Vegetable Butter Light Parameters Measured Using Visible Light and Laser Spectroscopy
Received: 01-Apr-2023 / Manuscript No. jabt-23-95534 / Editor assigned: 03-Apr-2023 / PreQC No. jabt-23-95534 / Reviewed: 17-Apr-2023 / QC No. jabt-23-95534 / Revised: 21-Apr-2023 / Manuscript No. jabt-23-95534 / Accepted Date: 27-Apr-2023 / Published Date: 28-Apr-2023 QI No. / jabt-23-95534
Abstract
Extra virgin olive oil (EVOO) and canola oil (CO) quality and composition variations with temperature are tested using UV-Vis absorption and fluorescence spectroscopy. The amplitudes of the absorbance and fluorescence signals gradually decreased as the temperature rose, indicating a change in the molecular structures of both types of oils. About the entire spectrum of pheophytin-a, b, carotenoids, lutein, and vitamin E in EVOO and linoleic acid and oleic acid in CO vanished at about 200°C, which was a considerable change. In a separate experiment, the transmission values for EVOO and CO were determined to be 33% and 75%, respectively, and it was shown that the output of the laser fluctuations in petroleum at a constant level (i.e., ambient) are linear depending on the intake. However, a nonlinear behaviour was seen in the transmission through heated oil, indicating a molecule optical response to temperature variations. The impact of oil adulteration and storage time were also assessed.
Keywords
Temperature; Optical Properties; UV-Vis Absorption; Fluorescence Spectroscopy; Laser; Extra Virgin Oil; Canola
Introduction
Vegetable oils come in a vast variety and are used for many daily tasks, including cooking, cosmetics, and medications. For instance, the majority of the fat in olive and canola oils is monounsaturated, while the majority of the fat in maize and soybean oils is polyunsaturated (i.e., contains more than one double bond), and the majority of the fat in coconut oil is saturated (i.e., no double bond in the molecule). An EVOO and CO are chosen for comparison in the research for this study. Olive oil is a complex substance that includes fatty acids CH3(CH2)nCOOH such monounsaturated oleic acid (about 83%) and polyunsaturated linoleic acid (about 21%), vitamins (like vitamin E), and water-soluble components. It is used all throughout the world, but is most popular in Mediterranean nations. A fatty acid is a carboxylic acid with a lengthy hydrocarbon chain and a carboxyl group at the end. The cultivar, altitude, time of harvest, and extraction method, such as cold pressing or refining, all affect the composition of olive oil. It is referred to as “virgin” olive oil if the olive oil is produced by pressing and does not go through any of the industrial processes used to produce “refined” oils like canola, sunflower, or soybean. “Pure,” “Light,” or “Olive Oil” are used to describe the lesser grades of olive oil. The main components of olive oil, such as omega-3 and omega-6 fatty acids, vitamins, and potent antioxidants [1-5] like polyphenols, are generally responsible for its health benefits for preventing cancer and cardiovascular disease. As a result, Compared to other vegetable oils, VOO exhibits a great resistance to oxidation. An EVOO is the least processed type of olive oil and has more monounsaturated fatty acids than the other types. It also has to have a free acidity level of 0.8% or less, which enhances the flavour. A notable minor component is -tocopherol, which shields the oil from oxidation at higher temperatures [5], [6] and correlates with the content of hydrophilic phenolic chemicals in EVOO. Brassica napus and Brassica rapa seeds are used to create canola oil (CO) in Canada. These cultivars differ chemically and physically from high erucic acid rapeseed oil due to their low erucic acid and glucosinolate content. Linolenic, linoleic, oleic, palmitic, steric, high oleic canola, and low linolenic canola make up the majority of canola oil’s chemical make-up. Light and the length of storage are two other factors, in addition to heating, which have a direct impact on oil quality. Due to the difficult duties required during the cultivation of olive trees, the harvesting, and the extraction processes, they are more expensive for these reasons. Their adulteration with other edible oils of lower commercial value typically occurs for this reason [7], [8]. As a result, an analytical method that is quick, affordable, and non-intrusive would be ideal, especially for online applications. Considerable amount of research and information is available in literature using different techniques including high-performance liquid chromatography (HPLC) [4], Fourier-transform infra-red spectroscopy (FTIR) [1], mass spectroscopy (MS) [1], nuclear magnetic resonance (MNR) [1- 2], inductively coupled plasma optical emission spectroscopy (ICPOES) [1-3], Raman spectroscopy [1-4] and optical spectroscopy such as absorption and fluorescence spectroscopy [1-5] [6-10]. In a nutshell, the principle behind fluorescence, a type of photoluminescence, is the excitation of molecules from their ground state through the absorption of light with energy proportional to the difference between the ground and excited states of a certain fluorophore. The two processes that result in the radiated photon having less energy than the incident photon are internal conversion and vibrational relaxation. The Stokes shift, which allows for efficient separation of the fluorescence emission signal from the Rayleigh-scattered excitation singlet, is what causes this shift to longer wavelengths. This shift is crucial for the sensitivity of fluorescence detection. There is a nanosecond-long lifetime. It is important to highlight that laser-induced fluorescence spectroscopy (LIFS), which often detects the presence of dyes and pigments, offers a number of advantages over absorption spectroscopy (AS). For instance, each molecule in LIFS has a unique optical signature that manifests as an emission as a result of absorption and excitation by a particular wavelength. If the wavelength is monochromatic and coherent, as in the case of a laser that can be configured to operate at only one wavelength, this benefit is even further increased. The molecular response would be extremely selective as a result. Its better selectivity with less background noise is seen as one of LIFS’s biggest advantages over conventional AS. Moreover, compared to AS, where the photons that are emitted are detected against a low background, LIF has higher sensitivity. The sensitivity is approximately 100–1000 times greater than AS, allowing for the detection of concentrations at parts per billion levels. LIFS is one of the most popular spectroscopic techniques used for biomedical applications, particularly in the diagnosis of cancer. It is one of many optical techniques used for biological and biomedical research. The goal of this research is to discuss the potential application of AS and LIFS to examine the optical response of EVOO and canola oil during heating, storage, and adulteration.
Materials and Procedure
For the experiment, fresh canola oil, two EVOO in dark green glass bottles—one fresh and the other after six months of storage— were employed. In order to conduct UV-Vis absorption spectroscopy (JENWAY-7205) between 200 and 900 nm, the oils were poured directly into a 10 mm cuvette. Prior to taking the measurements, the spectrometer was calibrated using a blank 10 mm cuvette filled with around 80% distilled water. To lessen the effects of environmental cooling, the samples were heated with a hot plate (Cole-Palmer) next to the spectrometer. Using a 10 mW 405 nm diode laser pointer, the Figure 1aforementioned steps were repeated for the laser-induced fluorescence spectroscopy (LIFS) (Flame-T-XRI-ES-Ocean optics). In order to compare and visualise the fluorescence with that of 405 nm, a 10 mW 532 nm laser pointer was also used. An optical fiber-coupled 4-channel laser (MCL-Thor Laboratories) operating at 406 nm with a variable output power was employed for the independent transmission experiment. The fluctuation of transmission with temperature was investigated using a low power 1 mW Newport He-Ne laser. A power sensor for an avalanche photodiode picked up the output signal (S121- C-Thor Labs). The cuvette was covered with a metal lid to shield it from ambient light noise during all LIFS experiments, which were conducted in a dark room at 20 C.
Results
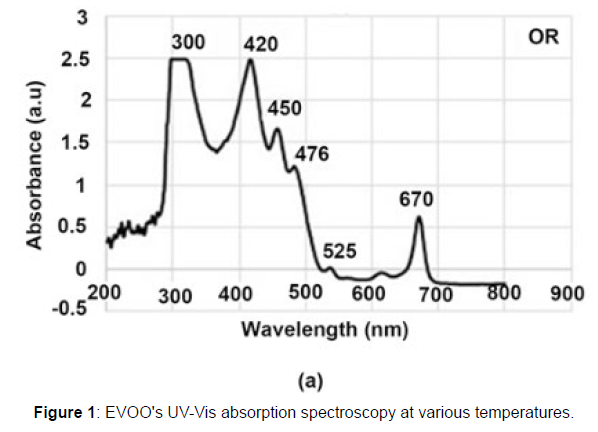
Figure 1 displays the numerous absorption peaks of fresh extra virgin olive oil at room temperature. The 300–400 band contains information regarding polyphenol antioxidants. Carotenoids are responsible for the absorption maxima at wavelengths around 425, 450, and 476 nm; nevertheless, the carotenoid’s 424 nm peak can readily overlap the chemical molecule pheophytin-407 a’s nm peak, which acts as an electron carrier in the photosystem of plants. The chlorophyll band has two maxima located at 430 and 664 nm (about 670 nm in our case), respectively, and the 525 nm peak is associated with vitamin E. Olive oil’s colour is substantially influenced by carotenoids and chlorophyll, which is a crucial aspect for customers.
Discussion
In this study, the effects of temperature on the content and quality of CO and EVOO, two commonly used vegetable oils, are discussed. There are numerous ways to apply heat, including frying, boiling, and microwave ovens. Hence, depending on the type of technology used as well as the intensity of heating and exposure period, various chemical changes are anticipated to occur in the context of cooking. These modifications include chemical processes like hydrolysis, oxidation, and polymerization, all of which have the potential to reduce nutritional value and, in some circumstances, over time; result in a major health issue like cancer owing to the production of free radicals.
Conclusion
Two varieties of EVOO and CO were tested for quality and changes in chemical structure using UV-Vis absorption and fluorescence spectroscopy. The outcomes demonstrated that both systems are capable of detecting composition and potential alterations. The amplitudes of the absorbance and fluorescence signals gradually decreased as the temperature rose, indicating a change in the molecular structures of both types of oils. These systems can also test for oil ageing and adulteration, although it is recommended that the optimal testing method be a combined one.
Acknowledgements
MEK expresses gratitude to Dr. K. Deshmukh for her assistance and discussion, as well as to Mr. Saeid Mohmedi, president of MIS Electronics Inc., for his support and funding of the project.
Competing Interests
The authors say they have no competing interests.
References
- García A, Ruiz Méndez MV, Romero C, Brenes M (2006) Effect of refining on the phenolic composition of crude olive oils. Journal of the American Oil Chemists' Society 83:159-164.
- Dabbou S, Rjiba I, Nakbi A, Gazzah N, Issaoui M, et al. (2010) Compositional quality of virgin olive oils from cultivars introduced in Tunisian arid zones in comparison to Chemlali cultivars. Scientia Horticulturae 124:122-127.
- Obidike I, Oluwakayinsola S (2013) Herbal medicines in the 21st century. Screening of Herbal medicines for potential toxicities. Intechopen 62-88.
- Okagu IU, Ndefo JN, Agbo MO (2021) Trado-Medical uses Chemical Constituents and Biological Activities of Newbouldia laevis (Bignoniaceae): A Review. Pharm Sci 1-52.
- Osigwe CC, Akah PA, Nworu CS (2017) Biochemical and Haematological Effects of the Leaf Extract of Newbouldia laevis in Alloxan-Induced Diabetic Rats. Journal of Biosciences and Medicines 5: 18-36.
- Lorke D (1983) A New Approach to Practical Acute Toxicity Testing. Arch Toxicol 54: 275–87.
- Langlois-Klassen D, Kipp W, Jhangri GS, Rubaale T (2007) Use of traditional herbal medicine by AIDS patients in Kabarole district, Western Uganda. American Tropical and Medical Hygiene 77: 757-763.
- Escrich E, Ramírez-Tortosa MC, Sánchez-Rovira P, Colomer R, Solanas M, et al. (2006) Olive oil in cancer prevention and progression. Nutrition Reviews 64:S40-S52.
- Bartoli R, Fernández-Bañares F, Navarro E, Castella E, Mane J, et al. (2000). Effect of olive oil on early and late events of colon carcinogenesis in rats: modulation of arachidonic acid metabolism and local prostaglandin E2 synthesis. Gut 46:191-199.
- Arnaud T, Gutiérrez F, Garrido A (2001) "Polyphenol Contribution to the Oxidative Stability of Virgin Olive Oil." 81:1463–1470.
Indexed at Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Khosroshahi MME (2023) Vegetable Butter Light Parameters Measured Using Visible Light and Laser Spectroscopy. J Anal Bioanal Tech 14: 510.
Copyright: © 2023 Khosroshahi MME. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 893
- [From(publication date): 0-2023 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 687
- PDF downloads: 206