Value of CD56, HBME-1, and CK19 Expression in Predicting the Risk of Papillary Thyroid Carcinoma (PTC) Occurrence in Hashimoto’s Thyroiditis (HT) Patients
Received: 20-Oct-2017 / Accepted Date: 28-Nov-2017 / Published Date: 05-Dec-2017
Abstract
Background: There were no previous studies tried to find the prediction of PTC occurrence in HT that will advise early thyroidectomy in HT cases with high risk of progression to PTC.
We aimed to use a panel of CD56, HBME-1, and CK-19 to detect their predictive ability for HT progression into PTC.
Patients and methods: we included 5 groups of paraffin blocks that were retrieved from 70 patients. first group included 20 cases of PTC, 2nd group included 20 samples from the same cases previously diagnosed as HT, 3rd group included 30 cases of HT. 4th group included 30 samples from the same cases previously diagnosed as HT and 5th group 20 cases of PTC without history of HT. Sections are stained by CD56, HMBE-1 and CK19 using immunohistochemistry.
Results: There is a significant difference between 2nd (HT that will be transformed to PTC) and 4th (HT that will not be transformed to PTC) groups as regards CD56, HMBE-1 and CK19 expression (P=0.012).
For differentiation between HT that will be transformed to PTC from HT that will not be transformed to PTC, negative CD56 expression was of highest sensitivity (90%) and diffuse positive HBME-1 expression was of highest specificity (95.7%).
Conclusion combination of negative CD56 expression and diffuse positive HMBE-1 could be used with high sensitivity and specificity in predicting PTC occurrence in certain cases of HT and these patients will be advised to made early total thyroidectomy to avoid PTC occurrence in the future.
Keywords: CD56; HBME-1; CK19; Papillary thyroid carcinoma; Hashimoto’s thyroiditis; Immuno-histochemistry
Introduction
The commonest thyroid malignancy is the papillary thyroid carcinoma (PTC) that forms about 80% of thyroid malignancies [1]. The commonest autoimmune disease of the thyroid gland is Hashimoto’s thyroiditis (HT) which the commonest cause of hypothyroidism [2]. The association and pathogenic relations between both HT and PTC remains controversial and still need further qualifications [3]. Dailey and colleagues first described that there is a relationship between both thyroid lesions in 1955 [4] and since then, there are many researchers tried to cover such scientific point but they provided conflicting results. It is essential also to mention that molecular analyses have indicated that PTC had high amount of lymphocytic infiltration that suggested the role of immunological factors in malignant progression [5,6]. Management of HT patients is mostly conservative with L-thyroxin [7], also performing total thyroidectomy is not preferred for such patients due to the presence of inflammatory response surrounds the thyroid gland and leads to more difficult surgical resection [8]. There are few indications for early surgical intervention in HT patients e.g. worsening of clinical symptoms that are related to the disease, malignancy suspicion, or a goiter with marked increase in size [8-10]. Although plethora of studies has identified the indications of surgery in patients with HT [11], performing early thyroidectomy is still a controversial method for their management. So it would be beneficial to use usual available bio-markers to predict which cases of HT will have a high liability of progression to PTC that will be helpful in performing early thyroidectomy for those patients even if no marker disfigurement or marked pressure symptoms.
An increasing number of promising bio-markers have emerged for differentiation between benign and malignant thyroid lesions, e.g. cluster of differentiation 56 (CD56), Hector Battifora mesothelial (HBME-1), and cytokeratin 19 (CK19). CD56 is an adhesion molecule that is present in the neural cells [12]. It is expressed in normal non neoplastic thyroid follicular cells with decreased its expression in thyroid malignancies mainly PTC [13]. HBME-1 exists in the microvilli of tracheal epithelium and mesothelial cells [14]. There are many previous studies that have clarified the role of HBME-1 expression in thyroid malignancies like PTC [15]. CK19 is an intermediate filament protein of type I that is widely expressed in normal epithelial cells [12]. A lot of researchers have clarified its strong and diffuse positivity in PTC [15]. Many of all those studies have assessed only the expression of single marker of them in benign and malignant thyroid lesions, but only few of them have explored the value of their combined expression [16,17]. Moreover most studies have assessed the roles of such markers in PTC diagnosis or to distinguish HT from PTC, but we noticed that there are no previous study which tried to assess the value of using such markers in prediction of PTC occurrence in HT patients that will advise performing early thyroidectomy in HT cases with high risk of progression to PTC before its occurrence that subsequently will decrease the malignancy risk in such patients.
Therefore, we aimed in the present study, to evaluate the usefulness of using a panel of the most sensitive and specific markers for papillary thyroid carcinoma, as previously mentioned, e.g. CD56, HBME-1 and CK-19 individually and in combination, to detect their ability to differentiate HT from PTC and detect their value in prediction of malignant progression of certain cases of HT to PTC.
Patients and Methods
In the period between January 2012 and December of 2016, 200 consecutive patients with either solitary thyroid nodule or multinodular goiter underwent total thyroidectomy in general surgery hospital, oncology unit, faculty of medicine Zagazig University and El Mansura University. All cases of total thyroidectomy were having marked pressure symptoms that advised performing total thyroidectomy. We include in our study 70 patients that had previous history of subtotal thyroidectomy. The contralateral lobe of the thyroid in the original studies of the 20 patients that had a diagnosis of HT and afterwards developed PTC and the 30 who had not developed PTC in the thyroidectomy specimens was radiologically free and shows no radiological abnormalities in most cases. In cases that were re-operated, the interval between the primary intervention and the completion of thyroidectomy ranged from 6-8 years.
The subtotal thyroidectomy and completion thyroidectomy specimens are sampled in totally, so as to exclude the possibility of microscopic PTC that could be seen in unstamped parts. In addition, to exclude the possibility of the presence of incidental papillary microcarcinoma in patients with CLT, as the absence of radiological findings before surgery does not exclude malignancy.
Our cases were divided into; 20 case of PTC with previous history of HT, 20 case of PTC without previous history of HT and 30 cases that have was diagnosed as HT in pathology department faculty of medicine Zagazig University. Data from all patients were retrospectively obtained from the files of the shared departments. All the included 70 thyroidectomy samples are processed and subjected to routine hematoxylin and eosin stain. We ordered all cases to bring their paraffin blocks that was acquired by subtotal thyroidectomy to do our research on them and on blocks retrieved from the total thyroidectomy samples. In such a method the results classification comprises 5 groups, First group 20 paraffin blocks of total thyroidectomy specimen that were recently diagnosed as PTC.
2nd group 20 paraffin blocks of the same cases historically diagnosed as HT by subtotal thyroidectomy since variable periods. 3rd group 30 paraffin blocks of total thyroidectomy specimen that that were recently diagnosed as HT. 4th group 30 paraffin blocks of the same cases historically diagnosed as HT by subtotal thyroidectomy since variable periods. 5th group 20 paraffin blocks of that were diagnosed as PTC without previous history of HT.
The collection and subsequent analysis of patients’ data was duly approved by IRB Committee in faculty of medicine, Zagazig University. The gross and histopathological features of each case were evaluated independently by two pathologists before arriving at the final diagnosis. We used T-N-M staging-system modified by the A-J-C-CCancer Staging, the seventh edition for surgical staging of PTC [18].
Macroscopic descriptions of thyroidectomy materials
The specimen size ranged in size from 6×5×4 cm in size to 10 × 9 × 8 cm in size and weight ranged from 100 gm to 500 gm. In most samples the thyroid glands were irregular in shape, contained macroscopic nodules ranged in size from 1×1×1 cm to 0.5×0.5×0.5 cm in size while the rest of samples the thyroid glands contained microscopic nodules.
Microscopic descriptions of thyroidectomy materials
Cases with HT was diagnosed by clinical and radiological signs, all HT patients already had clinical and biochemical evidence of HT e.g. clinical symptoms and signs of hypothyroidism, low T3 and T4, high TSH, positive anti-TPO and/or anti-thyroglobulin antibodies positivity, then by histo-pathological description of routine hematoxylin and eosin stained slides. Foci of follicular epithelial dysplasia (FED) that are foci with nuclear features found in few cases of HT that were transformed later on to PTC but not present in cases that are not transformed into PTC later on.
Immuno-histochemical staining: Sections from paraffin blocks that were retrieved from the 5 groups were deparaffinized in xylene and rehydrated in absolute alcohol. Antigen retrieval in citrate buffer (pH9 Lab vision cat#AP9003) was used after the sections were treated in a microwave at 8 W for six minutes, and the sections were then left to cool for20 min. Peroxidase blocks was done. After that we incubated the slides overnight with the primary anti-CD56 (clone 123C3; 1:100; Dako, Glostrup, Denmark); anti-HBME-1(clone HBME-1; 1:50; Dako, Glostrup, Denmark) and anti-CK-19 (1:100 (Dako Cytomation) antibodies at room temperature then washing them lightly by in phosphate buffered saline (PBS), pH 7.6. Followed by incubation with the secondary biotin conjugated antibody for one hour then with peroxidase conjugated streptavidin. Diamin benzidine tetrachloride (DAB) was added for 25 min, and finally the slides are counterstained in hematoxylin, then we dehydrated the slides, cleaned and mounted them [19]. Positive and negative control slides were included. Positive controls were sections from neuroblastoma, mesothelioma and skin for CD56, HBME-1 and CK19 respectively. Negative controls were done by removal of primary antibodies and their replacement with phosphate buffered saline (PBS) [20].
Interpretation of immunohistochemical staining of the studied markers:
1. Membranous expression with or without cytoplasmic staining of the cells qualified the case as positive for CD56 and CK19 [15,20].
2. Cytoplasmic expression with or without membranous staining, especially apical surface, that is the most valuable finding for HBME1of the cells qualified the case as positive for HBME-1 [21,22].
Scoring for the immune-markers by semi-quantitative assessment of markers expression;
3. For all antibodies, immune-reactivity was considered positive if >10% of follicular epithelial cells stained.
4. The immunoreactivity was scored as negative, focally positive (+: less than 25%), positive (++:25–50%) or diffusely positive (+++: more than 50%), based on the extent of the reaction [20-22].
Statistical analysis
Quantitative data were expressed as the mean ± SD and median (range), and qualitative data were expressed as absolute frequencies (number) and relative frequencies (percentage). Categorical data were compared using Chi-square test or Fisher’s exact test when appropriate. Paired categorical variables were compared using McNemar’s test. All tests were two sided. P-value <0.05 was considered statistically significant. Validity IHC was calculated using diagnostic performance depend on sample 2 × 2 contingency tables generation using the histological examination as the reference (Gold) standard. The sensitivities, specificities, positive predictive values, negative predictive values, and accuracies, with their respective 95% confidence intervals were calculated. All data were collected, tabulated and statistically analyzed using SPSS 20.0 for windows (SPSS Inc., Chicago, IL, USA) and MedCalc 13 for windows (MedCalc Software bvba, Ostend, Belgium).
Results
Patients criteria
We summarized the clinical data of our patients in Table 1. Our study included, 20 case of PTC with previous history of HT, they have included 2 males and 18 females, with their ages ranged from 29-45 years old, 5 cases were macroscopically presented with diffuse goiter and 15 presented with cold solitary thyroid nodule, 2 cases were diagnosed as follicular variant of PTC and 18 cases were diagnosed as Classic PTC, 20 case of PTC without previous history of HT they have included 4 males and 16 females, with their ages ranged from 35-50 years old, 3 cases were macroscopically presented with diffuse goiter and 17 presented with cold solitary thyroid nodule, 4 cases were diagnosed as follicular variant of PTC and 16 cases were diagnosed as Classic PTC and 30 cases that have was diagnosed as HT they have included 4 males and 26 females, with their ages ranged from 25-35 years old, 25 cases were macroscopically presented with diffuse goiter and 5 presented with solitary thyroid nodule.
| PTC with HT | PTC without HT | HT | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (N=20) | (N=20) | (N=30) | p1 | p2 | p3 | ||||
| No. | % | No. | % | No. | % | ||||
| Sex | |||||||||
| Male | 2 | 10% | 5 | 25% | 3 | 10% | |||
| Female | 18 | 90% | 15 | 75% | 27 | 90% | |||
| Age (years) | |||||||||
| Mean ± SD | 40.75 ± 7.12 | 41.20 ± 8.04 | 31.06 ± 4.89 | ||||||
| Median (range) | 38.50 (29-51) | 38.50 (29-52) | 30 (22-40) | ||||||
| HBME-1 (Historical HT) | |||||||||
| Negative | 4 | 20% | 22 | 73.30% | <0.001‡ | ||||
| Focal positive | 8 | 40% | 7 | 23.30% | |||||
| Diffuse positive | 8 | 40% | 1 | 3.30% | |||||
| HBME-1 (Recent) | |||||||||
| Negative | 2 | 10% | 3 | 15% | 22 | 73.30% | <0.001‡ | 0.619‡ | <0.001‡ |
| Focal positive | 4 | 20% | 6 | 30% | 7 | 23.30% | |||
| Diffuse positive | 14 | 70% | 11 | 55% | 1 | 3.30% | |||
| p-value | 0.031† | 1.000† | |||||||
| CK19 (Historical HT) | |||||||||
| Negative | 4 | 20% | 21 | 70% | <0.001‡ | ||||
| Focal positive | 5 | 25% | 7 | 23.30% | |||||
| Diffuse positive | 11 | 55% | 2 | 6.70% | |||||
| CK19 (Recent) | |||||||||
| Negative | 3 | 15% | 5 | 25% | 21 | 70% | <0.001‡ | 0.717‡ | <0.001‡ |
| Focal positive | 4 | 20% | 4 | 20% | 7 | 23.30% | |||
| Diffuse positive | 13 | 65% | 11 | 55% | 2 | 6.70% | |||
| p-value | 0.375† | 1.000† | |||||||
| CD56 (Historical HT) | |||||||||
| Negative | 10 | 50% | 5 | 16.70% | 0.012‡ | ||||
| Focal positive | 8 | 40% | 12 | 40% | |||||
| Diffuse positive | 2 | 10% | 13 | 43.30% | |||||
| CD56 (Recent) | |||||||||
| Negative | 16 | 80% | 17 | 85% | 5 | 16.70% | <0.001‡ | 0.834‡ | <0.001‡ |
| Focal positive | 2 | 10% | 2 | 10% | 12 | 40% | |||
| Diffuse positive | 2 | 10% | 1 | 5% | 13 | 43.30% | |||
| p-value | 0.070† | 1.000† | |||||||
Categorical variables were expressed as number (percentage); Continuous variables were expressed as mean ± SD and median (range); ‡Chi-square test; †McNemar's test; p1: PTC with HT vs. HT; p2: PTC with HT vs. PTC without HT; p3: PTC without HT vs. HT; p<0.05 is significant.
Table 1: Comparison between studied groups as regard demographic and immunohistochemistry staining.
All patients are composed of 10 (14%) males and 60(86%) females with age ranged from 29-51 years for patients with PTC with history of HT, 29-52 years for patients with PTC without history of HT and 22-40 years for patients with HT.
1. First group 20 paraffin blocks of total thyroidectomy specimen that were recently diagnosed as PTC, included 2 (10%) males and 18(90%) females.
2. 2nd group 20 paraffin blocks of the same cases historically diagnosed as HT by subtotal thyroidectomy since variable periods.
3. 3rd group 30 paraffin blocks of total thyroidectomy specimen that that were recently diagnosed as HT. included 3 (10%) males and 27(90%) females.
4. 4th group 30 paraffin blocks of the same cases historically diagnosed as HT by subtotal thyroidectomy since variable periods.
5. 5th group 20 paraffin blocks of that were diagnosed as PTC without previous history of HT. included 5 (25%) males and 15 (75%) females.
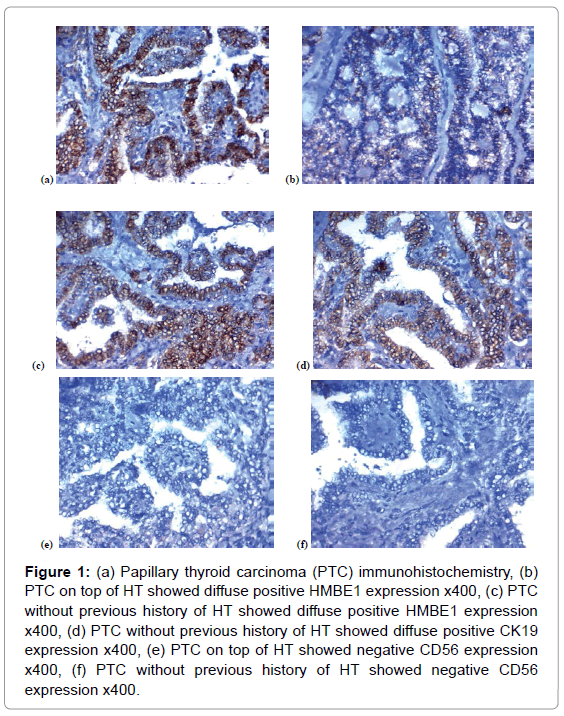
Immuno-histochemical expression in the studied thyroid: 1-CD56 expression in the studies lesions: Among the first group, negative CD56 expression was detected in 16 (80%), focal positive CD56 expression was observed in 2 (10%) and diffuse positive CD56 expression was found in 2 (10%) of cases of PTC that was on top of HT (Tables 1, 2, Figures 1E, 1F, 2E and 3C).
| CD56 IHC in historical specimen | CD56 IHC in recent specimen | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Focal positive | Diffuse positive | ||||||
| No. | % | No. | % | No. | % | No. | % | |
| PTC with HT | ||||||||
| Negative | 10 | 50% | 0 | 0% | 0 | 0% | 10 | 50% |
| Focal positive | 6 | 30% | 1 | 5% | 1 | 5% | 8 | 40% |
| Diffuse positive | 0 | 0% | 1 | 5% | 1 | 5% | 2 | 10% |
| Total | 16 | 80% | 2 | 10% | 2 | 10% | 20 | 100% |
| HT | ||||||||
| Negative | 5 | 16.70% | 0 | 0% | 0 | 0% | 5 | 16.70% |
| Focal positive | 0 | 0% | 12 | 40% | 0 | 0% | 12 | 40% |
| Diffuse positive | 0 | 0% | 0 | 0% | 13 | 43.30% | 13 | 43.30% |
| Total | 5 | 16.70% | 12 | 40% | 13 | 43.30% | 30 | 100% |
Categorical variables were expressed as number (percentage).
Table 2: Change in CD56 IHC between historical specimen and recent specimen.
Figure 1: (a) Papillary thyroid carcinoma (PTC) immunohistochemistry, (b) PTC on top of HT showed diffuse positive HMBE1 expression x400, (c) PTC without previous history of HT showed diffuse positive HMBE1 expression x400, (d) PTC without previous history of HT showed diffuse positive CK19 expression x400, (e) PTC on top of HT showed negative CD56 expression x400, (f) PTC without previous history of HT showed negative CD56 expression x400.
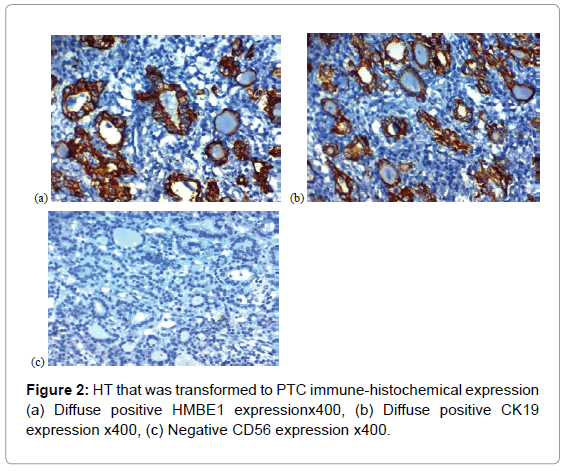
Among the second group, negative CD56 expression was detected in 10 (50%), focal positive CD56 expression was observed in 8 (40%) and diffuse positive CD56 expression was found in 2(10%) of cases of HT that found to transformed in to PTC later on. Cases with FED were all negative for CD56.
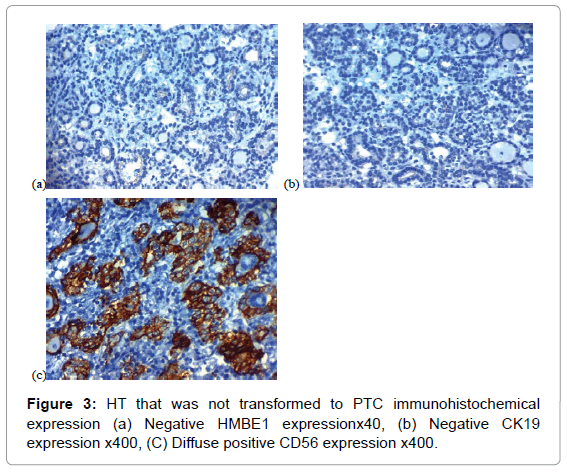
Among the third group, negative CD56 expression was detected in 5 (16.7%), focal positive CD56 expression was observed in 12 (40%) and diffuse positive CD56 expression was found in 13 (43.3%) of cases of HT that was not transformed in to PTC later on.
Among the 4th group, negative CD56 expression was detected in 5 (16.7%), focal positive CD56 expression was observed in 12 (40%) and diffuse positive CD56 expression was found in 13 (43.3%) of cases of HT that was not transformed in to PTC later on.
Among the 5th group, negative CD56 expression was detected in 17 (85%), focal positive CD56 expression was observed in 2 (10%) and diffuse positive CD56 expression was found in 1 (5%) of cases of PTC with no history of HT.
2-HBME-1 expression in the studied lesions HBME-1 signal was detected predominantly in the cytoplasm (Tables 1, 3, Figures 1A, 1B, 2A, 2B and 3A).
| HBME-1 IHC in historical specimen | HBME-1 IHC in recent specimen | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Focal positive | Diffuse positive | ||||||
| No. | % | No. | % | No. | % | No. | % | |
| PTC with HT | ||||||||
| Negative | 2 | 10% | 0 | 0% | 2 | 10% | 4 | 20% |
| Focal positive | 0 | 0% | 4 | 20% | 4 | 20% | 8 | 40% |
| Diffuse positive | 0 | 0% | 0 | 0% | 8 | 40% | 8 | 40% |
| Total | 2 | 10% | 4 | 20% | 14 | 70% | 20 | 100% |
| HT | ||||||||
| Negative | 22 | 73.30% | 0 | 0% | 0 | 0% | 22 | 73.30% |
| Focal positive | 0 | 0% | 7 | 23.30% | 0 | 0% | 7 | 23.30% |
| Diffuse positive | 0 | 0% | 0 | 0% | 1 | 3.30% | 1 | 3.30% |
| Total | 22 | 73.30% | 7 | 23.30% | 1 | 3.30% | 30 | 100% |
Categorical variables were expressed as number (percentage).
Table 3: Change in HBME-1 IHC between historical specimen and recent specimen.
Among the first group, negative HBME-1 expression was detected in 2 (10%), focal positive HBME-1 expression was observed in 4 (20%) and diffuse positive HBME-1 expression was found in 14 (70%) of cases of PTC that was on top of HT.
Among the second group, negative HBME-1 expression was detected in 4 (20%), focal positive HBME-1 expression was observed in 8 (40%) and diffuse positive HBME-1 expression was found in 8 (40%) of cases of HT that found to transformed in to PTC later on, and 4 cases of those with diffuse positive expression of HMBE1 carried foci of FED.
Among the third group, negative HBME-1 expression was detected in 22 (73.3%), focal positive HBME-1 expression was observed in 7 (23.3%) and diffuse positive HBME-1 expression was found in 1 (3.3%) of cases of HT that was not transformed in to PTC later on.
Among the 4th group, negative HBME-1 expression was detected in 22 (73.3%), focal positive HBME-1 expression was observed in 7 (23.3%) and diffuse positive HBME-1 expression was found in 1 (3.3%) of cases of HT that was not transformed in to PTC later on.
Among the 5th group, negative HBME-1 expression was detected in 3 (15%), focal positive HBME-1 expression was observed in 6 (30%) and diffuse positive HBME-1 expression was found in 11 (55%) of cases of PTC with no history of HT.
3-CK19 expression in the studied lesions CK19 expression was detected in the cell membrane with or without the cytoplasm (Tables 1, 4, Figures 1C, 1D, 2C, 2D and 3B).
| CK19 IHC in historical specimen | CK19 IHC in recent specimen | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Negative | Focal positive | Diffuse positive | ||||||
| No. | % | No. | % | No. | % | No. | % | |
| PTC with HT | ||||||||
| Negative | 3 | 15% | 1 | 5% | 0 | 0% | 4 | 20% |
| Focal positive | 0 | 0% | 2 | 10% | 3 | 15% | 5 | 25% |
| Diffuse positive | 0 | 0% | 1 | 5% | 10 | 50% | 11 | 55% |
| Total | 3 | 15% | 4 | 20% | 13 | 65% | 20 | 100% |
| HT | ||||||||
| Negative | 21 | 70% | 0 | 0% | 0 | 0% | 21 | 70% |
| Focal positive | 0 | 0% | 7 | 23.30% | 0 | 0% | 7 | 23.30% |
| Diffuse positive | 0 | 0% | 0 | 0% | 2 | 6.70% | 2 | 6.70% |
| Total | 21 | 70% | 7 | 23.30% | 2 | 6.70% | 30 | 100% |
Categorical variables were expressed as number (percentage).
Table 4: Change in CK19 IHC between historical specimen and recent specimen.
Among the first group, negative CK19 expression was detected in 3 (15%), focal positive CK19 expression was observed in 4 (20%) and diffuse positive CK19 expression was found in 13 (65%) of cases of PTC that was on top of HT (Figure 1A).
Among the second group, negative CK19 expression was detected in 4 (20%), focal positive CK19 expression was observed in 5 (25%) and diffuse positive CK19 expression was found in 11 (55%) of cases of HT that found to transformed in to PTC later on, 2 cases of those with focal positive CK19 expression and 4 cases of those with diffuse positive CK19 expression carried foci of FED.
Among the third group, negative CK19 expression was detected in 21 (70%), focal positive CK19 expression was observed in 7 (23.3%) and diffuse positive CK19 expression was found in 2 (6.7%) of cases of HT that was not transformed in to PTC later on.
Negative CK19 expression was detected in 21 (70%), focal positive CK19 expression was observed in 7 (23.3%) and diffuse positive CK19 expression was found in 2 (6.7%) of cases of HT that was not transformed in to PTC later on. Among the 5th group, negative CK19 expression was detected in 5 (25%), focal positive CK19 expression was observed in 4 (20%) and diffuse positive CK19 expression was found in 11 (55%) of cases of PTC with no history of HT (Figure 4).
No statistical significant difference was found between (1st and 5th groups), (2nd and 5th groups), (3rd and 4th groups) as regards all markers expression and (1st and 2nd groups) as regards CD56 and CK19 expression.
There is a highly significant statistical difference was found between 2nd and 4th group as regards CD56, HMBE1 and CK19 expression (P=0.012, 0.000 respectively).
There is a significant statistical difference was found between 1st and 2nd group as regards HMBE1expression (p=0.031).
There is a highly significant statistical difference was found between (1st and 4th groups), (1st and 3rd groups), (5th and 3rd groups) and (5th and 4th group) as regards all markers expression (0.000).
Specificity and sensitivity of each marker: Diagnostic validity of CD56 was of highest sensitivity (90%) in differentiating HT that will be transformed to PTC from HT that will not be transformed to PTC (Table 5). Diagnostic validity of HBME-1 was of highest specificity in differentiating HT that will be transformed to PTC from HT that will not be transformed to PTC.
| Group | IHC | TP | FP | TN | FN | SN | SP | Acc | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| HT (1) transformed to PTC vs. | HBME-1 | 14 | 1 | 29 | 6 | 65% | 95.70% | 80% | 93.30% | 82.90% |
| HT (2) that is not transformed to PTC | (diffuse=HT(1)) | (49.9-90.1) | (90.2-100) | (76.4-95.6) | (80.7-100) | (70.4-95.3) | ||||
| CK19 | 13 | 2 | 28 | 7 | 60% | 83.30% | 82% | 86.70% | 80% | |
| (diffuse=HT(1)) | (44.1-85.9) | (84.4-90) | (71.4-90.6) | (69.5-100) | (66.7-93.3) | |||||
| CD56 | 18 | 17 | 13 | 2 | 90% | 40% | 50% | 51.40% | 86.70% | |
| (-ve/focal=HT(1)) | (76.9-100) | (25.6-55) | (48.5-70.5) | (34.9-68) | (38.9-74.4) | |||||
| PTC vs. | HBME-1 | 14 | 9 | 11 | 6 | 70% | 55% | 62.50% | 60.90% | 64.70% |
| HT (1) same patients | (diffuse=PTC) | (49.9-90.1) | (33.2-76.8) | (47.5-77.5) | (40.9-80.8) | (42-87.4) | ||||
| CK19 | 13 | 11 | 9 | 7 | 65% | 45% | 55% | 54.20% | 56.30% | |
| (diffuse=PTC) | (44.1-85.9) | (23.2-66.8) | (39.6-70.4) | (34.2-74.1) | (31.9-80.6) | |||||
| CD56 | 18 | 18 | 2 | 2 | 90% | 10% | 50% | 50% | 50% | |
| (-ve/focal=PTC) | (76.9-100) | (0-23.1) | (34.5-65.5) | (33.7-66.3) | (1-99) | |||||
| PTC vs. | HBME-1 | 14 | 1 | 29 | 6 | 70% | 96.70% | 86% | 93.30% | 82.90% |
| HT (2) | (diffuse=PTC) | (49.9-90.1) | (90.2-100) | (76.4-95.6) | (80.7-100) | (70.4-95.3) | ||||
| CK19 | 13 | 2 | 28 | 7 | 65% | 93.30% | 82% | 86.70% | 80% | |
| (diffuse=PTC) | (44.1-85.9) | (84.4-100) | (71.4-92.6) | (69.5-100) | (66.7-93.3) | |||||
| CD56 | 18 | 17 | 13 | 2 | 90% | 43.30% | 52% | 51.40% | 86.70% | |
| (-ve/focal=PTC) | (76.9-100) | (25.6-61.1) | (48.5-75.5) | (34.9-68) | (38.9-74.4) |
TP: True Positive, FP: False Positive; TN: True Negative, FN: False Negative, SN: Sensitivity, SP: Specificity, Acc: Accuracy, PPV: Positivdle Predictive Value, NPV: Negative Predictive Value HT that is transformed into PTC=(1) HT that is not transformed into PTC=(2).
Table 5: Validity of HBME-1, CK19 and CD56 IHC.
Discussions
Plethora of researchers have assessed the association between HT and PTC, some of them found a significant positive correlation [2,3], but others have not found any correlation between both conditions [23]. The importance of finding such association is that the presence of goiter of any size in a patient with HT should raise the possibility of developing PTC later on, and also indicated the need for deeper investigations to exclude coexistence of malignancy in such cases.
The most recent hypothesis that we tried to prove here in our study is if we can predict the liability for malignant progression of certain cases of HT to PTC that will be of a great help to the patient as we will advise the surgeon to do early total thyroidectomy for those HT cases with more liability for malignant progression.
Akhtar and Scognamiglio [24] found that HT and PTC have the same pluripotent stem cells origins, which proved that the association between conditions is antibody specific and may have an oncogenic role [25]. It was found that elevated levels of TSH in HT patients could be also being risk factors for cancer [26].
So many previous researchers have tried to differentiate PTC from HT by immunohistochemistry and have succeeded in such issue using certain available sensitive and specific markers, but there are no previous study tried to use such immunohistochemical markers to predict the progression of HT to PTC, which could identify a group of patients that will be in a certain need to do early total thyroidectomy for management of HT to avoid malignant transformation in to PTC later on.
CD56 has been found to be related to follicular epithelium differentiation, and many previous authors reported high CD56 expression in normal non neoplastic thyroid tissue and some benign thyroid lesions [13,27]. In accordance with those studies, we currently report a high positive CD56 expression in (83.3%) of HT cases that found to not transform to PTC. On the other hand, negative CD56 expression was observed in 80-85% of PTC that has occurred on top of HT and that has occurred de novo respectively and we have detected highly significant difference between PTC and HT cases that was not transformed to PTC (P<0.001). Similarly, previous studies reported negative CD56 expression in most of their studied PTC cases [27,28].
We found that negative CD56 expression was detected in 10 (50%), focal positive CD56 expression was observed in 8 (40%) and diffuse positive CD56 expression was found in 2(10%) of cases of HT that found to transformed in to PTC later on. There was no statistically significant difference between CD56 expressions in PTC cases and HT cases that was transformed into PT later on, but CD56 distinguished the HT group that did not transformed in to PTC later from the HT group that was transformed in to PTC later so it can be used to categorize HT into cases with high incidence of malignant transformation into PTC and cases with low in cadence of such transformation. The sensitivity and the specificity for CD 56 in distinguishing the HT group that did not transformed in to PTC later from the HT group that was transformed in to PTC later were 90% and 40% respectively, so CD56 was of highest sensitivity as a negative marker in differentiating HT that will be transformed to PTC from HT that will not be transformed to PTC that will be very helpful to us in prediction of cases with high incidence of malignant transformation into PTC and cases with low incidence of such transformation.
HBME-1 is a component of the microvilli that is located on the surface of mesothelial cells [15]. Previous studies demonstrated that HBME-1 overexpression as detected by immunohistochemistry, was observed in thyroid cancers and it was a sensitive marker for PTCs [29,30].
That was similar to us as we proved that HMBE1 was positive in 85-90% of cases of PTC that has occurred de novo and that has occurred on top of HT respectively while it was negative in most cases of HT (73.3%) that was not transformed to PTC later on and we have detected highly significant difference between PTC and HT cases that was not transformed to PTC (P<0.001). Our results proved that HBME-1 has been reported to be one of the most promising markers [16,31,32] reported HBME1 positivity in 70% classic PTC, and Prasad et al. demonstrated HBME1 expression in 85% PTC [16]. This was slightly differing from Arturs et al. [28] who detected negative HBME- 1expression in all benign lesions, while they observed its positive expression in all cases of PTC.
In our study when we used HMBE1 in prediction of HT to PTC we found that the sensitivity and the specificity for HBME-1 in distinguishing the HT group that did not transformed in to PTC later from the HT group that was transformed in to PTC later were 65% and 95.7% respectively, so HBME-1 was of highest specificity in differentiating HT that will be transformed to PTC from HT that will not be transformed to PTC that will be very helpful to us in prediction of cases with high incidence of malignant transformation into PTC and cases with low incidence of such transformation, and this was in agreement with Saleh et al. [15] who showed that HBME-1 was a sensitive and specific marker to differentiate benign from malignant lesions was higher than any markers .
We found that negative HBME-1 expression was detected in 4 (20%), focal positive HBME-1 expression was observed in 8 (40%) and diffuse positive HBME-1 expression was found in 8 (40%) of cases of HT that found to transformed in to PTC later on, and there was no statistically significant difference between HBME1 expression in PTC cases and HT cases that was transformed into PT later on, but HBME1 expression distinguished the HT group that did not transformed in to PTC later from the HT group that was transformed in to PTC later so it can be used to categorize HT into cases with high incidence of malignant transformation into PTC and cases with low incidence of such transformation.
CK-19 (keratin 19), a keratin family member that plays an essential role in the structure and integrity of most epithelial cells, but its role in the diagnosis of PTC is still a point of research [23-35].
Some studies have found that negative CK19 expression was found in all benign thyroid lesions de Matos et al. while Cheung et al. demonstrated that 20% of benign thyroid lesions were focally CK19 positive [33]. The study by Nasr et al. also noted a 68% CK19 positivity in benign lesions, but staining intensity was weak. In all these cases, CKI9 staining was patchy and moderate. Zhu et al. suggested that CK- 19 was not a specific marker of PTC [36].
Sahoo et al. and Guyetant et al. have demonstrated that all cases of PTC showed strong CK19 positivity [37,38]. Prasad et al., showed a high sensitivity and specificity of CK19 in PTC [17], so the chief benefit of CK19 lies in its diagnostic ability of PTC and its negative staining is a sign against PTC. Negative staining for CK19, therefore, is strong evidence against PTC. We have proved results similar to most of these previous studies as we found that CKI9 was positive in 75- 85% of cases of PTC that has occurred de novo and that has occurred on top of HT, respectively while it was negative in most cases of HT (70%) that was not transformed to PTC later on and we have detected highly significant difference between PTC and HT cases that was not transformed to PTC (P<0.001).
The sensitivity and the specificity for CK-19 in distinguishing the HT group that did not transform in to PTC later from the HT group that was transformed in to PTC later were 60% and 83.3% respectively
CH-19 was of moderate sensitivity and specificity in differentiating HT that will be transformed to PTC from HT that will not be transformed to PTC that will be also helpful in addition to HMBE1 and CD56 to us in prediction of cases with high incidence of malignant transformation into PTC.
In contrast to our results that HT increased risk of PTC occurrence, Segal et al. suggest that HT does not increase but rather delays PTC occurrence due to the presence of circulating antibodies which may be a significant factor which could prevent cancer development and also hinder nodal metastases in transformed cases [39], these results are in contrast to the investigations by Di Pasquale et al., that proved a strong autoimmune background in all cases of PTC coexisting with HT [40]. So further studies are needed to prove and clarify our results.
In summary,
1. The commonest thyroid malignancy is PTC and the commonest autoimmune disease of the thyroid gland is HT.
2. The association and pathogenic relations between both HT and PTC remains controversial.
3. Dailey and colleagues first described that there is a relationship between both thyroid lesions and since then, there are many conflicting results regarding such issue.
4. Management of HT patients is mostly conservative and performing total thyroidectomy is not preferred due to the presence of inflammatory response surrounds the thyroid gland and leads to more difficult surgical resection
5. Although plethora of studies has identified the indications of surgery in patients with HT performing early thyroidectomy is still a controversial method for their management.
6. It would be beneficial to use usual available bio-markers to predict which cases of HT will have a high liability of progression to PTC that will be helpful in performing early thyroidectomy for those patients even if no marker disfigurement or marked pressure symptoms.
7. We used bio-markers have emerged for differentiation between benign and malignant thyroid lesions e. g. CD56, HBME-1, and CK19.
8. Most studies have assessed the roles of such markers in PTC diagnosis or to distinguish HT from PTC, but we noticed that there are no previous study which tried to assess the value of using such markers in prediction of PTC occurrence in HT patients that will advise performing early thyroidectomy in HT cases with high risk of progression to PTC before its occurrence that subsequently will decrease the malignancy risk in such patients.
Conclusion
We detected that a panel of CD56 negative expression and HMBE1 diffuse expression is considered the most sensitive and specific for prediction of PTC occurrence in certain HT cases.
References
- Liu Z, Wang L, Yi P, Wang CY, Huang T (2014) Risk factors for central lymph node metastasis of patients with papillary thyroid microcarcinoma: a meta-analysis. Int J Clin Exp Pathol 7: 932-937.
- Jankovic B, Le KT, Hershman JM (2013) Clinical review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab 98: 474-482.
- Kim KW, Park YJ, Kim EH, Park SY, Park do J, et al. (2011) Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto's thyroiditis. Head Neck 33: 691-695.
- Dailey ME, Lindsay S, Skahen R (1955) Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. Arch Surg 70: 291-297.
- Gasbarri A, Sciacchitano S, Marasco A, Papotti M, Di Napoli A, et al. (2004) Detection and molecular characterisation of thyroid cancer precursor lesions in a specific subset of Hashimoto's thyroiditis. Br J Cancer 91: 1096-1104.
- Prasad ML, Huang Y, Pellegata NS, de la Chapelle A, Kloos RT (2004) Hashimoto's thyroiditis with papillary thyroid carcinoma (PTC)-like nuclear alterations expresses molecular markers of PTC. Histopathology 45: 39-46.
- Cipolla C, Sandonato L, Graceffa G (2005) Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg 71: 874-878.
- Shih ML, Lee JA, Hsieh CB, Yu JC, Liu HD, et al. (2008) Thyroidectomy for Hashimoto’s thyroiditis: complications and associated cancers. Thyroid 18: 729-734.
- Tajiri J (2006) Radioactive iodine therapy for goitrous Hashimoto’s thyroiditis. J Clin Endocrinol Metab 91: 4497-4500.
- Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, et al. (2008) Higher Serum TSH Level in Thyroid Nodule Patients is Associated with Greater Risks of Differentiated Thyroid Cancer and Advanced Tumor Stage. Journal of Clinical Endocrinology and Metabolism 93: 809-814.
- Shimizu K, Nakajima Y, Kitagawa W, Akasu H, Takatsu K, et al. (2003) Surgical therapy in Hashimoto’s thyroiditis. J Nippon Med Sch 70: 34-39.
- Dina ED, Ahmed N, Salem A (2008) Application of CD56, P63 and CK19immunohistochemistry in the diagnosis of papillary carcinoma ofthe thyroid. Diagn Pathol 3-5.
- Rasha MA, Lobna SS (2012) Potential diagnostic utility of CD56 and claudin-1 in papillary thyroid carcinoma and solitary follicular thyroidnodules. J Egypt Natl Cancer Inst 24: 175-184.
- Debdas B, Ram ND, Uttara C, Banerjee U (2012) Cytokeratin 19 immunore-activity in the diagnosis of papillary thyroid carcinoma. Indian J MedPaediatr Oncol 33: 107-111.
- Saleh HA, Feng J, Tabassum F, Al-Zohaili O, Husain M, et al. (2009) Differential expresÂsion of galectin-3, CK19, HBME1, and Ret oncoprotein in the diagnosis of thyroid neoplasms by fine needle aspiration biopsy. Cytojournal 6: 18.
- Prasad ML, Pellegata NS, Huang Y, Decaestecker C, André S, et al. (2005) Galectin-3, fibronectin-1, CITED-1, HBME1 and cytokeratin-19 immunohistochemistry is useful for the differential diagnosis of thyroid tumors. Mod Pathol 18: 48-57.
- de Matos PS, Ferreira AP, de Oliveira FF, Assumpc LV (2005) Usefulness of HBME-1, cytokeratin 19 and galectin-3 immu-nostaining in the diagnosis of thyroid malignancy. Histopathology47: 391-401.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, et al. (2010) AJCC Cancer Staging Manual 7th ed. New York, NY: Springer-Verlag.
- Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J HistochemCytochem 29: 577-580.
- Park WY, Jeong SM, Lee JH, Kang HJ, Sin DH, et al. (2009) Diagnostic value of decreased expression of CD56 protein in pap-illary carcinoma of the thyroid gland. Basic Appl Pathol 2: 63-68.
- Yasuhiro I, Hiroshi Y, Chisato T, Miya A, Kobayashi K, et al. (2005) HBME-1 expression in follicular tumor of the thyroid: an inves-tigation of whether it can be used as a marker to diagnose follicular carcinoma. Anticancer Res 25: 179-182.
- Mauro P, Jaime R, Roberta DP, Bartolazzi A, Rosai J (2005) Galectin-3 and HBME-1 expression in well-differentiated thyroid tumors with fol-licular architecture of uncertain malignant potential. Mod Pathol 18: 541-546.
- Anil C, Goksel S, Gursoy A (2010) Hashimoto's thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid 20: 601-606.
- Akhtar M, Scognamiglio T (2007) Solid cell nests: role in thyroid disease. Adv Anat Pathol 14: 141-142.
- Azizi G, Malcho CD (2011) Autoimmune thyroid disease: a risk factor for thyroid cancer. Endocrine Practice 17: 201-209.
- Ling Z, Hui L, Qinghai J, Zhu YX, Wang ZY, et al. (2012) The clinical features of papillary thyroid cancer in Hashimoto's thyroiditis patients from an area with a high prevalence of Hashimoto's disease. BMC Cancer 12: 610.
- Shin MK, Kim JW, Ju Y (2011) CD56 and high molecular weight keratin asdignostic markers of papillary thyroid carcinoma. Korean J Pathol 45: 477-484
- Arturs O, Zenons N, Ilze S, Volanska G, Gardovskis J, et al. (2012) Immuno-histochemical expression of HBME-1, E-cadherin, and CD56 inthe differential diagnosis of thyroid nodules. Medicina (Kaunas) 48: 507-514.
- Nga ME, Lim GS, Soh CH, Kumarasinghe MP (2008) HBME-1 and CK19 are highly discriminaÂtory in the cytological diagnosis of papillary thyroid carcinoma. Diagn Cytopathol 36: 550-556.
- Mase T, Funahashi H, Koshikawa T, Imai T, Nara Y, et al. (2003) HBME-1 immuÂnostaining in thyroid tumors especially in folÂlicular neoplasm. Endocr J 50: 173-177.
- Nasr MR, Mukhopadhyay S, Zhang S, Katzenstein AL (2006) Immunohis-tochemical markers in diagnosis of papillary thyroid carcinoma:utility of HBME 1 combined with CK-19 immunostaining. Mod Pathol 19: 1631-1637
- Cheung CC, Ezzat S, Freeman JL, Rosen IB, Asa SL. Immunohis-tochemical diagnosis of papillary thyroid carcinoma. Mod Pathol 14: 338-342.
- Song Q, Wang D, Lou Y, Li C, Fang C, et al. (2011) Diagnostic significance of CK19, TG, Ki67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of China. Diagn Pathol 6: 126.
- Chung SY, Park ES, Park SY, Song JY, Ryu HS (2014) CXC motif ligand 12 as a novel diagnostic marker for papillary thyroid carcinoma. Head Neck 36: 1005-1012.
- Wu G, Wang J, Zhou Z, Li T, Tang F (2013) Com-bined staining for immunohistochemical markÂers in the diagnosis of papillary thyroid carciÂnoma: improvement in the sensitivity or speciÂficity? J Int Med Res 41: 975-983.
- Zhu X, Sun T, Lu H, Zhou X, Lu Y, et al. (2010) Diagnostic significance of CK19, RET, galectin-3 and HBME-1 expression for papillary thyroid carcinoma. J Clin Pathol 63: 786-789.
- Sahoo S, Hoda SA, Rosai J, DeLellis RA (2001) Cytokeratin 19 immunoreac-tivity in the diagnosis of papillary thyroid carcinoma. Am J Clin Pathol 116: 696-702.
- Guyetant S, Michalak S, Valo I, Saint-André JP (2003) Diagnosis ofthe follicular variant of papillary thyroid carcinoma sig-nificance of immunohistochemistry. Ann Pathol 23: 11-20.
- Segal K, Ben-Bassat M, Avraham A, Hard-El G, Sidi J (1985) Hashimoto’s thyroiditis and carcinoma of the thyroid gland. Intl Surg 70: 205-209.
- Di Pasquale M, Rothstein JL, Palazzo JP (2001) Pathologic features of Hashimoto’s-associated papillary thyroid carcinomas. Human Pathol 32: 24-30.
Citation: Harb OA (2017) Value of CD56, HBME-1, and CK19 Expression in Predicting the Risk of Papillary Thyroid Carcinoma (PTC) Occurrence in Hashimoto’s Thyroiditis (HT) Patients. J Oncol Res Treat 2: 117.
Copyright: © 2017 Harb OA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 8818
- [From(publication date): 0-2017 - Nov 15, 2025]
- Breakdown by view type
- HTML page views: 7555
- PDF downloads: 1263