Varietal Differences in Flowering, Pod Setting and Photosynthesis in Soybean Under High Temperature Conditions
Received: 17-Aug-2018 / Accepted Date: 04-Sep-2018 / Published Date: 12-Sep-2018 DOI: 10.4172/2329-8863.1000392
Keywords: CO2 assimilation rate; Chlorophyll fluorescence; High temperature; Flowering number; Pod number; Pod setting; Transpiration rate; Seed yield; Soybean
Introduction
Under nearly all scenarios, global surface temperature is likely to exceed 1.5°C relative to 1850-1900 at the end of the 21st century [1]. Lobell and Asner [2] predicted that soybean yield in the US will decrease by roughly 17% with a 1°C increase in the growing season temperature. In northeast China, soybean yields have declined significantly due to warming trends since 1987 [3]. Experimental studies show that high temperatures cause several negative effects on soybean growth and/or yield seed yield was slightly reduced by high temperature stress when induced only during the initial seed filling period [4]. An increase in temperature decreased seed weight, which was mainly due to a reduction in seed size [5]. Total dry matter, seed yield, and harvest index were reduced by an increase in temperature when using a temperature gradient chamber [6]. Pollen morphology and viability were also negatively affected by high temperatures [7], which resulted in a lower pod-set and seed weight concomitant with a decreased photosynthetic rate [8].
Several commercial heat tolerant cultivars of rice have been released in Japan [9], and others are being developed in the IRRI [10], Vietnam [11], and Bangladesh [12]. Heat-tolerant genotypes of potato have also been developed [13]. For soybean, however, heat tolerant breeding projects are yet to be undertaken, although genes related to heat tolerance have been explored [14]. In addition to such exploration, it is important to evaluate the phenotypic avoidance and/or adaptation mechanisms of heat tolerance in the photosynthetic apparatus and other agronomic characteristics However, only few studies have been conducted on the varietal differences in such characteristics, despite being the essential information for breeding. Therefore, the varietal differences for phenotypic characteristics associated with heat tolerance in soybean should be evaluated.
Photosynthesis is a key phenomenon that substantially contributes to crop yield [15]. There have been reviews on CO2 gas exchange characteristics; however, several studies suggest that photosynthetic rate could be used as a potential indicator of heat tolerance, while other studies report little to no association of this physiological trait with heat tolerance. This may be because of the variation in photosynthetic capacity for different species under varying degrees of stress tolerance. In soybean, there are only a few studies about photosynthesis under heat stress conditions [16-18]; therefore, it is necessary to evaluate CO2 assimilation rates (AN) under heat stress conditions. In addition to AN, transpiration is one of the main factors for avoiding heat stress [19]. It has been shown that the differences between soybean and cotton adaptation mechanisms in arid conditions depend on transpiration ability.
Chlorophyll fluorescence measurements are commonly used to study the functions of the photosynthetic apparatus [20,21]. In particular, the maximum quantum yield of photosystem II (PSII) (Fv/Fm) is used as a sensitive indicator of photosynthetic performance, such as photoinhibition [21]. In addition to Fv/Fm, actual quantum yield of PSII (ΦPSII) and qN are also evaluated for the efficiency of PSII photochemistry and non-photochemical quenching, respectively, to evaluate their relationship with heat dissipation of excessive energy [21]. In this experiment, we evaluated the varietal differences of physiological characteristics, including photosynthetic apparatus, and agricultural characteristics, including flowering, pod number, and yield under high temperature conditions.
Materials and Methods
During the year previous to this experiment, 80 cultivars from the world soybean core collection, which were derived from the Genebank Project, National Agriculture and Food Research Organization in Japan, were grown in greenhouse conditions, then 7 cultivars were selected with the following criteria: beginning flowering (R1) was from August 10-13, stem height was less than 80 cm, under the conditions in Matsudo, Chiba, Japan (lat 36″N, long 140″E). In addition to these 7 cultivars, 2 Japanese cultivars, Enrei and Tachinagaha, were used in this experiment (Table 1).
| Cultivar | Origin |
|---|---|
| Enrei | Japan |
| Tachinagaha | Japan |
| Chunhoku 2 | Rep Korea |
| Shirosota | Korean Peninsula |
| Chieneum Kong | Rep Korea |
| Kongnamul Kong | Rep Korea |
| Heukdaelip | Rep Korea |
| Heamnam | Rep Korea |
| Uronkon | Korean Peninsula |
Table 1: Materials.
The experiment was conducted in two greenhouses at the Faculty of Horticulture, Chiba University in 2015. Six pots were used for each treatment for a single cultivar. Three seeds were sown in a 1/5000 Wagner pot (height 198 cm, average diameter 16 cm) on June 24 and were thinned to one plant per pot after emergence. The plants were grown in the open air before the beginning of flowering (R1), after which two air temperature treatments were used starting on August 6. The pots in the high temperature (HT) experiment and the control were grown in the greenhouses. The HT treatment had a maximum air temperature of 41°C and was controlled by opening the windows of the greenhouse; the control group had an air temperature similar to the ambient temperature and was controlled by maintaining open windows. The mean difference of the daily mean air temperature between the HT and the control group was 0.95°C during the experimental period. After R1, flower number and flowering period were measured daily for every pot. The pots were irrigated up to 2-3 times a day, according to the soil conditions. At the harvesting time, 3 pots per treatment for a cultivar were harvested individually, and the yield and yield components were measured. Seed yield was determined after oven drying at 80°C for 72 h. The rates of yield and yield components were calculated as follows: (the control-the treatment)/the control.
Chlorophyll fluorescence parameters including the quantum yield of Photosystem II (PSII)(ΦPSII), maximum quantum yield of PSII (Fv/ Fm), and non-photochemical quenching (qN ) were measured for the Chieneum Kong, Chuuhoku 2, Tachinagaha, and Uronkon cultivars by using a chlorophyll fluorometer (PAM-2000, Waltz, Germany) on August 18. CO2 assimilation rate (AN), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration rate (E) for Chuuhoku 2, Tachinagaha, and Uronkon were also measured using a portable photosynthesis system (LI-6400, Li-Cor, USA) on August 21. The uppermost fully expanded leaves were used for the measurement from 0900 to 1400 h.
Results
Flowering period and number and pod number and setting rate
The growth stages for each cultivar are shown in Table 2. The HT group showed delayed growth stages. The HT group showed a delay in the beginning pod and the full maturity stages by 1 to 10 days and -1 to 17 days, respectively.
| Cultivar | Growth stage | ||||
|---|---|---|---|---|---|
| R1 | R3 | R8 | |||
| Control | HT | Control | HT | ||
| Enrei | 31 Jul | 11 Aug | 12 Aug | 5 Oct | 9 Oct |
| Tachinagaha | 31 Jul | 11 Aug | 12 Aug | 25 Oct | 6 Nov |
| Chunhoku 2 | 3 Aug | 9 Aug | 18 Aug | 11 Oct | 19 Oct |
| Shirosota | 3 Aug | 9 Aug | 14 Aug | 25 Oct | 25 Oct |
| Chieneum Kong | 3 Aug | 10 Aug | 15 Aug | 9 Oct | 8 Oct |
| Kongnamul Kong | 3 Aug | 10 Aug | 18 Aug | 24 Oct | 4 Nov |
| Heukdaelip | 4 Aug | 10 Aug | 16 Aug | 26 Oct | 12 Nov |
| Heamnam | 5 Aug | 10 Aug | 13 Aug | 24 Oct | 29 Oct |
| Uronkon | 5 Aug | 18 Aug | 28 Aug | 26 Oct | 5 Nov |
Table 2: Growth stages (R1, R3 and R8) of each cultivar in the control and high temperature treatment (HT). R1: Beginning flowering stage, R3: Beginning pod stage and R8: Full maturity stage.
Table 3 shows flower number, pod number, and pod setting rate the flower number per plant tended to be higher in the HT group than in the control Kongnamul Kong, Heukdaelip, and Uronkon showed significantly more flower numbers in the treated group than in the control.
| Cultivar | Flower Number (plant-1) | Pod Number (plant-1) | Pod Setting Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | HT | Control | HT | Control | HT | ||||
| Enrei | 105c | 121d | ns | 49c | 43cd | ns | 49a | 36a | ns |
| Tachinagaha | 112c | 132d | ns | 22d | 38cd | ns | 20de | 28ab | ns |
| Chunhoku 2 | 289a | 360ab | ns | 145a | 115a | ns | 50a | 33a | * |
| Shirosota | 174bc | 198cd | ns | 55c | 55bcd | ns | 32c | 28ab | ns |
| Chieneum Kong | 280a | 335ab | ns | 81b | 64bc | ns | 30cd | 19bc | * |
| Kongnamul Kong | 216ab | 275bc | * | 89b | 92ab | ns | 41ab | 34a | ns |
| Heukdaelip | 159bc | 293abc | * | 54c | 21d | ns | 36bc | 7d | * |
| Heamnam | 219ab | 251bcd | ns | 56c | 74bc | * | 26cde | 29a | ns |
| Uronkon | 282a | 407a | * | 52c | 67bc | ns | 18e | 17cd | ns |
Table 3: Flower number, pod number and pod setting rate affected by high temperature. Values in each column followed by the same letter are not significantly different at 5% level by LSD. * and ns indicate 5% level of significance and no significance between the control and high temperature treatment (HT), respectively.
There was no incremental increase in pod number for the HT group compared to the control. Only Heamnam showed a significant increase in the treatment group compared to the control. Pod setting rate showed a decreasing tendency in the HT group compared to the control. Chunhoku 2, Chieneum Kong, and Heukdaelip had a significantly lower pod setting percentage in the HT treatment than in the control. However, Enrei, Tachinagaha, Chunkoku 2, Shirosota, Kongnamul Kong, and Heamnam showed 30% for pod setting even in the treatment groups. Conversely, Heukdaelip showed a lower pod setting percentage in the HT treatment than in the control.
Seed number, 100 seed weight, and yield
There was no significant difference between the control and the treatment groups for seed number, except for Heukdaelip. Most of the cultivars had a lower number of seeds in the treatment group than in the control group, but the other cultivars, including Shirosota, Kongnamul Kong, Heamnam, and Uronkon, had a larger number of seeds in the treatment group in the control groups (Table 4). Only the Heukdaelip cultivar showed a significant decrease in the HT treatment compared to the control. Every cultivar showed a significant decrease in 100 seed weight in the treatment group compared to the control, although Kongnamul Kong had a relatively larger seed size in the treatment group than in the control. Seed yield showed a decreasing trend in the HT treatment when compared to the control; Enrei, Chieneum Kong, and Heukdaelip had significantly smaller seed yields in the treatment than in the control. Conversely, Kongnamul Kong, Heamnam, and Uronkon showed a smaller decrease and a relatively higher yield in the HT treatment than in the control. There was no significant correlation between yield and the yield components.
| Cultivar | Seed Number (plant-1) | 100 Seed Weight (g) | Yield (g plant-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | HT | Control | HT | Control | HT | ||||
| Enrei | 91d | 67cd | ns | 27c | 20c | * | 24ab | 13cd | * |
| Tachinagaha | 60d | 50d | ns | 33a | 28a | * | 20b | 14cd | ns |
| Chunhoku 2 | 265a | 211a | ns | 10e | 8f | ** | 25ab | 16bc | ns |
| Shirosota | 75d | 84cd | ns | 29bc | 19c | ** | 21ab | 16bc | ns |
| Chieneum Kong | 137bc | 123bc | ns | 14d | 10e | * | 20b | 12cd | * |
| Kongnamul Kong | 146b | 165ab | ns | 15d | 14d | * | 23ab | 23a | ns |
| Heukdaelip | 97cd | 31d | * | 30abc | 25b | * | 29a | 8d | * |
| Heamnam | 86d | 102bcd | ns | 28c | 21c | * | 24ab | 21ab | ns |
| Uronkon | 81d | 95cd | ns | 32ab | 23b | ** | 26ab | 22ab | ns |
Table 4: Yield and yield components affected by high temperature. Values in each column followed by the same letter are not significantly different at 5% level by LSD. *, ** and ns indicate 5%, 1% level of significance and no significance between the control and high temperature treatment (HT), respectively.
Table 5 shows the correlation coefficients among the decreasing rate of yield and the yield components in the HT treatment [(the control- HT plot)/the control]. There was a highly significant correlation between yield and seed number, indicating the decrease in yield was caused mainly by the decrease in seed number. Yield also showed positive correlations with pod number and pod setting rate, although these values were not significant. The decrease in pod setting rate resulted in a reduced pod number increasing flower number in the HT treatment resulted in a reduction of pod setting rate.
| Flower Number | Pod Number | Pod Setting Rate | Seed Number | Seed Weight | |
|---|---|---|---|---|---|
| Yield | -0.53 | 0.62 | 0.63 | 0.92** | 0.14 |
| Flower Number | -0.54 | -0.67* | -0.63 | 0.26 | |
| Pod Number | 0.99** | 0.61 | 0.06 | ||
| Pod Setting Rate | 0.64 | 0.01 | |||
| Seed Number | -0.25 |
Table 5: Correlation coefficients of decreasing rates of yield with yield components by high temperature. * and ** indicate 5% and 1% level of significance (n=9).
Photosynthetic characteristics
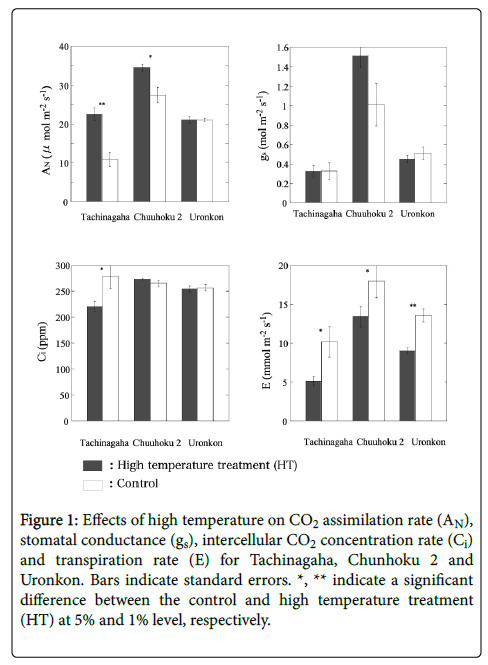
Figure 1 shows the CO2 assimilation rate (AN), stomatal conductance (gs), intercellular CO2 concentration rate (Ci), and the transpiration rate (E). There was no significant difference in AN between the treatments, however, Tachinagaha and Chunhoku 2 had a smaller AN in the HT treatment than in the control. There was no significant difference in gs between the control and the HT treatment, although higher values were noted in Chunkuku 2 than in the other two cultivars. Chunhoku 2 and Uronkon showed no significant difference in Ci between the control and the treatment; however, Tachinagaha had a smaller Ci value in the control than in the HT treatment. The HT treatment had higher E values for every cultivar than those of the control, where Chunboku 2 had the highest values followed by Uronkon.
Figure 1: Effects of high temperature on CO2 assimilation rate (AN), stomatal conductance (gs), intercellular CO2 concentration rate (Ci) and transpiration rate (E) for Tachinagaha, Chunhoku 2 and Uronkon. Bars indicate standard errors. *, ** indicate a significant difference between the control and high temperature treatment (HT) at 5% and 1% level, respectively.
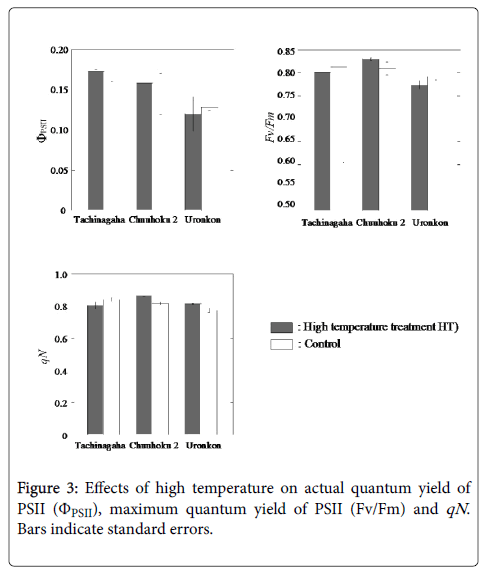
The actual quantum yield of PSII (ΦPSII) was not significantly different between the control and the HT treatment, indicating that high temperature did not reduce the efficiency of electron transport in PSII in any of the cultivars. There was no varietal difference in ΦPSII. The maximum quantum yield of PSII was not significantly different between the control and treatment groups. Every plot had more than 0.79, assuming there was no photoinhibition by the HT treatment. There was also no significant difference in qN between the control and treatment groups. The degree of heat dissipation in PSII was similar in both the control and HT groups and among the cultivars.
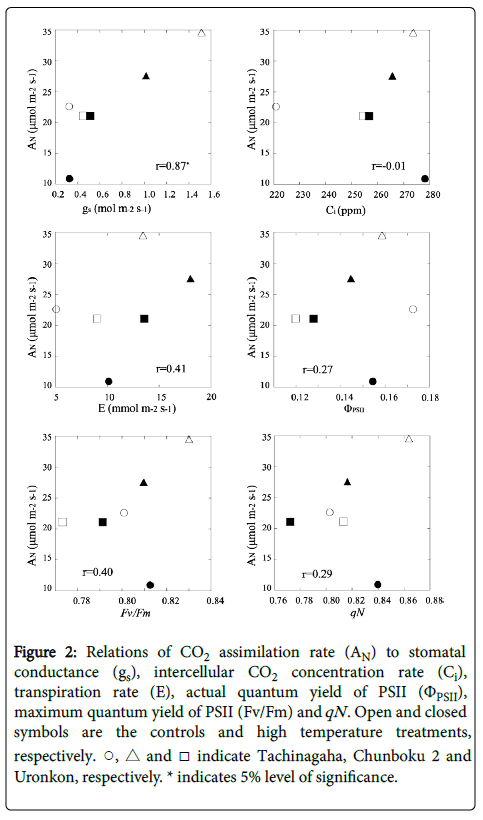
Figure 2 shows the relationships of AN to gs, Ci, E, ΦPSII, Fv/Fm, and qN . The AN was closely related to gs in both the control and HT treatments, indicating that cultivars with a high stomatal conductance tend to have a high CO2 assimilation rate. The Chunboku 2 cultivar in both the control and treatment groups showed high AN value because of the high gs in both the groups. The other photosynthetic characteristics did not show a relationship with AN.
Figure 2: Relations of CO2 assimilation rate (AN) to stomatal conductance (gs), intercellular CO2 concentration rate (Ci),transpiration rate (E), actual quantum yield of PSII (ΦPSII), maximum quantum yield of PSII (Fv/Fm) and qN . Open and closed symbols are the controls and high temperature treatments, respectively. ○, △ and □ indicate Tachinagaha, Chunboku 2 and Uronkon, respectively. * indicates 5% level of significance.
Discussion
The HT treatments in this experiment showed decreased pod setting rates and then decreased seed number, which resulted in a decrease in yield and an increase in flower number. The decrease in yield from the HT treatment was caused mainly by the decrease in seed number, which was followed by a decrease in pod number and pod setting rate. The decrease in pod number depended mainly on the decrease in pod setting rate. The seed set rate primarily depended upon the function of pollen and the ovule, successful pollination, fertilization, and postfertilization processes [8]. For soybean, studies have shown that pollen viability and germination decreases with high temperatures [8,22]. Conversely, it has also been reported that there is a decreased amount of assimilates available for growth due to high respiration rates associated with pod setting rates rather than due to pollen viability [23]. In this experiment, some cultivars, including Kongnamul Kong, Heamnam, and Uronkon, showed a small decrease in yield, even under the HT treatment. The pod setting rates in the HT treatment were not significantly different from those in the control. These cultivars might, therefore, have a low effect on pollen viability and decreased amounts of assimilates even under the HT treatment. Conversely, the cultivars with significantly low pod setting rates, including Heukdaelip and Chunhoku 2, might have low pollen viability and/or excessive consumption of assimilates due to respiration in the HT treatment.
The sustained decrease in Fv/Fm indicates the occurrence of photoinhibitory damage in response to one or more environmental stresses [21]. There was no significant decrease in Fv/Fm in the HT treatment compared to the control, and the photosynthetic apparatus may have not been damaged by the HT treatment in this experiment. Inamullah and Isoda [24] compared soybean and cotton under water stress and high temperature conditions and reported that the photosynthetic apparatus in soybean was protected from photoinhibition under water stress because of its high ability to down-regulate PSII activity and activate xanthophyll cycle pigment conversion to dissipate excess excitation energy as heat. In this experiment, however, there was no significant difference between the actual quantum yield and qN , i.e., no marked down-regulation of PSII and heat dissipation in the HT treatment when compared to that of the control (Figure 3).
Therefore, the carbon reactions of photosynthesis might not be largely affected by the efficiency of light reactions in this experiment. The decrease in AN by the HT treatment when compared to that of the control was observed in Tachinagaha and Chunhoku 2 compared to the control. In the HT treatment, Chunhoku 2 showed a decrease in gs but no decrease in C, while Tachinagaha showed no decrease in gs and an increase in C. It is, therefore, assumed that the main factor in the decrease of AN in Chunkoku 2 is gs, and the decrease of AN in Tachinagaha is caused by something different. The high CO2 concentration in the leaf with a low CO2 assimilation rate in Tachinagaha indicates low rubisco activity. Uronkon had no significant decrease in AN in the HT treatment compared to the control. Isoda et al. [25] reported that leaf temperature in soybean was regulated by the combination of leaf movement and transpiration. Some cultivars regulated leaf temperature mainly through leaf para-heliotropic movement, whereas others by both transpiration and leaf movement. In this experiment, transpiration rates increased in the HT treatment group compared to the control. The cultivars used in the HT treatment might, therefore, regulate leaf temperature by transpiration, especially for Uronkon that showed an increase of E in the HT treatment compared to the control, which resulted in the prevention of photoinhibition and no decrease in AN. Wang et al. [26] reported varietal differences in the transpiration ability of cotton and suggested that a cultivar with higher transpiration ability tended to have higher dry matter production in arid conditions. Therefore, we can assume that a higher transpiration ability in soybean may be also associated with a higher adaptability for high temperature conditions.
References
- ICPP (2013) Climatic Change 2013. The Physical Science Basis. Summary for Policymakers, p:18.
- Lobell DB, Asner GP (2003) Climate and management contributions to recent trends in US agricultural yields. Science 299: 1032-1032.
- Zheng HF, Chen LD, Han XZ (2009) The effects of global warming on soybean yields in a long-term fertilization experiment in Northeast China. The Journal of Agricultural Science 147: 569-580.
- Ferris R, Wheeler TR, Ellis RH, Hadley P (1999) Seed yield after environmental stress in soybean grown under elevated CO2 . Crop Science 39: 710-718.
- Heinemann AB, de HN Maia A, Dourado-Neto D, Ingram KT, Hoogenboom G (2006) Soybean (Glycine max (L.) Merr.) growth and development response to CO2 enrichment under different temperature regimes. European Journal of Agronomy 24: 52-61.
- Tacarindua CR, Shiraiwa T, Homma K, Kumagai E, Sameshima R (2013) The effects of increased temperature on crop growth and yield of soybean grown in a temperature gradient chamber. Field Crops Research 154: 74-81.
- Koti S, Reddy KR, Reddy VR, Kakani VG, Zhao D (2004) Interactive effects of carbon dioxide, temperature, and ultraviolet-B radiation on soybean (Glycine max L.) flower and pollen morphology, pollen production, germination, and tube lengths. Journal of Experimental Botany 56: 725-736.
- Djanaguiraman M, Prasad PV, Boyle DL, Schapaugh WT (2013) Soybean pollen anatomy, viability and pod set under high temperature stress. Journal of Agronomy and Crop Science 199: 171-177.
- Tanamachi K, Miyazaki M, Matsuo K, Suriyasak C, Tamada A, et al. (2016) Differential responses to high temperature during maturation in heat-stress-tolerant cultivars of Japonica rice. Plant Production Science 19: 300-308.
- Mackill DJ, Ismail AM, Pamplona AM, Sanchez DL, Carandang JJ, et al. (2010) Stress tolerant rice varieties for adaptation to a changing climate. Crop Environment & Bioinformatics 7: 250-259.
- Buu BC, Ha PTT, Tam BP, Nhien TT, Van Hieu N, et al. (2014) Quantitative trait loci associated with heat tolerance in rice (Oryza sativa L.). Plant Breeding and Biotechnology 2: 14-24.
- Masuduzzaman ASM, Ahmad HU, Haque M, Ahmaed NME (2016) Evaluation of rice lines tolerant to heat during flowering stage. Journal of Rice Research 4: 170.
- Benites FRG, Pinto CABP (2011) Genetic gains for heat tolerance in potato in three cycles of recurrent selection. Crop Breeding and Applied Biotechnology 11: 133-140.
- Zhu B, Ye C, Lü H, Chen X, Chai G, et al. (2006) Identification and characterization of a novel heat shock transcription factor gene, GmHsfA1, in soybeans (Glycine max). Journal of Plant Research 119: 247-256.
- Ashraf MHPJC, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51: 163-190.
- Djanaguiraman M, Prasad PV, Boyle DL, Schapaugh WT (2011) High-temperature stress and soybean leaves: leaf anatomy and photosynthesis. Crop Science 51: 2125-2131.
- Vu JCV, Allen Jr LH, Boote KJ, Bowes G (1997) Effects of elevated CO2 and temperature on photosynthesis and Rubisco in rice and soybean. Plant, Cell & Environment 20: 68-76.
- Vu JC, Allen Jr LH, Widodo W (2007) Leaf photosynthesis and Rubisco activity and kinetics of soybean, peanut, and rice grown under elevated atmospheric CO2, supraoptimal air temperature, and soil water deficit. Curr Top Plant Biol 7: 27-41.
- Isoda A, Wang P (2002) Leaf temperature and transpiration of field grown cotton and soybean under arid and humid conditions. Plant Production Science 5: 224-228.
- Fracheboud Y, Haldimann P, Leipner J, Stamp P (1999) Chlorophyll fluorescence as a selection tool for cold tolerance of photosynthesis in maize (Zea mays L.). Journal of Experimental Botany 50: 1533-1540.
- Maxwell K, Johnson GN (2000) Chlorophyll fluorescence-a practical guide. Journal of Experimental Botany 51: 659-668.
- Salem MA, Kakani VG, Koti S, Reddy KR (2007) Pollen-based screening of soybean genotypes for high temperatures. Crop Science 47: 219-231.
- Kitano M, Saitoh K, Kuroda T (2006) Effects of high temperature on flowering and pod set in soybean. Scientific Report of Faculty of Agriculture, Okayama University 95: 49-55.
- Ullah I, Isoda A (2005) Adaptive responses of soybean and cotton to water stress II. Changes in CO2 assimilation rate, chlorophyll fluorescence and photochemical reflectance index in relation to leaf temperature. Plant Production Science 8: 131-138.
- Isoda A, Yoshimura T, Ishikawa T, Nojima H, Takasaki Y (1994) Effects of leaf movement on radiation interception in field grown leguminous crops: III. Relation to leaf temperature and transpiration among soybean cultivars. Japanese Journal of Crop Science 63: 657-663.
- Wang C, Isoda A, Li Z, Wang P (2004) Transpiration and leaf movement of cotton cultivars grown in the field under arid conditions. Plant Production Science 7: 266-270.
Citation: Isoda A, Komaki K (2018) Varietal Differences in Flowering, Pod Setting and Photosynthesis in Soybean Under High Temperature Conditions. Adv Crop Sci Tech 6:392. DOI: 10.4172/2329-8863.1000392
Copyright: © 2018 Isoda A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3592
- [From(publication date): 0-2018 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 2815
- PDF downloads: 777