is enough to make sure the transition between acceptor (ethyl-methyl-amino group) and donor (phenyl ring) whereas the observed UV-Visible band was identified at 260 nm. The experimental CT band was shifted to higher wavelength region since the source material was in solid phase. In solvent phase, the CT band is identified at 249 nm with oscillator strength of 0.07 at the same energy gap. The attained result of CT complex in gas as well as solvent phase showed the strong interaction between donor (methoxy) and acceptor (phenyl). The absorption band of present compound was transparently occurring in the UV spectrum in R-band (German, radikalartig) and consistently being with anti-depression activity. In this case, the identification of absorption band in quartz-UV region predicted that, the symmetrical placement of methoxy entities in opposite sides of the ring was playing the important role of such pharmaceutical action. The electronic excitation parameters are presented in the Table 6 and the absorption band was displayed in the Figure 7.
| λ (nm) | E (eV) | ( f ) | Major contribution | Assignment | Region | Bands |
|---|---|---|---|---|---|---|
| Gas | ||||||
| 250.38 | 4.9519 | 0.0562 | H®L (92%) | n→π* | Quartz-UV | R-band (German, radikalartig) |
| 235.5 | 5.2647 | 0.0003 | H®L (89%) | n→π* | ||
| 219.55 | 5.6472 | 0.0201 | H®L (86%) | n→π* | ||
| DMSO | ||||||
| 249.61 | 4.9671 | 0.0704 | H®L (90%) | n→π* | Quartz-UV | R-band (German, radikalartig) |
| 236.95 | 5.2326 | 0.0002 | H®L (90%) | n→π* | ||
| 221.64 | 5.5939 | 0.015 | H®L (87%) | n→π* | ||
| Chloroform | ||||||
| 250.13 | 4.9568 | 0.074 | H®L (86%) | n→π* | Quartz-UV | R-band (German, radikalartig) |
| 236.38 | 5.2452 | 0.0003 | H®L (85%) | n→π* | ||
| 220.84 | 5.6142 | 0.0207 | H®L (78%) | n→π* | ||
Table 6: Theoretical electronic absorption spectra of 2,5-Dimethoxy-4-ethylamphetamine (absorption wavelength λ (nm), excitation energies E (eV) and oscillator strengths (f)) using TD-DFT/B3LYP/6-311Gmethod.
The rearrangement of electronic orbitals on par with the equilibrium force of attraction existing between the dipoles of the compound induced the local electric field which making instantaneous polarization causing ECD.
The interaction of chromophores and auxochrome with base compound providing smaller energy increments for transition to excited states modify the chemical activity of the compound which can be identified in the ECD spectra. As in the Figure 7, the ECD absorption band was identified at 220 nm which was nearly equal energy absorption as UV-visible energy transition. This effect explored the unique chemical reactivity.
Molecular Electrostatic Potential (MEP) maps
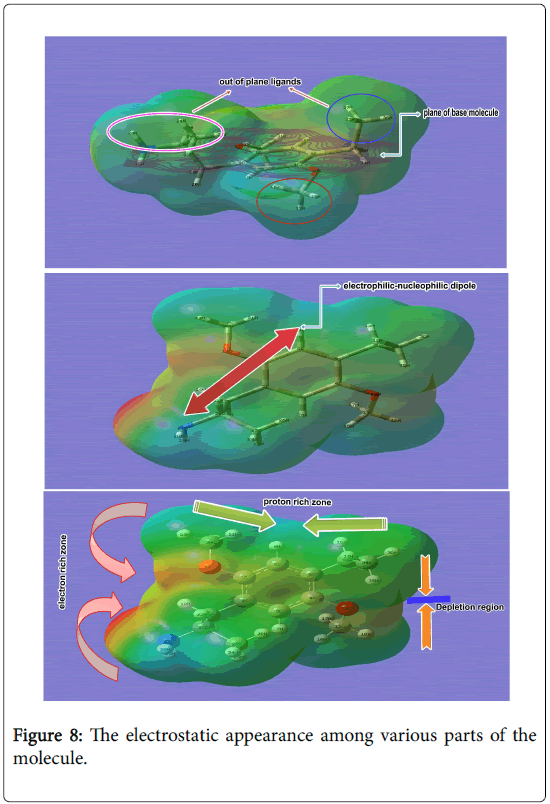
The asymmetrical charge reorientation of the molecule has been organized by the restoring chemical equilibrium forces from the arrangement of different dipoles in various part of the compound.
Such an elevated charge orientation over the molecule was produced by homo and hetero nuclear bonds of the ring and ligand groups.
Here, the main frame of molecule was substituted by three dissimilar atomic groups and thereby the asymmetric charge orientation causing strong electrostatic potential between two extreme charge levels. The electrostatic appearance among various parts of the molecule was shown in the Figure 8.
The faded electron rich and electron deficient zones were distinguished by the red to blue colour region on the molecule. The electron rich showed intensive red and proton wealthy part identified by concentrated blue.
In the Figure 8, the electron bustle zone was captured over the O of methoxy group and N of amino group. The moderate negative region was concealed over the ring carbons and further decayed when moved towards chain.
The protonic content was incarcerated on the hydrogen zones over the methyl group. It was copious in around the edge of the molecule and deficient in carbon bonded side. This faze situation was induced by the hydrogen bond chaos on methoxy and methyl groups.
Due to the electron pulling away from the ring, the electrostatic energy was found to be uniform at the centre part of the ring and acted as defect free energy grid. In each and every molecule has strong ligand which is the root cause of the major property of the compound.
In this case, the strong electrophilic-nucleophilic dipole was found between ring C-H and N of amino group and o of methoxy group. The out of plane ligand usually making strong receptor activity when docking is made.
Here, methoxy and ethyl-methyl chain appeared as out of plane ligand which was indicated in the Figure 7.
Polarizability and hyperpolarizability analysis
The chemical force of attraction stabilized the polarized orbitals in different coordinates of the molecule which facilitate the strong physico-chemical property and can be measured by computing Polarizability and first order hyperpolarizability as in the Table 7.
| Parameter | a.u. | Parameter | a.u. |
|---|---|---|---|
| αxx | -102.4282 | βxxx | -15.8134 |
| αxy | 0.2259 | βxxy | -20.5931 |
| αyy | -79.5913 | βxyy | -2.9661 |
| αxz | -5.8184 | βyyy | 8.6568 |
| αyz | 2.4708 | βxxz | 26.1358 |
| αzz | -102.6729 | βxyz | 2.1077 |
| αtot | 198.432 | βyyz | -11.5149 |
| Δα | 268.436 | βxzz | -4.7675 |
| μx | 0.1763 | βyzz | -0.9354 |
| μy | -0.4912 | βzzz | -1.493 |
| μz | 0.7768 | βtot | 187.7 |
| Δμ | 0.9358 |
Table 7: The dipole moments μ (D), the polarizability α(a.u.), the average polarizability αo (esu), the anisotropy of the polarizability Δα (esu), and the first hyperpolarizability β(esu) of 2,5-Dimethoxy-4-ethylamphetamine.
The calculated value of the dipole moment was found to be very less (0.935 Debye) since the multi pole moments were found to be dispersed in different dimensions. The ligand in the compound oriented in different sides and the resultant dipole moment was very low. The calculated showed that, the major entities were found to be on x and y coordinates of the compound which point out the direction of the chain and methoxy group.
The calculated average polarizability and anisotropy of the polarizability is 198 x10−30esu and 268 x 10−30esu, respectively. The hyperpolarizabilityï¢ is one of the important key factors of stabilization of frontier molecular orbital interaction system. The B3LYP/6-311+ +G(d,p) calculated first hyperpolarizability value (ï¢) is 187.7 x10−33esu. From this observation, it was clear that, the hyper asymmetrical polarization was taking place abruptly to empower the frontier molecular orbitals for the stimulation of pharmaceutical property.
Thermodynamical functions analysis
Normally, the thermo dynamical analysis on aromatic compound is very important since they provide the necessary information regarding the chemical reactivity [12]. The thermodynamic functional parameters were depicted in the Table 8. The variation of thermodynamic functional parameters with temperature was shown in Table 8. The calculated entropy, specific heat capacity and enthalpy were found to be varied with positive temperature coefficient. When the temperature increased from 100K to absolute temperature 298.15, the functional parameters were varied unhurriedly whereas from 350 to 1000K, the thermodynamical functions established to swing as linear pattern and rather constant at maximum temperature. This view of variation showed the consistent chemical reactivity and considerable chemical hardness of the present compound. The Gibbs free energy is always negative temperature coefficient and here, since it was found to be true, the present compound has strong and unique chemical property and endless chemical reaction.
| T(K) | (cal mol-1 K-1) | (calmol-1K-1) | (kcalmol-1) | Gibbs free energy ΔG=ΔH-TΔS KJmol1 |
|---|---|---|---|---|
| 100 | 359.47 | 132.87 | 8.09 | -35938.9 |
| 200 | 477.67 | 214.28 | 25.61 | -95508.4 |
| 298.15 | 577.18 | 289.86 | 50.32 | -172036 |
| 300 | 578.98 | 291.32 | 50.85 | -173643 |
| 400 | 673.54 | 369.21 | 83.91 | -269332 |
| 500 | 763.7 | 439.82 | 124.44 | -381726 |
| 600 | 849.36 | 500.04 | 171.52 | -509444 |
| 700 | 930.36 | 550.72 | 224.13 | -651028 |
| 800 | 1006.78 | 593.58 | 281.41 | -805143 |
| 900 | 1078.86 | 630.1 | 342.64 | -970631 |
| 1000 | 1146.91 | 661.42 | 407.25 | -1146503 |
Table 8: Thermodynamic parameters at different Temperatures for 2,5-Dimethoxy-4-ethylamphetamine.
NBO transition analysis
The NBO data of the compound was derived from perturbed and non-perturbed frontier molecular orbitals in which the electronic energy was exchanged. The energy was transferred among various energy domains for standardize the significant orbitals for obtaining desired physical and chemical characteristics [38]. In this venture, the donor and acceptors of electronic orbitals were identified and their energy transitions were tabulated in Table 9.
| Donor[i] | Type of bond | Occupancy | Acceptor[j] | Type of bond | E2[kcal/mol] | Ej – Ei [au] | F(I j) [au] |
|---|---|---|---|---|---|---|---|
| C1-C2 | σ | 1.97172 | C2-C3 | σ* | 3.39 | 1.28 | 0.059 |
| C1-C2 | σ | 1.97172 | C3-C4 | σ* | 19.39 | 0.28 | 0.066 |
| C1-C2 | σ | 1.97172 | C5-C6 | σ* | 19.43 | 0.29 | 0.067 |
| C1-C6 | π | 1.976 | C1-C2 | π* | 4.57 | 1.28 | 0.068 |
| C1-C6 | π | 1.976 | C2-C13 | π* | 3.4 | 1.1 | 0.055 |
| C1-C6 | π | 1.976 | C5-C6 | π* | 3.52 | 1.28 | 0.06 |
| C2-C3 | π | 1.96654 | C1-C2 | π* | 3.61 | 1.27 | 0.061 |
| C2-C3 | π | 1.96654 | C1-O12 | π* | 4.19 | 0.98 | 0.057 |
| C2-C3 | π | 1.96654 | C3-C4 | π* | 3.59 | 1.25 | 0.06 |
| C2-C3 | π | 1.96654 | C4-O10 | π* | 4.04 | 0.98 | 0.056 |
| C2-C13 | σ | 1.97109 | C1-C6 | σ* | 3.26 | 1.15 | 0.055 |
| C3-C4 | σ | 1.67521 | C2-C3 | σ* | 3.65 | 1.28 | 0.061 |
| C3-C4 | σ | 1.67521 | C2-C13 | σ* | 3.27 | 1.1 | 0.054 |
| C3-C4 | σ | 1.67521 | C4-C5 | σ* | 4.6 | 1.28 | 0.069 |
| C3-C4 | σ | 1.67521 | C5-C9 | σ* | 3.22 | 1.1 | 1.1 |
| C3-C4 | σ | 1.67521 | C1-C2 | σ* | 20.49 | 0.29 | 0.07 |
| C3-C4 | σ | 1.67521 | C5-C6 | σ* | 20.92 | 0.29 | 0.071 |
| C3-H7 | σ | 1.97462 | C1-C2 | σ* | 4.27 | 1.11 | 0.061 |
| C3-H7 | σ | 1.97462 | C4-C5 | σ* | 3.76 | 1.11 | 0.058 |
| C4-C5 | π | 1.97208 | C3-C4 | π* | 4.32 | 1.26 | 0.066 |
| C4-C5 | π | 1.97208 | C5-C6 | σ* | 3.2 | 1.29 | 0.057 |
| C5-C6 | σ | 1.96709 | C1-C6 | σ* | 3.44 | 1.25 | 0.059 |
| C5-C6 | σ | 1.96709 | C1-O12 | σ* | 4.06 | 0.98 | 0.056 |
| C5-C6 | σ | 1.96709 | C1-C2 | σ* | 20.34 | 0.29 | 0.069 |
| C5-C6 | σ | 1.96709 | C3-C4 | σ* | 19.38 | 0.28 | 0.067 |
| C5-C9 | σ | 1.97355 | C3-C4 | σ* | 3.31 | 1.15 | 0.055 |
| C6-H8 | σ | 1.97461 | C1-C2 | σ* | 3.78 | 1.11 | 0.058 |
| C6-H8 | σ | 1.97461 | C4-C5 | σ* | 4.24 | 1.11 | 0.061 |
| C9-H14 | σ | 1.97872 | C5-C6 | σ* | 3.27 | 1.08 | 0.053 |
| C9-H15 | σ | 1.98004 | C4-C5 | σ* | 3.01 | 1.07 | 0.051 |
| C13-H19 | σ | 1.97768 | C21-C23 | σ* | 3.32 | 1.07 | 0.053 |
| C23-H26 | σ | 1.98554 | C21-N27 | σ* | 3.48 | 0.86 | 0.049 |
| O10 | n | 1.9678 | C3-C4 | σ* | 4.67 | 1.14 | 0.065 |
| O10 | n | 1.9678 | C3-C4 | σ* | 17.03 | 0.34 | 0.073 |
| O10 | n | 1.9678 | C11-H16 | σ* | 4.52 | 0.72 | 0.052 |
| O10 | n | 1.9678 | C11-H17 | σ* | 5.67 | 0.72 | 0.058 |
| O12 | n | 1.96751 | C1-C6 | π* | 4.65 | 1.14 | 0.065 |
| O12 | n | 1.96751 | C1-C12 | π* | 16.37 | 0.35 | 0.073 |
| O12 | n | 1.96751 | C30-H31 | σ* | 4.5 | 0.72 | 0.052 |
| O12 | n | 1.96751 | C30-H32 | σ* | 5.67 | 0.72 | 0.058 |
| N27 | n | 1.96142 | C23-H26 | σ* | 6.81 | 0.68 | 0.061 |
| C3-C4 | σ | 0.02637 | C1-C2 | σ* | 316.66 | 0.01 | 0.082 |
Table 9: The calculated NBO of 2,5-Dimethoxy-4-ethylamphetamine by second order Perturbation theory.
Usually, the electron density delocalized among occupied Lewis type (bond or lone pair) orbitals and unoccupied (anti-bonding and Rydberg) non-Lewis orbital in order to stabilize donor acceptor interaction [39]. Here, in ring system, the transition from C1-C2 to C3-C4 and C5-C6 and they assigned to σ-σ* in which 19.39 kcal/mol energy was transferred from first chain to second chain in order to connect the major ligand groups. In same system, another transitions from C3-C4 to C1-C2 and C5-C6 which were assigned to σ-σ* with energy of 20.50 kcal/mol respectively. In these transitions, the received energy was exchanged from methoxy group to chain and another methoxy group on another side. Similarly, the transitions were taking place from C5-C6 to C1-C2 and C3-C4 by σ-σ* interaction with the energy of 20.3 and 19.3 kcal/mol respectively. In this case, the energy was transferred in order to blend the Lewis of chain and methoxy group. It was very rare to take place the transitions from lone pair to other system. Here it was happened from C5-C6 to C1-C2 and C3-C4 with the exchanged energies of 20.3 and 19.3 kcal/mol respectively for the back donation of interaction energy (from chain to methoxy group). The huge amount of energy of 316.6 kcal/mol was transferred from C3-C4 to C1-C2 for the two symmetrical chain and methoxy groups. In these transitions, the electronic energies exchanged between ligand to ligand via the ring and also the residue energy was transferred from the chain to the ring system. Thus the energy was exchanged back and forth among the orbitals to make desirable physical and chemical property of the study compound.
Physico-chemical properties
The chemical properties and molecular reactivity descriptors of the present compound were computed from Frontier molecular energy levels. The entire parameters were presented in the Table 10. The resultant dipole moment is the measuring scale of asymmetric charge orientation of compound and the Total determined dipole moment was found to be 0.93 and 2.34 dyne in IR and UV-visible region respectively. In this case, the base compound is benzene; its dipole moment is almost zero. Here, the total compound composed by multiple ligands with benzene ring. Due to the symmetrical substitutions in ortho and meta positions in the ring, the total computed dipole moment was found to be very low and it ensured that symmetric charge orientation for the desired pharmaceutical property. The energy gap of the frontier molecular orbitals measured usually, the chemical stability of the compound; the same was determined to be 2.66 and 2.86 eV in IR and UV-Visible region respectively. Both the values showed moderate chemical stability and also it was appeared in non-reactive Quartz UV region.
| Parameter | B3LYP 6311G | UV-Visible | Electrophilicity charge transfer (ECT) (ΔNmax)A-(ΔNmax)B |
|---|---|---|---|
| Etotal (Hartree) | 7.13 | -7.13 | 1.321 |
| EHOMO (eV) | 5.442 | 6.059 | |
| ELUMO (eV) | 0.117 | 0.333 | |
| DEHOMO-LUMO gap (eV) | 5.325 | 5.726 | |
| EHOMO-1 (eV) | 6.343 | 6.13 | |
| ELUMO+1 (eV) | 6.556 | 6.58 | |
| DEHOMO-1-LUMO+1 gap (eV) | 12.9 | 0.449 | |
| Chemical hardness (h) | 2.662 | 2.863 | |
| Electronegativity (χ) | 2.662 | 2.863 | |
| Chemical potential (μ) | 2.662 | 2.863 | |
| Chemical softness(S) | 10.65 | 11.452 | |
| Electrophilicity index (ω) | 1.331 | 1.431 | |
| Dipole moment | 0.935 | 2.34 |
Table 10: Calculated energies, chemical hardness, electro negativity, Chemical potential, Electrophilicity index of 2,5-Dimethoxy-4-ethylamphetamine.
The electron affinity of the molecule is very important for the determination of the reaction ability of receptor protein and was found to be 5.44 which were elevated to the extreme and the reaction capability of the present compound is energetic. The ionization potential of the compound is significant to evaluate chemical-bond reorganization. The ionization potential was found to be 0.11 which is very small and was main reason for the low dipole moment and it was enough to maintain the chemical bond stability. Generally, the chemical hardness is a scale of obstacle for transformation of charge whereas the electronegativity is measure of the tendency to attract electrons by inter-chemical bond [39]. Here, both parameters were found to be 2.66 which was moderate and illustrated the good reactive character and it was not possible to add further additive drug properties.
The electrophilicity index is an indicator of energy flow via frontier molecular orbitals. In this case, the electrophilicity index was recognized to be 1.331 eV, but the same was 2.09 eV for benzene ring. The derived energy was very low due to the symmetrical existence of the ligand groups. From this point of view, it was clear that, the maximum energy exchanged between ligand via ring for creating the prosperous pharmaceutical application. Here, the benzene acted as base compound and it was substituted with ethyl-methyl-amino groups and methoxy groups in balanced form and the electrophilicity charge transfer of the compound was found to be + 1.321 which emphasized the maximum charge flow from ligand to ligand via benzene. This also major reason for the present compound is an antidepression agent.
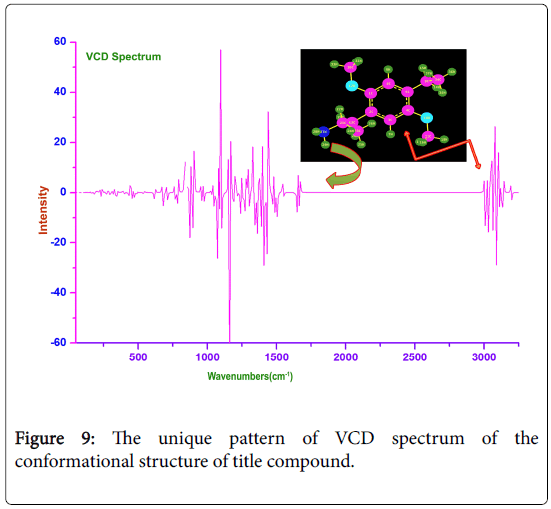
VCD verification
The good Chirality of the compound can have good biological and pharmaceutical property with hiding of toxicity. The architecture of the chirality reflects masking of side effect. The regular peak sequence on both sides was created by circularly polarized infrared radiation during a vibrational transition. Generally, the peaks are found to be in unique sequential pattern. In addition to that, there were few small opaque parts identified in different region of the spectrum which reflect the unwanted properties. This is mainly due to the flaw in optimization which can be removed by re optimizing the structure. The unique pattern of VCD spectrum of the conformational structure of title compound was displayed in the Figure 9. The VCD of present compound showed the R- enantiomer and emphasized the optical and chemical purity of the present substance.
Conclusion
The present compound; 2,5-Dimethoxy-4-ethylamphetamine was the primary derivative of Amphetamine. In order to evaluate and determining unknown properties, the basic Amphetamine was substituted by suitable ligand and different analyses have been made on the chemical structure. The molecular deformation analysis gave the complete information regarding the structure activity on par with the ligand. The charge reorientation among bonded entities revealed the asymmetric movement of the charges which was favoured for inducement of peculiar drug property. The vibrational assignments of the compound explicit the fundamental IR and Raman frequencies which were consistently emphasized the correct compositional bonds which composed the compound. The chemical reaction path arrangement of different carbons was ensured from the discrete chemical shift and the background reason was extracted. The orbital interaction lobe formation favoured for the chemical process to produce desirable drug property was predicted from the cascade arrangement of HOMO-LUMO. The chromophores reactivity on the base compound causing the electronic shift in UV-Visible spectra was discussed in detail. The electronic energy transition from donor and acceptor orbitals was studied. The consumption of energy between ligand and base compound was measured and maximum energy flow among the orbitals for the completion of the drug property was determined.
Conflict of Interest
As a corresponding Author, I hereby declare that there is no conflict with other fields and other persons belong to field.
References
- Snyder SH, Faillace LA, Weingartner H (1969) A new psychotropic agent. Arch Gen Psychiatry. 21: 95-101.
- Weingartner H, Snyder SH, Faillace LA, Markley H (1970) Altered free associations: Some cognitive effects of DOET (2, 5â?dimethoxyâ?4â?ethylamphetamine). BehavSci 15: 297-303.
- Snyder SH, Weingartner H, Faillace LA (1971) DOET (2, 5-dimethoxy-4-ethylamphetamine), a new psychotropic drug: Effects of varying doses in man. Arch Gen Psychiatry 24: 50-55.
- Heal DJ, Smith SL, Gosden J, Nutt DJ (2013) Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol 27: 479-496.
- Adderall XR (2013) Prescribing Information, United States Food and Drug Administration. Shire US Inc. 12-13.
- In Sydor A, Brown RY (2009) Molecular neuropharmacology: A foundation for clinical neuroscience (2nd ed.) McGraw-Hill Medical, New York. 367.
- Moorthy N, Prabakar PJ, Ramalingam S, Pandian GV, Anbusrinivasan P (2016) Vibrational, NMR and UV–visible spectroscopic investigation and NLO studies on benzaldehydethiosemicarbazone using computational calculations. J PhysChem Solids 91: 55-68.
- Xavier S, Periandy S (2015) Spectroscopic (FT-IR, FT-Raman, UV and NMR) investigation on 1-phenyl-2-nitropropene by quantum computational calculations. SpectrochimActa A MolBiomolSpectrosc. 149: 216-230.
- Moorthy N, Prabakar PJ, Ramalingam S, Govindarajan M, Gnanamuthu SJ (2016) Spectroscopic analysis, AIM, NLO and VCD investigations of acetaldehyde thiosemicarbazone using quantum mechanical simulations. J PhysChem Solids. 95: 74-88.
- Hiremath CS, Yenagi J, Tonannavar J (2007) FT-Raman and infrared spectra and vibrational assignments for 3-chloro-4-methoxybenzaldehyde, as supported by ab initio, hybrid density functional theory and normal coordinate calculations. SpectrochimActa A MolBiomolSpectrosc. 68: 710-717.
- George G, Ramalingam S, Periandy S, Gokulakrishnan V (2016) Spectroscopic investigation and chemical properties analysis on anticancer compound; α, α, ά, ά-Tetrabromo-p-Xylene with computational analysis. J MolStruct 1106: 37-52.
- Ali MM, George G, Ramalingam S, Periandy S, Gokulakrishnan V (2015) Vibrational [FT-IR, FT-Raman] analysis, NMR and mass–Spectroscopic investigation on 3, 6-Dimethylphenanthrene using computational calculation. J MolStruct 1099: 463-481.
- Rauhut G, Pulay P (1995) Transferable scaling factors for density functional derived vibrational force fields. [Erratum to document cited in CA122:199802]. J. Phys. Chem 99: 14572–14572.
- Meyer W, Pulay P (1974) Hartree-Fock calculation of the harmonic force constants and equilibrium geometry of formaldehyde. TheoreticaChimicaActa 32: 253-264.
- Hameka HF, Famini GR, Jensen JO, Newhouse EI (1990) Computations of vibrational infrared frequencies of selected amines. Pennsylvania Univ Philadelphia
- Ebenezar JD, Ramalingam S, Raja CR, Helan V (2013) Precise spectroscopic [IR, Raman and NMR] investigation and gaussian hybrid computational analysis (UV-visible, NIR, MEP Maps and Kubo Gap) on L-valine. J TheorComputSci 1: 1-3.
- Varsányi G (1974) Assignments for vibrational spectra of 700 benzene derivatives. Hilger.
- Socrates G (2004) Infrared and Raman characteristic group frequencies: tables and charts. John Wiley & Sons.
- Becke AD (1993) Densityâ?functional thermochemistry. III. The role of exact exchange. J ChemPhys 98: 5648-5652.
- Altun A, Gölcük K, Kumru M (2003) Structure and vibrational spectra of p-methylaniline: Hartree-Fock, MP2 and density functional theory studies. J MolStruc-Theochem 637: 155-169.
- Muthu S, Ramachandran G (2012) Vibrational spectroscopic investigation on the structure of 2-ethylpyridine-4-carbothioamide. SpectrochimActa A MolBiomolSpectrosc 93: 214-222.
- Dani VR (1995) Organic Spectroscopy. Tata McGraw- Hill publishing company, New Delhi.
- Singh RN, Kumar A, Tiwari RK, Rawat P (2013) Study of spectroscopic, reactivity and NLO properties of synthesized dipyrromethane containing cyanovinylhydrazide using experimental and theoretical approaches. J MolStruct. 1048: 448-459.
- Kalsi PS (1993) Spectroscopy of Organic Compounds, Wiley Eastern Limited, New Delhi.
- Ramalingam S, Periandy S (2011) Spectroscopic investigation, computed IR intensity, Raman activity and vibrational frequency analysis on 3-bromoanisole using HF and DFT (LSDA/MPW1PW91) calculations. SpectrochimActa A MolBiomolSpectrosc 78: 835-843.
- Cotlhup NB, Daly LH, Weberly SE (1964) Introduction to Infrared and Raman Spectroscopy, Academic Press, Inc, New York.
- Sundaraganesan N, Kumar KS, Meganathan C, Joshua BD (2006) Vibrational spectroscopy investigation using ab initio and density functional theory analysis on the structure of 2-amino-4, 6-dimethoxypyrimidine. SpectrochimActa A MolBiomolSpectrosc 65: 1186-1196.
- Babu VA, Lakshmaiah B, Ramulu KS, Rao GR (1987) Substituted Benzenes. 13. Normal Coordinate Analysis of Anisoles. Indian j pure applphy 25: 58-65.
- Abood NA, AL-Askar M, Saeed BA (2012) Structures and vibrational frequencies of Imidazole,benzimidazole and its 2-alkyl Derivatives determined by DFT Calculations. Basrah Journal of Science 30: 119-131.
- Shakila G, Periandy S, Ramalingam S (2011) Molecular structure and vibrational analysis of 3-Ethylpyridine using ab initio HF and density functional theory (B3LYP) calculations. SpectrochimActa A MolBiomolSpectrosc 78: 732-739.
- Gamer G, Wolff H (1973) Raman and infrared spectra of gaseous secondary aliphatic amines [(CH32NH,(CH3) 2ND,(C2H5) 2NH and C2H5NHCH3]. SpectrochimActa A 29: 129-137.
- Stewart JE (1959) Vibrational spectra of primary and secondary aliphatic amines. J ChemPhys 30: 1259-1265.
- Sharma BK (2000) Instrumental Methods of Chemical Analysis, Krishna Prakashan Media 110-150.
- Spire A, Barthes M, Kellouai H, De Nunzio G (2000) Far-infrared spectra of acetanilide revisited. Physica D 137: 392-401.
- Silverstein M, Webster FX (2003) Spectrometric Identification of Organic Compounds (6th edn) John Wiley, Asia 75-105.
- Bellamy LJ (1975) The Infrared Spectrum of Complex Molecules (3rd edn), Chapman and Hall, London.
- Karunakaran V, Balachandran V (2014) Experimental and theoretical investigation of the molecular structure, conformational stability, hyperpolarizability, electrostatic potential, thermodynamic properties and NMR spectra of pharmaceutical important molecule: 4′-Methylpropiophenone. SpectrochimActa A MolBiomolSpectrosc 128: 1-4.
- Madanagopal A, Periandy S, Gayathri P, Ramalingam S, Xavier S (2017) Molecular structure activity on pharmaceutical applications of Phenacetin using spectroscopic investigation. J MolStruct 1127: 611-625.
- Al-Sehemi AG, Irfan A, Alrumman SA, Hesham AE (2016) Antibacterial activities, DFT and QSAR studies of quinazolinone compounds. Bull ChemSoc Ethiop 30: 307-316.
Relevant Topics
- Agglutination
- Anovulation
- Bacteriostasis
- Biological Processes
- Biopharmaceuticals
- Catabolism
- Catalysis in Organic Synthesis
- Defoliation
- Deossification
- Digestion
- Drug Discovery Process
- Eburnation
- Ecchymosis
- Effacement
- Erythropoiesis
- Eutrophication
- Green Chemistry in Process Research
- Introversion
- Intussusception
- Molecular and Cellular Biology
- Molecular Pharmacy
- Nanomedicine and Nanoparticle Drug Delivery
- Pharmaceutical Nanotechnology
- Pharmacokinetics and Pharmacodynamics
- Protein Protein interactions
Recommended Journals
Article Tools
Article Usage
- Total views: 3515
- [From(publication date):
December-2017 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 2616
- PDF downloads : 899