Research Article Open Access
Validated Liquid Chromatographic Method for Simultaneous Determination of Metformin, Pioglitazone, Sitagliptin, Repaglinide, Glibenclamide and Gliclazide - Application for Counterfeit Drug Analysis
Ehab F Elkady1, Asmaa A El-Zaher1, Hanan M Elwy2 and Mahmoud A Saleh2*
1Pharmaceutical Chemistry Department, Faculty of Pharmacy, Cairo University, Kasr El-Aini St., Egypt
2National Organization for Drug Control and Research (NODCAR), Egypt
- *Corresponding Author:
- Mahmoud A. El Makarim M. Saleh
Makram Ebeid, 8th district, 11762
Nasr city, Cairo, Egypt
Tel: +2-01114516158
E-mail: mahmoud.saleh88@hotmail.com
Received date: October 26, 2015 Accepted date: November 12, 2015 Published date: November 19, 2015
Citation: Elkady EF, El-Zaher AA, Elwy MH, Saleh MA (2015) Validated Liquid Chromatographic Method for Simultaneous Determination of Metformin, Pioglitazone, Sitagliptin, Repaglinide, Glibenclamide and Gliclazide - Application for Counterfeit Drug Analysis. J Anal Bioanal Tech S13:007. doi:10.4172/2155-9872.S13-007
Copyright: © 2015 Elkady EF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A rapid, precise and selective RP-LC method was developed for simultaneous determination of the widely used oral antidiabetic; metformin hydrochloride (MTF), with some commonly prescribed oral antidiabetics, namely; sitagliptin phosphate (SIT), pioglitazone hydrochloride (PGZ), gliclazide (GLZ), glibenclamide (GLB) and repaglinide (RPG). The chromatographic separation carried out using gradient elution mode with acetonitrile: 0.05M potassium dihydrogen phosphate (MKP) and 0.01M sodium octane sulphonate (SOS) (pH 3.55) at flow rate 0.85 ml/min on Kromasil 100-C18, (30 × 0.4 cm, 10 μm) at 40°C. UV detection was carried out at 220 nm. The method was validated according to ICH guidelines. Linearity, accuracy and precision were satisfactory over the concentration ranges (μg/ ml) of 0.05-205 for MTF, 0.05-100 for PGZ, GLB and SIT, 0.1-100 for RPG and 1-100 μg/ml for GLZ. The correlation coefficients were >0.99 for all analytes. Limits of quantification (LOQs) found were 0.002, 0.003, 0.009, 0.012, 0.007 and 0.024 μg/ml for MTF, SIT, PGZ, GLZ, GLB and RPG respectively. The developed method is specific and accurate for quality control and routine analysis of the cited drugs in their pharmaceutical preparations. It is recommended for application in the quality control of the herbal antidiabetic products to detect possible counterfeits.
Keywords
Anti-diabetic drugs; Counterfeits; Liquid chromatography; Metformin hydrochloride; Pharmaceutical preparations
Abbreviations
AUP: Area under peak; GLB: Glibenclamide; GLZ: Gliclazide; LOD: Limit of detection; LOQ: Limit of quantitation; MKP: Potassium dihydrogen phosphate; MTF: Metformin hydrochloride; PGZ: Pioglitazone hydrochloride; RPG: Repaglinide; SIT: Sitagliptin phosphate; SOS: Sodium octane sulphonate; MRM: Multiple reaction monitoring; DPP-4: Dipeptidyl peptidase 4
Introduction
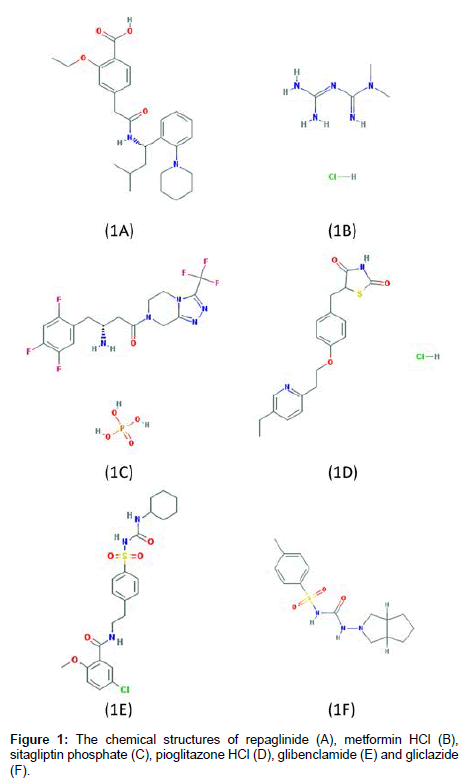
Repaglinide (RPG), S(+) 2-ethoxy-4(2((3- methyl-1-(2-(1- piperidinyl) phenyl)-butyl) amino)-2-oxoethyl) benzoic acid (Figure 1A), belongs to the meglitinide class of antidiabetics for the treatment of type 2 diabetes. Metformin hydrochloride (MTF), N,Ndimethylimidodicarbonimidic diamide hydrochloride (Figure 1B) [1], is considered to be the main drug in mixed or combination therapies of diabetes. MTF belongs to the biguanide class of oral anti-diabetic [2]. Sitagliptin phosphate (SIT), 1,2,4-triazolo[4,3-a]pyrazine,7-[(3R)-3- amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8- tetrahydro-3- (trifluoromethyl), phosphate (Figure 1C) [3] belongs to dipeptidyl peptidase 4(DPP-4) inhibitors [4]. Pioglitazone hydrochloride (PGZ), ((RS) -5- (4- [2-(5-ethylpyridin-2-yl) ethoxy] benzyl) thiazolidine-2,4- dione (Figure 1D), is one of the thiazolidinedione antidiabetic that is used either alone or in combination with other oral antidiabetic drugs [2]. Glibenclamide (GLB), 5-chloro-N-[2-[4-[[[(cyclohexyl (amino) carbonyl]-amino]sulphonyl] phenyl] ethyl-2- methoxy benzamide (Figure 1E) [5], and gliclazide (GLZ), 1-(3-azabicyclo[3.3.0]oct-3-yl)- 3-(p-tolylsulfonyl) urea (Figure 1F) [6] belong to sulfonylurea drugs that are given orally in the treatment of type 2 diabetes mellitus [7].
Literature survey revealed that MTF and RPG have been simultaneously determined using HPLC methods in rabbit plasma [8] and pharmaceutical formulations [1,9-11]. They have been also simultaneously determined by LC-tandem MS in human plasma [12] and by LC-MS/MS-ESI in rat plasma [13]. Spectrophotometric methods have been reported for the simultaneous estimation of MTF and RPG [14,15]. MTF and SIT have been simultaneously determined using HPLC [4,16-20] and spectrophotometric techniques [3,21-24] and together with degradation products by spectrophotometric and spectrofluorimetric methods [25]. MTF has been simultaneously determined with PGZ by spectrophotometric methods [26,27] and by HPLC in binary mixture [28-31] and with other components by HPLC [32-35]. GLZ has been simultaneously determined with MTF by spectrophotometric methods [36-38] and by LC in dosage forms [39-43] and in human plasma [44,45]. MTF and GLB have been simultaneously determined by spectrophotometry [46,47] and by LC in dosage forms [48-50] and in human plasma [51]. It is worth noting that many mixtures composed of more than two drugs of the present mixture were simultaneously determined using RP-HPLC technique in dosage forms [52,53], in plasma [54] and in both-dosage forms and human plasma or serum [55,56]. Spectrophotometric determinations have been reported for mixtures composed of more than two drugs [57-59]. To the best of our knowledge, no method has been reported for the simultaneous determination of all six components in the mixture of the proposed LC method. Thus, the aim of this method was to develop and validate an analytical method using the most preferable RP-LC technique for the simultaneous determination of the well-known oral anti-diabetic metformin hydrochloride, along with commonly prescribed oral antidiabetic drugs from different pharmacological classes as mentioned before, namely; sitagliptin phosphate, pioglitazone hydrochloride, gliclazide, glibenclamide and repaglinide. Examples of pharmaceutical products include, but not limited to, Glucovance 500/5, Januvia 100 (or 50), Janumet 50/500 (or 50/1000) Actos 30, Diamedizen 60, Prandimet 1/500 (or 2/500) and Novonorm 0.5 (or 2). Besides, the proposed method is applied to the detection of counterfeits in herbal antidiabetic therapy.
Materials and Methods
Materials and reagents
All the chemicals used were of analytical reagent grade, and the solvents were of HPLC grade. Elnasr Pharmaceutical Company supplied MTF (certified to contain 99.9%). Nutra Specialties Private limited supplied SIT (certified to contain 99.8%). Mepaco Pharmaceutical Company supplied PGZ (certified to contain 99.2%). Zhejang Jiuzhou supplied GLZ (certified to contain 100.2%). Amrya Pharmaceutical Company supplied GLB (certified to contain 99.5%). Marcyrl Pharmaceutical Company supplied RPG (certified to contain 100.2%). Sodium octane sulphonate (SOS) was obtained from Sd Fine Chemical Limited - India. Acetonitrile was obtained from Poch limited-Product of Poland. Actos® 30 mg tablets; (Takeda Pharmaceutical Company) each labeled to contain 30 mg PGZ, Glucovance® 500/5 mg tablets; (Merck Serono Pharmaceutical Company) each labeled to contain 500 mg MTF and 5 mg GLB, Novonorm® 0.5 mg tablets; (Novonordisk Pharmaceutical Company) each labeled to contain 0.5 mg RPG, Januvia®100 mg tablets; (MSD Pharmaceutical Company) each labeled to contain 123.9 mg SIT and Diamedizen® 60 mg tablets each labeled to contain 60 mg GLZ; were purchased from commercial sources in local market. Potassium dihydrogen phosphate (MKP), sodium hydroxide and o-Phosphoric acid 85% (Sigma-Aldrich) were used.
Instrumentation
The LC system used was Agilent HPLC system 1100 series equipped with G1311A quaternary pump, G1314A variable wavelength detector and Agilent 1260 Infinity G1322A degasser. Agilent Chemstation PC program was used for the instrument control, data acquisition and analysis. Separation was achieved on Teknokroma; Kromasil 100- =C18, 10 μm, 30 × 0.4 cm. Hanna instruments® pH-meter-Romania was used for pH adjustment of the buffer. Water was purified for HPLC analysis using Purelab-flex water system-UK. Mobile phase was mixed and degassed using Crest® sonicator-USA and filtered through 0.2 μm Sartorius AG® membrane filter.
Chromatographic conditions
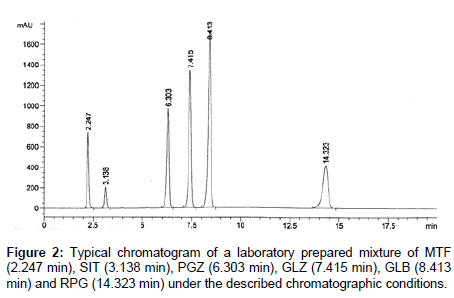
Chromatographic separation of the six oral antidiabetic drugs (MTF, SIT, PGZ, GLZ, GLB and RPG) was achieved on Agilent 1100 HPLC system using Teknokroma; Kromasil 100-C18 (30 × 0.4 cm, 10 μm). The mobile phase consisted of acetonitrile (solution A) and buffer (solution B); the latter was composed of 0.05 M MKP and 0.01M SOS, pH adjusted to 3.55 by 85% ortho-phosphoric acid. The45 mobile phase flow was pumped at 0.85 ml/min through the column adjusted at temperature 40°C on a gradient program as follows; Time/ Buffer %: 0/50, 3/35, 7/30, 15/30, 20/50. The injection volume was 20 μl. The effluent was detected at 220 nm. The run time for each injection was 20 minutes. Post run time for equilibration was 4 minutes. Data acquisition was performed on Agilent LC Chemstation software. The retention times were 2.24, 3.13, 6.3, 7.41, 8.41 and 14.32 for MTF, SIT, PGZ, GLZ, GLB and RPG (Figure 2).
Standard stock solutions preparation
Accurately weighed 25 mg of MTF and 10 mg of each of RPG, PGZ, GLZ, GLB and SIT; were separately transferred into 100 ml volumetric flasks, dissolved in and completed to volume with solvent mixture C which consists of equal volumes of acetonitrile and buffer (0.05 M MKP+ 0.01 M SOS, pH: 3.55). (0.25 mg/ml for MTF and 0.1 mg/ml for SIT, PGZ, GLZ, GLB and RPG).
Construction of calibration graphs
Accurately measured aliquots were transferred from the standard stock solutions of MTF, SIT, PGZ, GLZ, GLB and RPG; into separate sets of 10 ml volumetric flasks to prepare concentrations equivalent to 0.05-205 μg/ml for MTF, 0.05-100 μg/ml for SIT, PGZ and GLB, 0.1-100 μg/ml for RPG and 1-100 μg/ml for GLZ. Solvent mixture C was used to complete each flask to mark. The calibration graphs were obtained by plotting the area under the peak versus the concentration of each drug in μg/ml and the corresponding regression equations were derived.
Assay of cited drugs in bulk
The procedure described under “Construction of Calibration Graphs” was repeated for concentrations equivalent to 15-90 μg/ml for each of MTF, SIT, PGZ, GLB and RPG and 30-90 μg/ml for GLZ. The mean recovery values were calculated using the corresponding regression equations.
Preparation of the laboratory prepared synthetic mixtures
Laboratory prepared mixtures of the six drugs were prepared by mixing aliquots taken from stock standard solutions in the ratio of 100: 24.8 (and 12.4): 6: 12: 1: 0.4 (and 0.1) for MTF, SIT, PGZ, GLZ, GLB and RPG respectively. The volumes were taken into a series of 10 ml volumetric flasks to obtain concentrations of (130-202 μg/ml), (20-50 μg/ml), (7.7-12 μg/ml), (16-25 μg/ml), (1.3-2.2 μg/ml) and (0.5-0.8 μg/ml) for MTF, SIT, PGZ, GLZ, GLB and RPG respectively. Concentrations of the six drugs were calculated using the corresponding regression equations.
Pharmaceutical samples preparation
Ten tablets of each of Glucovance, Januvia, Actos 30, Diamedizen 60 and Novonorm 0.5 were separately weighed, powdered using mortar and pestle to fine particle size. An amount of the powder equivalent to 500 mg, 123.9 mg, 30 mg, 60 mg, 5 mg and 0.5 mg for MTF, SIT, PGZ, GLZ, GLB and RPG; respectively were separately weighed and transferred into a set of 100 ml volumetric flasks. Approximately 50 ml of solvent mixture C were added, sonicated for 10 minutes with frequent shaking. Solutions were diluted to volume with solvent mixture C and were filtered through 0.45 μm filter paper. The first 10 ml of the filtrates were discarded then the filtrates were diluted to the appropriate concentrations (μg/ml) (10-180), (5-96), (29-90), (30-95), (5-75) and (1.5-72) for MTF, SIT, PGZ, GLZ, GLB and RPG respectively. Standard addition technique was also carried out. The mean recovery values of the pharmaceutical products and added standards were calculated using the corresponding regression equations.
Herbal samples preparation
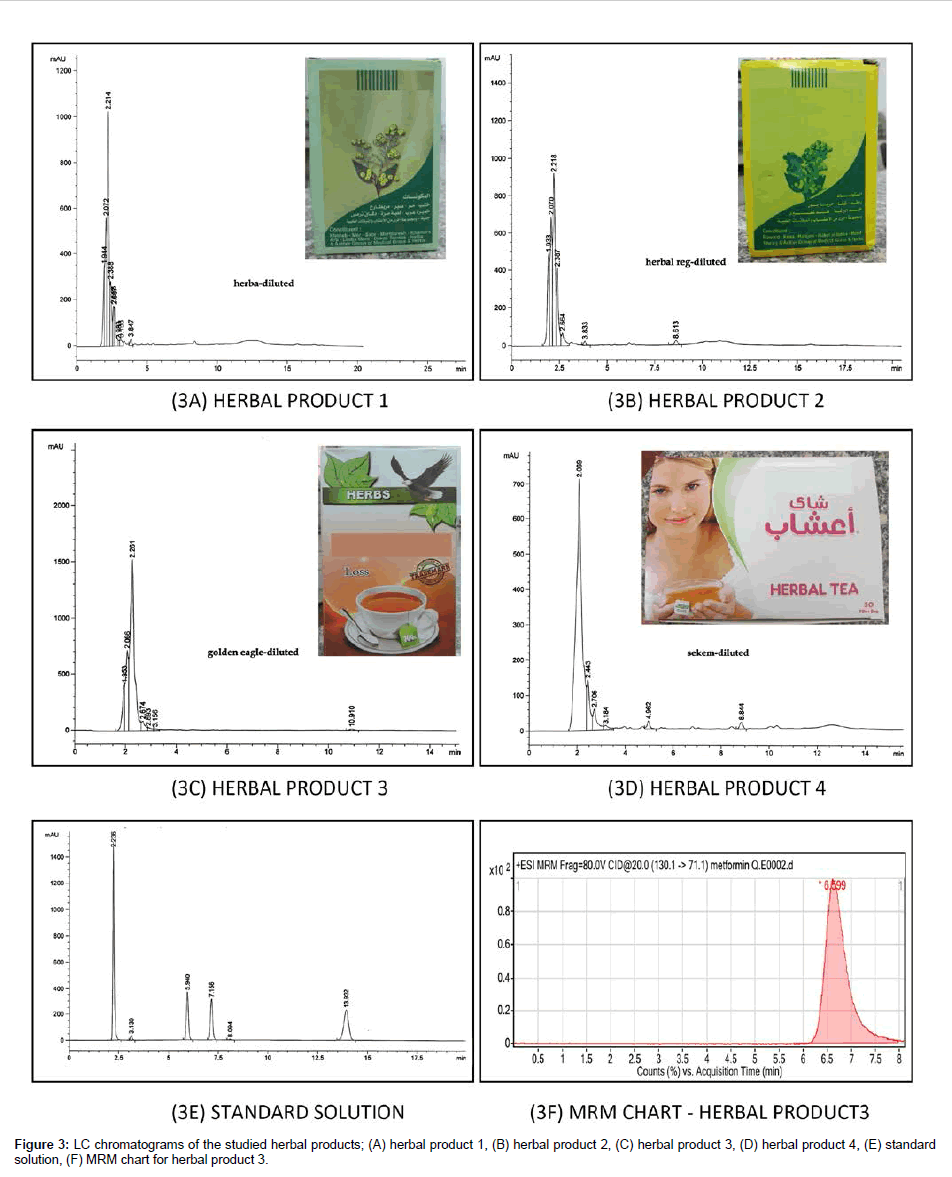
Four Samples of herbal bulk powders and tea bags (Figure 3A-3D) that were under suspicion of adulteration with undeclared synthetic drugs were purchased from local herbal druggists and retail outlets. According to the labels on the boxes, the products contain herbal ingredients and were declared as anti-diabetic and anti-obesity formulations. These products were mainly from suburbs of Cairo.
One teaspoonful from each of the 3 herbal bulk powders and one teabag from the fourth sample were each transferred into a 100 ml volumetric flask, extracted in 50 ml solvent mixture C by sonication for 10 minutes with frequent shaking, then diluted to volume with the same solvent, mixed and filtered through 0.45 μm filter paper. The first 10 ml of the filtrates were discarded then an aliquot of 2 ml was transferred from each preparation to a series of 10 ml volumetric flasks and diluted to volume with solvent mixture C. Another series of corresponding dilutions that were spiked with 10 μg/ml MTF, SIT, PGZ, GLZ, GLB and RPG were also prepared.
Results and Discussion
The main concern of this work was to develop a fast and validated LC method suitable for the determination of some well-known antidiabetic MTF, along with commonly prescribed oral antidiabetic drugs from different pharmacological classes. Besides, the proposed method will be useful for the detection of counterfeits in antidiabetic therapy.
Optimization of chromatographic conditions
During the optimization cycle, different columns with different lengths and internal diameters were tried namely, Waters symmetry C18 (30 × 0.4 cm, 10 μm) and Supelco supelcosil C18 (25 × 0.46 cm, 5 μm) but finally satisfactory separation was obtained on Teknokroma kromasil 100-C18 (30 × 0.4 cm, 10 μm) column. Methanol and acetonitrile were examined individually and simultaneously as organic modifiers and acetonitrile was found to be more suitable, individually, as it allowed better separation of the six analytes under investigation. Isocratic mode of elution with different ratios of organic to aqueous phases was tried and it was found unsuitable so gradient elution mode was attempted. Different gradient elution modes were tested in order to achieve proper separation of the cited analytes in a reasonable run time. The described gradient elution mode stated in section 2.3 was selected. The use of 0.05M MKP buffer was necessary in this method in order to influence the ionization of the analytes and to help in their co-elution. Also, it kept the pH constant as each of MTF, PGZ, GLZ, GLB and RPG is obviously affected by the mobile phase composition and pH. The effect of pH on the separation of the analytes was studied. It was found that pH higher than 4.59 was not suitable as due to improper separation of the analyzed compounds. pH was adjusted at 3.55 for the best separation of the six analytes in a reasonable run time (<15 min) and with good resolution between all peaks (Figure 2). Different flow rates were studied and flow rate of 0.85 mL min-1 was found to be optimum. Quantitation was achieved with UV-detection at 220 nm. Many trials were done to enhance symmetry and sharpness of peaks and hence improve the resolution of the cited drugs. Addition of 10mM sodium octane sulphonate, as ion pairing agent, produced significant improvement. The use of optimized mobile phase containing acetonitrile and buffer (0.05 M MKP + 0.01 M SOS, pH: 3.55) (50:50 v/v) instead of methanol as a solvent led to good peaks` symmetry, sharpness and best resolution between the cited analytes (Figure 2). The retention times for MTF, SIT, PGZ, GLZ, GLB and RPG were 2.24, 3.13, 6.30, 7.41, 8.41 and 14.32 min respectively. The system suitability tests were used to verify that the conditions of the chromatographic system were adequate for the separation and hence for the analysis [60] (Table 1).
| Parameter | Components/Data | |||||||
|---|---|---|---|---|---|---|---|---|
| MTF | SIT | PGZ | GLZ | GLB | RPG | |||
| N | 5790 | 6217 | 16037 | 20063 | 21138 | 15655 | ||
| Tailing factor (T) | 1.449 | 1.234 | 1.04 | 1.057 | 1.014 | 0.934 | ||
| % RSD of 6 injections | 0.0157 | 0.298 | 0.531 | 0.7480 | 0.1262 | 0.333 | ||
| R | Normal | - | 6.46 | 16.2 | 6.23 | 4.41 | 17.45 | |
| pH of buffer (± 0.1) | 3.45 | - | 6.28 | 15.67 | 6.97 | 4.45 | 16.91 | |
| 3.65 | - | 6.46 | 16.79 | 5.59 | 4.49 | 17.79 | ||
| Flow rate (± 0.1) | 0.75 | - | 6.79 | 16.22 | 6.26 | 4.39 | 17.88 | |
| 0.95 | - | 6.14 | 16 | 6.11 | 4.45 | 17.11 | ||
| Acetonitrile % (± 2) | 52% | - | 5.46 | 15.99 | 5.66 | 3.87 | 16.87 | |
| 48% | - | 7.26 | 15.84 | 6.69 | 4.88 | 17.8 | ||
(N: Number of theoretical plates; R: Resolution factor; T: Tailing factor; RSD: Relative standard deviation, Flow rate (ml/min))
Table 1: System suitability and Robustness tests for the proposed LC method.
Validation of the method
Validation of the proposed RP-HPLC method was performed with respect to linearity, limit of detection (LOD), limit of quantitation (LOQ), accuracy, precision and robustness according to ICH guidelines [60].
Linearity: Linearity was assessed for the six oral antidiabetic drugs at concentration ranges 0.05-205 μg/ml for MTF, 0.05-100 μg/ml for SIT, PGZ and GLB, 0.1-100 μg/ml for RPG and at 1-100 μg/ml for GLZ. A linear relationship was established at these ranges between Area under the peak (AUP) and concentration. Good linearity was proved by high values of coefficient of determinations. The calibration data are shown in Table 2.
| Parameter | Components/Data | ||||||
|---|---|---|---|---|---|---|---|
| MTF | SIT | PGZ | GLZ | GLB | RPG | ||
| Linearity range* | 0.05-205 | 0.05-100 | 0.05-100 | 1-100 | 0.05-100 | 0.1-100 | |
| Retention time | 2.24 | 3.13 | 6.30 | 7.41 | 8.41 | 14.32 | |
| Wavelength of detection | 220 nm | ||||||
| Regression equation | AUPMTF=64.2C*+33.34 | AUPSIT=5.77C+0.44 | AUPPGZ=47.17C-19.55 | AUPGLZ=39.13C-29.4 | AUPGLB=69.23C-1.41 | AUPRPG=52.89C-3.41 | |
| Correlation coefficient (R2) | 0.9996 | 0.9999 | 0.9993 | 0.9997 | 0.9999 | 0.9999 | |
| LOD* | 0.001 | 0.001 | 0.003 | 0.004 | 0.002 | 0.007 | |
| LOQ* | 0.002 | 0.003 | 0.009 | 0.012 | 0.007 | 0.024 | |
| Sb* | 0.457 | 0.022 | 0.427 | 0.282 | 0.175 | 0.198 | |
| Sa* | 21.463 | 1.046 | 20.089 | 15.867 | 8.23 | 9.816 | |
| Precision | Intraday R.S.D % | 0.011-0.092 | 0.036-0.64 | 0.05-0.22 | 0.035-0.23 | 0.077-0.36 | 0.03-0.45 |
| Interday R.S.D% | 0.32 -0.71 | 0.5-2.0 | 0.7-1.8 | 0.5-1.6 | 0.18-1.9 | 0.2-1.6 | |
| Accuracy | Drug in bulk | 100.9 ± 0.737 | 99.9 ± 0.921 | 100 ± 1.969 | 101.5 ± 0.621 | 100.1 ± 1.27 | 101 ± 0.364 |
| Drug in lab prepared mixture | 101.2 ± 0.643 | 100.1 ± 0.75 | 100.9 ± 0.932 | 98.7 ± 0.385 | 100.4 ± 1.501 | 100.2 ± 1.578 | |
| Drug in dosage form* | 102% ± 0.033 | 91.7% ± 0.024 | 95.2 ± 3.118 | 100.1% ± 0.013 | 104% ± 0.009 | 101.3% ± 0.033 | |
| Drug added | 100.9% ± 1.481 | 99.5% ± 1.11 | 98.9% ± 0.008 | 100.3% ± 1.343 | 99.7% ±1.246 | 98.9% ± 1.218 | |
(Linearity range (μg/ml); LOD (μg/ml); LOQ (μg/ml)), C: Concentration (μg/ml), Sa: Standard error of intercept, Sb: Standard error of slope; Dosage form: mentioned for each component in section 2.1 and for preparation technique in section 2.8
Table 2: Characteristics and Results of the Proposed RP-LC Method.
Limit of detection (LOD) and Limit of quantitation (LOQ): According to ICH Q2 (R1) recommendations [60], LOD was considered as the minimum concentration with a signal to noise ratio of 3, while LOQ was taken as a minimum concentration with signal to noise ratio of 10. LOD and LOQ were calculated experimentally. Results are shown in Table 2.
Accuracy and precision: Accuracy of the method was proved by recovery study of each of MTF, SIT, PGZ, GLZ, GLB and RPG in bulk, in their synthetic mixtures of their medicinally recommended ratios and by standard addition technique. Results of recovery tests are summarized in Table 2. Repeatability (intra-day) and intermediate precision (inter-day) were assessed using three concentrations and three replicates of each concentration. The relative standard deviations were found to be small indicating reasonable repeatability and intermediate precision of the proposed method (Table 2).
Robustness: Robustness test was performed by applying small deliberate changes to the chromatographic conditions. The most important parameters studied under this test were flow rate (changed by ± 0.1), mobile phase composition-acetonitrile percent (changed by ± 2 in all levels of the gradient program) and pH value of the buffer component of the mobile phase (changed by ± 0.1). Each of the previous parameters were changed while keeping the other parameters constant. The resolution factors between each two successive peaks were calculated for each change. The resolution factors under different parameters of robustness are summarized in Table 1. Robustness test revealed good values of resolution that were not significantly changed by minor changes in the chromatographic conditions, which indicates the good robustness of the proposed LC method.
Application to detection of counterfeits: By comparing the chromatograms of the herbal products (Figure 3A-3D) with the chromatogram of the standard solution of the cited antidiabetics (Figure 3E), the presence of a peak at the retention times of MTF in herbal products (1-3) was observed. Besides, the presence of a small peak at the retention time of SIT was also observed in the chromatogram of herbal products (1, 3, and 4). MTF is relatively cheap so the adulteration with MTF maybe suspected and further investigation is suggested to assure the adulteration with MTF and SIT with different spectroscopic techniques as MS, NMR and IR spectroscopy. Besides, the investigated herbal products were spiked with MTF and SIT among the other components; PGZ, GLZ, GLB and RPG which increased the height of MTF peak at 2.2 min for herbal products (1-3) and increased the height of SIT peak at 3.1 min for herbal products (1,3,4). Spiking the herbal extracts with the standard materials confirmed suitability of detection of the investigated antidiabetics in the present study in the herbal matrix. Herbal products (1-3) were subjected to mass spectrometric analysis to confirm or deny adulteration with SIT and MTF that were suspected due to the presence of peaks at their respective retention times in HPLC-UV analysis. Adulteration in herbal products 1 and 2 was denied by the mass spectrometric analysis. Meanwhile, adulteration of herbal product 3 with MTF was confirmed due to the presence of an intense peak of metformin in the multiple reaction monitoring (MRM) chart (Figure 3F). Quantitation was carried out by a pre-developed method for MTF. It was calculated that each 1 g of herbal product 3 is equivalent to ca. 40 mg of MTF. As the matrix of the herbal product was not available, selectivity study of the developed method in the herbal matrices was not feasible.
Conclusion
A quick, selective, specific and validated RP-LC method for the simultaneous determination of six commonly used oral antidiabetics, namely; metformin hydrochloride, sitagliptin phosphate, pioglitazone hydrochloride, gliclazide, glibenclamide and repaglinide. This method is characterized by simplicity, accuracy, preciseness besides its wide range of applications and it can be used for routine analysis and quality control of the cited drugs separately or in combinations in many dosage forms. The developed method proved suitability for the detection of counterfeit drugs.
References
- Bhaskar Reddy L, Raveendra Reddy P, Useni Reddy M, Reddy LM (2011) Novel RP-HPLC method for metformin HCL, Glipizide and Repaglinidepharmaceuctial drug products. International Journal of Research and Reviews in Pharmacy and Applied Sciences 1: 131-139.
- El-Bagary RI, Elkady EF, Ayoub BM (2011) Liquid chromatographic methods for the determination of vildagliptin in the presence of its synthetic intermediate and the simultaneous determination of pioglitazone hydrochloride and metformin hydrochloride. Int J Biomed Sci7: 201-208.
- Amruta BL, Minal RG, SawantSD (2012) Simultaneous UV Spectrophotometric Method for Estimation of Sitagliptin phosphate and Metformin hydrochloride in Bulk and Tablet Dosage Form. Der PharmaChemica 4: 854-859.
- Govindasamy J,NyolaN (2012) Simultaneous estimation of sitagliptin phosphate monohydrate and metformin hydrochloride in bulk and pharmaceutical formulation by RP-HPLC. J Pharm Educ Res 3: 24-28.
- Anandkimar RT, Gurupadayya BM, Neeraj S,Vishwanathan (2013) Method development and validation of metformine, pioglitazone and glibenclamide in tablet dosage form by using RP-HPLC. Biochem Anal Biochem 2: 1-5.
- Havele S,Sunil D (2010) Development and validation of a HPLC method for the determination of metformin hydrochloride, gliclazide and piogliglitazone hydrochloride in multicomponent formulation.Webmedcentral Pharmaceutical Sciences 1: 1-16.
- SweetmanSC (2009) Martindale-The Complete Drug Reference, 36th edn. Pharmaceutical Press, London, UK.
- Ramanjireddy T, Dhachinamoorthi D, BannothCK (2010) Method Development and Validation of Metformin and Repaglinide in Rabbit Plasma by RP-HPLC. Fabad J Pharm Sci 35: 69-75.
- Madhavi J, Ami S, SoniLK (2012) Development and validation of RP-HPLC methods for simultaneous estimation of metformin hydrochloride and repaglinide in tablet dosage form. International Journal of Pharmaceutical and Research Science 1: 251-258.
- Navya S (Year N/A) Simultaneous estimation of repaglinide and metformin hydrochloride in tablet dosage form by reverse phase high performance liquid chromatography. The Experiment 2: 109-115.
- Patel Deepa R, Patel Laxmanbhai J, PMM (2011) Stability indicating HPLC method for simultaneous determination of repaglinide and metformin hydrochloride in pharmaceutical dosage form. Asian Journal of Research in Chemistry 4: 500-505.
- Liang XR, Dai XJ, Zhang YF, Ding JF, Chen XY, et al. (2013) Liquid chromatography-tandem mass spectrometry simultaneous determination of repaglinide and metformin in human plasma and its application to bioequivalence study.Yao XueXueBao 48: 547-553.
- Sharma K, Pawar G, Yadam S, Giri S, Rajagopal S, et al. (2013) LC-MS/MS-ESI method for simultaneous quantitation of metformin and repaglinidie in rat plasma and its application to pharmacokinetic study in rats.Biomed Chromatogr 27: 356-364.
- Patel JR, SuhagiaBN, Patel BH (2007) Simultaneous spectrophotometric estimation of metformin and repaglinide in a synthetic mixture. Indian J Pharm Sci 69: 844-846.
- Prasanth VG, SusammaCicyEapen, SheejaVelayudhanKutty, Rai A (2012) Development and validation of UV spectroscopic methods for the estimation of repaglinide and metformin hydrochloride in synthetic mixture. International Journal of Pharmaceutical Science and Health Care 2: 150-158.
- Hitesh PI,MhaskeAA (2012) RP-HPLC Method for Simultaneous Determination of Metformin Hydrochloride, Rosiglitazone and Sitagliptin –Application to Commercially Available Drug Products. International Journal of Pharmaceutical Sciences and Research 3: 3267-3276.
- Sachin LP, Jayant RB, BhalgatCM (2013) Development and Validation of a Stability Indicating RP-HPLC Method for Simultaneous Determination of Sitagliptin and Metformin in Tablet Dosage Form. International Journal of Research in Pharmaceutical and Biomedical Sciences 4: 590-596.
- Loni AB, GhanteMR, SawantDSD (2012) Method development and validation for simultaneous determination of sitagliptin phosphate and metformin hydrochloride by RP-HPLC in bulk and tablet dosage form. Asian Journal of Pharmaceutical Sciences and Research- AJPSR 2: 23-37.
- RamanjaneyuluJuvvigunta, Nagarjuna ReddyG, DhanalakshmiD, Ramesh B (2013) A new analytical method development and validation for simultaneous estimation of sitagliptin and metformin hydrochloride in tablet dosage form by RP-HPLC. International Journal of Pharma Sciences 3: 360-364.
- Lathareddy Y, RaoNS (2013) Stability-indicating RP-HPLC method and its validation for analysis of metformin &sitagliptin in bulk and pharmaceutical dosage form. World Journal of Pharmacy and Pharmaceutical Sciences 2: 3691-3709.
- Ghazala Khan, Dinesh Sahu, Agrawal YP, NeetuSabarwal, Avnish Jain, et al. (2011) Simultaneous Estimation of Metformin and Sitagliptin In Tablet Dosage Form. Asian Journal of Biochemical and Pharmaceutical Research 1: 352-358.
- Narendra N, JeyabalanGS (2012) Method development of simultaneous estimation of sitagliptin and metformin hydrochloride in pure and tablet dosage form by uv-vis spectroscopy. World Journal of Pharmacy and Pharmaceutical sciences 1: 1392-1401.
- Sujani PV, Phanindra SS, Anasuya P, Padamanabha Reddy Y,DevannaN (2013) Development and Validation of Simultaneous Equation Method for the Estimation of Metformin amdSitagliptin by UV Spectroscopy. Research and Reviews: Journal of Pharmacy and Pharmaceutical Sciences2: 72-75.
- Safaa MR, Mamdouh RR, Ghada YM, AleemAAE (2012) Spectrophotometric determination of sitagliptin and metformin in their pharmaceutical formulation. International Journal of Comprehensive Pharmacy 3: 1-4.
- Ramzia IB, Ehab FE,AyoubBM (2011) Spectroflourometric and spectrophotometric methods for the determination of sitagliptin in binary mixture with metformin and ternary mixture with metformin and sitagliptin alkaline degradation product. Int J BiomedSci 7: 62–69.
- Sujana K, Swathi Rani G, Bhanu Prasad M, Reddy MS (2010) Simultaneous Estimation of Pioglitazone Hydrochloride and Metformin Hydrochloride using UV Spectroscopic Method. J Biomed Sci and Res. 2: 110-115.
- Goswami L, Mukhopadhyay S, Durgapal S (2010) Simultaneous estimation of metformin and pioglitazone by ultraviolet spectrophotometry.Indian J Pharm Sci 72: 508-510.
- JajowSwapna, ChandakaMadhu, MallepelliSrivani, Sumalatha M, Nehalatha Y, et al. (2012) Analytical Method Development and Method Validation for the Simultaneous Estimation of Metformin hydrochloride and Pioglitazone hydrochloride in Tablet Dosage Form by RP-HPLC. Asian J Pharm Ana 2: 85-89.
- Lakshmi KS, Rajesh T, Sharma S (2009) Simultaneous determination of metformin and pioglitazone by reversed phase HPLC in pharmaceutical dosage forms. International Journal of Pharmacy and Pharmaceutical Sciences 1: 162-166.
- Sahoo PK, Sharma R, Chaturvedi SC (2008) Simultaneous Estimation of Metformin Hydrochloride and Pioglitazone Hydrochloride by RPHPLC Method from Combined Tablet Dosage Form.Indian J Pharm Sci 70: 383-386.
- Srinivas P, Venkataramana K, MangammaK (2012) Simultaneous determination of metformin and pioglitazone tablets in pharmaceutical dosage form by RP-HPLC method. International Journal of Pharmaceutical, Chemical and Biological Sciences 2: 104-109.
- Deepti Jain, Jain S, Jain D, Amin M (2008) Simultaneous Estimation of Metformin Hydrochloride, Pioglitazone Hydrochloride, and Glimepiride by RP-HPLC in Tablet Formulation. J ChromatogrSci 46: 501-504.
- Freddy HH, VairalDL (2010) Simultaneous estimation of metformin hydrochloride, rosiglitazone and pioglitazone hydrochloride in the tablets dosage form. International Journal of Applied Biology and Pharmaceutical Technology 1.
- Sultana N, SaeedArayne M, Nighat S, Siddiqui FA, HussainA (2011) Development and Validation of New Assay Method for the Simultaneous Analysis of Diltiazem, Metformin, Pioglitazone and Rosiglitazone by RP-HPLC and its Applications in Pharmaceuticals and Human Serum. J ChromatogrSci49: 774-779.
- Sankar ASK, VetrichelvanTV, VenkappayaD (2012) Simultaneous quantification and validation of five antidiabetic and hyperlipidemic drugs using isocratic RP-HPLC and its application to the marketed formulations. Journal of Pharmacy Research 5: 1217-1220.
- Ketan PD, Parthika AN,AgrawalYK (2011) Development and validation of spectrophotometric method for simultaneous estimation of gliclazide and metformin hydrochloride in bulk and tablet dosage form by simultaneous equation method. International Journal of Pharmaceutical Sciences and Research 2: 1559-1563.
- DhabalePN, SeerviCR (2010) Simultaneous UV Spectrophotometric Method for Estimation of Gliclazide and Metformine Hydrochloride in Tablet Dosage Form. Journal of ChemTech Research 2: 813-817.
- Radhika B, Rahul B, Mahendra KS, Vipin S, BhatK (2012) UV-spectrophotometric-assisted chemometric methods for the simultaneous determination of metformin hydrochloride and gliclazide in pharmaceutical formulations.Pharmaceut Anal Acta 3: 1-5.
- El-Wasseef DR (2012) Simultaneous determination of metformin, nateglinide and gliclazide in pharmaceutical preparations using micellar liquid chromatography.Int J Biomed Sci 8: 144-151.
- Fatema K, MdZakiur R, Tasnuva H, Mohammad Abdul KA, Reza MS (2010) Development and validation of a simple method for simultaneous estimation of metformin hydrochloride and gliclazide in tablets by using reversed phase high performance liquid chromatography. Dhaka University Journal of Pharmaceutical Sciences 9: 83-89.
- Vasudevan M, Ravi J, Ravisankar S, Suresh B (2001) ION-pair liquid chromatography technique for the estimation of metformin in its multicomponent dosage forms.J Pharm Biomed Anal 25: 77-84.
- Rao BU, NikaljeAP (2011) Determination of gliclazide in a tablet dosage form in the presence of metformin hydrochloride by ion pair – reversed phase liquid chromatographic technique. J Anal Bioanal Tech1: 105
- AnushaAkula, Prajwala N, SandhyaM, RaoDUM (2013) Development and validation of RP-HPLC method for simultaneous estimation of metformin hydrochloride and gliclazide in bulk and combined doasage form. International Journal of Pharmacy and Pharmaceutical Sciences 5: 511-517.
- Maria-cristinaR, Mihaela I, Lavinia H, Elena I, Valentina A, et al. (2009) Validation of a HPLC method for the simultaneous analysis ofmetformin and gliclazide in human plasma. Farmacia 57: 728-735.
- ZhongGP, Bi HC, Zhou S, Chen X, Huang M (2005) Simultaneous determination of metformin and gliclazide in human plasma by liquid chromatography-tandem mass spectrometry: application to a bioequivalence study of two formulations in healthy volunteers. J Mass Spectrom 40: 1462-1471.
- PatilSudarshan S,Bonde CG (2009) Development and validation of analytical method for simultaneous estimation of glibenclamide and metformin HCl in bulk and tablets using UV–visible spectroscopy. International Journal of ChemTech Research 1: 905-909.
- Sohrabi MR, Kamali N,Khakpour M (2011) Simultaneous spectrophotometric determination of metformin hydrochloride and glibenclamide in binary mixtures using combined discrete and continuous wavelet transforms. AnalSci 27: 1037-1041.
- Subhashini E, Sundhar BS (2014) New analytical method development and validation for the simultaneous estimation of metformin and glibenclamide in bulk and tablet dosage form using RP-HPLC. Rasayan 7: 55-63.
- Havele SS, Dhaneshwar SR (2014) Determination of glibenclamide, metformin hydrochloride and rosiglitazone maleate by reversed phase liquid chromatographic technique in tablet dosage form Chem. IndChemEng Q 20: 39-47.
- Rao BU, Nikalje AP (2010) Determination of glipizide, glibenlamide and glimeperide in a tablet dosage form in the presence of metformin hydrochloride by ion pair –reversed phase liquid chromatographic technique. J AnalBioanal Tech 1: 1-5.
- Georgita C, Albu F, David V, Medvedovici A (2007) Simultaneous assay of metformin and glibenclamide in human plasma based on extraction-less sample preparation procedure and LC/(APCI) MS.J Chromatogr B AnalytTechnol Biomed Life Sci 854: 211-218.
- BandarkaraFS, KhattabaIS (2010) Simultaneous estimation of glibenclamide, gliclazide, and metformin hydrochloride from bulk and commercial products using a validated ultra-fast liquid chromatography technique. Journal of Liquid Chromatography and Related Technologies 33: 1814-1830.
- Chaturvedi PK, Sharma R (2008) Development and validation of an RP-HPLC method for simultaneous analysis of a three-component tablet formulation containing metformin hydrochloride, pioglitazone hydrochloride, and glibenclamide.ActaChromatographica 20: 451-461.
- Aburuz S, Millership J, McElnay J (2005) The development and validation of liquid chromatography method for the simultaneous determination of metformin and glipizide, gliclazide, glibenclamide or glimperide in plasma.J Chromatogr B AnalytTechnol Biomed Life Sci 817: 277-286.
- Sultana N, Naveed S,Saeed A (2013) Development and validation of a simple and efficient RPLC method for analysis of captopril, metformin, pioglitazone and glibenclamide in API, formulations and human serum. Pharm Anal Acta 4: 1-6.
- Venkatesh P, Harisudhan T, Choudhury H, Mullangi R,Srinivas NR (2006) Simultaneous estimation of six anti-diabetic drugs-glibenclamide, gliclazide, glipizide, pioglitazone, repaglinide and rosiglitazone: development of a novel HPLC method for use in the analysis of pharmaceutical formulations and its application to human plasma assay. Biomed Chromatogr 20: 1043-1048.
- ChaturvediPK, Sharma R (2008) Simultaneous spectrophotometric estimation and validation of three component tablet formulation containing pioglitazone hydrochloride, metformin hydrochloride and glibenclamide. Analytical Letters 41: 2133-2142.
- NarsimhaRao D, Shanmugasundaram P, VaishnavH (2011) Method Development and Validated of Simultaneous Estimation of Metformin Hydrochloride, Pioglitazone Hydrochloride and Glibenclamide in Pure and Tablet dosage form by Spectrophotometric Multi component Method. International Journal of ChemTech Research 3: 2011-2017.
- Dhole SM, Pramod BK, AmnerkarND (2013) UV Spectrophotometric Absorption Correction Method for the Simultaneous Estimation of Pioglitazone HCl, Metformin HCl and Glibenclamide in Multicomponent Formulation. International Journal of Analytical and Bioanalytical Chemistry 3: 18-22.
- IPM Biotech(1996) ICH Topic Q 2 B. Validation of Analytical Procedures: Methodology.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18227
- [From(publication date):
specialissue-2015 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 17114
- PDF downloads : 1113