UTI-Related Bacteremia Due to Enterococcus faecalis: A Retrospective Case Control Study of Potential Risk Factors
Received: 10-May-2015 / Accepted Date: 08-Jun-2015 / Published Date: 12-Jun-2015 DOI: 10.4172/2161-1165.1000188
Abstract
Background: Bacteremia related to urinary tract infections (UTI) is associated with significant levels of morbidity and mortality as well as extensive financial costs for hospitals. Enterococcus faecalis accounts for the majority of enterococcal bacteremia with urinary tract infections reported as a frequent point of infection.
Aim: To investigate different epidemiological characteristics and hospital procedures as potential risk factors for the development of UTI-related bacteremia due to E. faecalis.
Methods: In a retrospective case control study, performed in the Capital Region of Denmark between 2010 and 2013, we compared 51 patients, with UTI-related bacteremia due to E. faecalis, to 56 patients, with urinary tract infections due to E. faecalis but without bacteremia, through univariate and multivariate analyses.
Findings: In the univariate analysis male-sex was positively associated with UTI-related bacteremia due to E. faecalis with an odds ratio of five and the association was highly significant (p-value<0.001). Catheterization, admission to a urological ward and urological surgery were also positively associated with bacteremia and the associations were likewise significant (for all, P<0.05). In the multivariate analysis, male-sex and urological surgery were found to be independent significant predictors of UTI-related bacteremia due to E. faecalis.
Conclusion: Male sex, urological surgery and catheterization are factors associated with UTI-related bacteremia due to E. faecalis.
Keywords: Bacteremia; Catheterization; Enterococcus faecalis ; Risk factors; UTI-Related; Urologic surgery; Urinary tract infection
163318Introduction
During the last decades Enterococcus species have become of increasing medical interest as human pathogens causing an increasing number of infections in both Europea and the US [1-3]. Enterococcus spp. are commensal inhabitants of the human gastrointestinal tract and rated as the second most common cause of urinary tract infections (UTI) and the third most common cause of bacteremia [4]. Enterococcus faecalis accounts for 65-90% of clinical Enterococcus spp. isolates [5,6]. Furthermore, UTIs are reported as a frequent focus of infection for enterococcal bacteremia [2,7].
Enterococcal infections are difficult to treat due to their intrinsic resistance towards cephalosporins and frequently acquired resistance to glycopeptides, penicillins and aminoglycosides [8]. Antibiotic-resistance in enterococci influences clinical outcomes drastically. Increase in mortality-rates for antibiotic-susceptible strains from 27% to 52% for strains resistant to glycopeptides has been reported (2). Today, Enterococcus spp. and in particular E. faecalis is recognized as an important human pathogen causing high levels of morbidity and mortality as well as extensive economic costs for hospitals. The emergence of E. faecalis as a common cause of bacteremia, emphasize the need for more knowledge about UTI-related bacteremia due to E. faecalis.
Previous studies have investigated UTI-related bacteremia in general and enterococcal bacteremia, but none have investigated UTI-related bacteremia due to E. faecalis specifically. The aim of this retrospective Danish population-based case-control study is to investigate a number of potential risk factors for UTI-related bacteremia due to E. faecalis.
Methods
Research ethical approvals
Research ethical approvals had been given by The Regional Committee of Danish Data Protection Agency.
Setting
This retrospective case-control study was done for patients with UTI-related bacteremia due to E. faecalis in the Capital Region of Denmark between 2010 and 2013. A single tertiary referral hospital in the region (Rigshospitalet) did not participate in the study. The Capital Region of Denmark has a population of 1.7 million people. Each Danish citizen has a unique personal identification number, which make cross-database linkage possible for each individual [9,10].
Identification of patients
We defined a case of UTI-related bacteremia due to E. faecalis bacteremia as any adult patient (>18 years) meeting the following criteria: (i) A positive blood sample containing E. faecalis taken no later than three weeks after a positive urine sample for E. faecalis or Enterococcus spp. of >103-bacteria/ml urine. (ii) Hospital admission within 30 days of the positive blood sample.
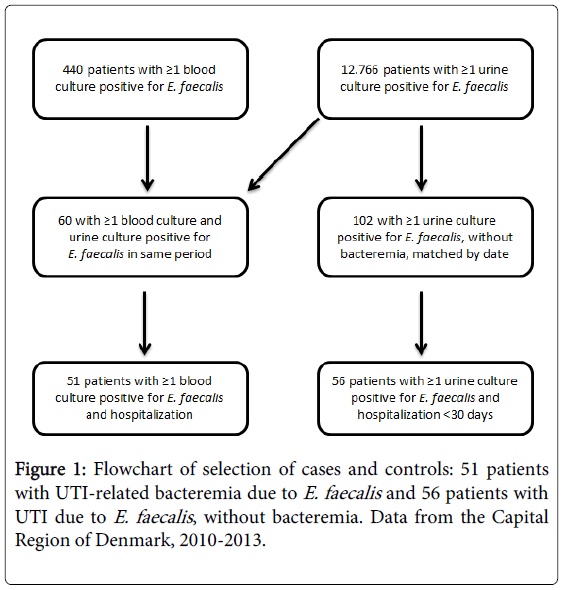
Subsequently, we picked patients for the control group by matching each of the bacteremia-cases with patients who had a urine sample positive for E. faecalis in the same week as the case-patient had the positive blood sample. The criteria for the control group were: (i) A urine sample positive for E. faecalis. (ii) No concurrent blood culture positive for enterococcal infection of the bloodstream. (iii) Hospital admission <30 days to the positive urine sample. Figure 1 shows the flowchart of the selection of cases and controls.
We reviewed medical records for each patient for the period of 30 days prior to the positive blood sample for the cases and 30 days prior to the positive urine sample for the control group, respectively. We noted details on hospital admission and stay, patient diagnoses and medical and surgical procedures to identify potential risk factor for UTI-related bacteremia due to E. faecalis.
The variables investigated included quantity of E. faecalis in patients’ urine samples, epidemiologic characteristics of patients, catheterization (such as urinary catheters, nephrostomy or JJ-stents), urological surgery (such as cystoscopy, prostate or ureteral surgery) and surgery overall.
The microbiological data available did not contain isolate information. Thus, we were not able to retrieve information on antibiotic susceptibility. Furthermore, data on prophylactic antibiotic treatment in association to surgical procedures were not available.
Statistical analysis
We compared cases and controls on specific descriptive and demographic characteristics at first. Subsequently we analyzed the data by means of univariate analysis and multivariate logistic-regression analysis for associations as well as statistical significance of these associations.
We did a univariate and a multivariate analysis for the following potential risk factors: Male sex, E. faecalis quantity of urine sample, catheterization, admission to urological ward, urological procedures or surgery and surgery overall. A p-value of <0.05 was considered significant and the software STATISTICA (StatSoft) used to calculate p-values.
Results
Patient characteristics are listed in Table 1. Results for the univariate and multivariate analyses are found in Tables 2 and 3, respectively. Male-sex was positively associated with the risk of UTI-related bacteremia due to E. faecalis with an odds ratio of five. Catheterization, admission to a urological ward and urological surgery were all also positively associated with bacteremia and the association was significant (for all, P<0.05) (Table 2).
| Characteristic | No. of cases/value | % of cases | No. of controls/value | % of cases |
|---|---|---|---|---|
| Age, mean ± SD, years | 77 (±12.5) | 68.5 (±17.3) | ||
| Median of E. faecalis quantity in urine sample (range: 103-105/ml) | 105/ml | 105/ml | ||
| Male | 43 | 84 | 29 | 52 |
| Catheterization | 29 | 57 | 17 | 30 |
| Admission to urological ward | 17 | 33 | 9 | 16 |
| Urological surgery | 10 | 20 | 3 | 5 |
| Surgery | 16 | 31 | 22 | 36 |
Table 1: Descriptive and demographic characteristics, 51 cases vs. 56 controls.
| Risk factors | No. of cases | (%) | No. of controls | (%) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Male | 43 | 84% | 29 | 52% | 5.0 (2.0-12.5) | <0.001 |
| Catheterization <30 days | 29 | 57% | 17 | 30% | 3.0 (1.4-6.7) | 0.006 |
| Admission to urological ward <30 days | 17 | 33% | 9 | 16% | 2.6 (1.04-6.56) | 0.037 |
| Urological surgery <30 days | 10 | 20% | 3 | 5% | 4.3 (1.1-16.7) | 0.024 |
| Other surgery <30 days | 16 | 31% | 22 | 39% | 0.7 (0.3-1.6) | 0.39 |
Table 2: Univariate analysis of risk factors present for E. faecalis bacteremia <30 days prior to the positive blood or urine sample, 51 cases vs. 56 controls.
| Risk factors | p-value |
|---|---|
| Male | 0.003 |
| Catheterization <30 days | 0.067 |
| Urologicalsurgery <30 days | 0.044 |
Table 3: Multivariate analysis, multiple logistic regression, alpha: 0.05.
In the multivariate analysis, male-sex and urological surgery were found to be independent significant predictors of UTI-related bacteremia due to E. faecalis (Table 3).
Discussion
A better understanding of the factors increasing the risk of enterococcal bacteremia might enable the healthcare system to take appropriate precautions when treating patients at particular risk. In this retrospective Danish population-based case-control study, we investigated the potential risk factors for UTI-related bacteremia due to E. faecalis.
Genitourinary infections are specifically associated with bacteremia due to E. faecalis. Previously identified predictors for UTI-related bacteremia are indwelling urethral catheters, obstructive urological diseases, male sex, immunosuppressant therapy, cigarette use within 5 years, diabetes mellitus for patients older than 70 and prior antibiotic treatment [11-13]. This study extends prior work by investigating surgery overall, male sex, catheterization and urologic surgical procedures as risk factors, specifically for UTI-related bacteremia due to E. faecalis.
We found male sex and urological surgery to be independent risk factors for UTI-related bacteremia due to E. faecalis. Furthermore, catheterization and admission to a urological ward were positively associated with bacteremia.
Previous studies have evaluated different gender distributions for enterococcal bacteremia and have found a male majority ranging from 53% to 68% of cases [2,14-17]. Men might be at an overall greater risk of UTI-related bacteremia due to E. faecalis, compared to bacteremia from other Enterococcus species and other UTI-related bacteremia in general [13]. Gender-specific differences in the risk for bacteremia can be used to take appropriate precautions when treating or operating male patients. By taking into account, the level of risk estimated for the individual patient, the hospital can chose appropriate antibiotic prophylaxis before urological surgery or catheterization. We did not have access to gender ratio data at the urological wards in the Capital Region of Denmark nor did we have any microbiological data on strains available. Such information would have been useful in order to investigate if the majority of males, with UTI-related bacteremia due to E. faecalis found in this study, were simply representing the overall gender distribution of patients undergoing manipulation of the urinary tract through e.g. catheterization or urological surgery.
Other studies examining the effect of urinary catheters on enterococcal bacteremia or UTI-related bacteremia also found a significant association, supporting the findings of the univariate analyses of this study [7,14,17-19]. The positive association of urinary catheterization and bacteremia is likely to be a result of a disruption of the urothelium exposing the blood stream for bacteria from the urinary tract. Jerkeman and Braconier, 1992, found that urinary catheters were more common in a bacteremic patient group compared to a non-bacteremic group [15]. Similar to catheterization, urological surgery entails a risk to disrupt the urothelium and expose the blood stream for bacteria, setting the patient at risk for bacteremia. In this study, 20% of the patients with UTI-related bacteremia had urological surgery within 30 days prior to the positive blood sample. Notably, the data from the patients examined did not show any association between surgery overall and UTI-related bacteremia due to E. faecalis. In support of this, Caballero-Granado et al. did not find surgical treatment of all locations to be a risk factor for enterococcal bacteremia [20] and another study found postoperative bacteremia to be associated with urological procedures [21].
In the case of preventing bacteremia following manipulation of the urinary tract, a urine culture prior to catheterization or urological operation could reveal infections of the urinary tract. Subsequently, the treating physicians could initiate appropriate antibiotic prophylaxis based on typing of the bacteria and identification of resistance-patterns.
In the two urological wards in the Capital Region of Denmark, it is mandatory to take a pre-operative urine sample from patients undergoing extracorporeal shock wave lithotripsy, endoscopy of the urinary tract or surgery of the kidney. Several studies have confirmed the association of enterococcal urinary tract infections with bacteremia. However, the Danish guidelines for antimicrobial prophylaxis prior to urological procedures like extracorporeal shock wave lithotripsy, transurethral procedures, prostate biopsies and insertion of catheters does not cover enterococci. The guidelines advise for the use of gentamicin and ciprofloxacin for prophylaxis, but E. faecalis is frequently resistant towards these antibiotics [22,23].
A single-dose of antimicrobial prophylaxis has been shown to be effective to prevent infections related to urological surgery [24]. Another important aspect of antibiotic treatment is that inappropriate use bears the risk to prompt development and spread of antibiotic resistance [20]. All this call for individualized antibiotic prophylaxis based on urine samples prior to manipulation of the urinary tract. Trials with a randomized controlled study-design are necessary to assess the efficacy of individual antimicrobial prophylaxis prior to urological procedures to prevent enterococcal bacteremia.
This study has some limitations and thus the findings should be considered in the context of these.
First, the retrospective study-design gives rise to some uncertainty regarding the identified cases. We did not have information on the focus of infection for the cases of bacteremia. A prospective study is a more robust study design, which could have enabled a contemporary examination of each patient by medical staff. Second, we did not have access to the necessary antimicrobial resistance patterns or molecular typing methods to confirm that the E. faecalis isolates from the blood and urine were identical for each of the patients in the case group. Thus, we cannot be certain that each bacteremia case reflected a primary urinary tract infection. However, the convergent E. faecalis infection of both blood stream and urinary tract make the association plausible. Third, we did not evaluate the antibiotics administered to the patients in the two groups. However, this would only have been useful if we had information on the resistance patterns of the strains isolated from each patient. Fourth, the statistical strength of this study is low due to the small size of the patient groups evaluated and thus the findings might be limited. Larger studies are necessary to confirm the results. The results of this study should therefore not be interpreted as more than indicators of associations between the investigated variables and the risk of bacteremia due to E. faecalis.
Finally, there are other risk factors previously identified for enterococcal bacteremia and UTI-related bacteremia. To illuminate the interactions of the different risk factors they must all be evaluated in the same study. Other enterococcus species should also be included, in particular E. faecium, to analyze similarities and differences between the two predominant species’.
Conclusion
In conclusion, we found male sex, urological surgery and catheterization to be associated with UTI-related bacteremia due to E. faecalis. Individual antimicrobial prophylaxis for specific patient subgroups at risk might be a way to reduce this problem. Appropriate antibiotic treatment following a urine sample to identify a possible urinary tract infection can ideally enhance the safety of patients at risk of bacteremia due to E. faecalis.
References
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis309-317.
- Pinholt M, Ostergaard C, Arpi M, Bruun NE, Schønheyder HC, et al. (2013) Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006-2009: a population-based cohort study. ClinMicrobiol Infect1-7.
- Caballero-granado FJ, Cisneros JM, Luque R, Villanueva JL, Pe R, et al. (1998)Infecciosas LASE: Comparative Study of Bacteremias Caused by Enterococcus spp . with and without High-Level Resistance to Gentamicin 36:520-525.
- Noble CJ (1978) Carriage of group D streptococci in the human bowel. J ClinPathol 31:1182-1186.
- Moellering RC (1992) Emergence of Enterococcus as a Significant Pathogen 14:1173-1176.
- Fisher K, Phillips C (2009) The ecology, epidemiology and virulence of Enterococcus. Microbiology155:1749-1757.
- Graninger W, Ragette R (1992) Nosocomial bacteremia due to Enterococcus faecalis without endocarditis. Clin Infect Dis 15:49-57.
- Werner G, Coque TM, Franz CMaP, Grohmann E, Hegstad K, et al. (2013) Antibiotic resistant enterococci-Tales of a drug resistance gene trafficker. Int J Med Microbiol 303:360-379.
- Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB (2006) The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull 53:441-449.
- Pedersen CB (2011) The Danish Civil Registration System. Scand J Public Health 39:22-5.
- Krieger JN, Kaiser DL, Wenzel RP (1983) Urinary tract etiology of bloodstream infections in hospitalized patients. J Infect Dis 148:57-62.
- Saint S, Kaufman SR, Rogers MaM, Baker PD, Boyko EJ, et al. (2006) Risk factors for nosocomial urinary tract-related bacteremia: A case-control study. Am J Infect Control 34:401-407.
- Greene MT, Chang R, Kuhn L, Rogers MaM, Chenoweth CE, et al. (2012) Predictors of hospital-acquired urinary tract-related bloodstream infection. Infect Control HospEpidemiol 33:1001-1007.
- Malone DA, Wagner RA, Myers JP, Watanakunakorn C (1986)Enterococcalbacteremia in two large community teaching hospitals. Am J Med 81:601-606.
- Jerkeman M, Braconier JH (1992) Bacteremic and non-bacteremic febrile urinary tract infection - a review of 168 hospital-treated patients. Infection 20:143-145.
- Chang R, Greene MT, Chenoweth CE, Shuman E, Rogers MAM, et al. (2012) Epidemiology of Hospital-Acquired Urinary Tract-Related Bloodstream Infection at a University Hospital32:1127-1129.
- Kayser FH (2003) Safety aspects of enterococci from the medical point of view. Int J Food Microbiol 88:255-262.
- Chang R, Greene M (2011) Epidemiology of hospital-acquired urinary tract-related bloodstream infection at a university hospital. HospEpidemiol 32:1127-1129.
- Noskin GA, Peterson LR, Warren JR (1995) Enterococcus faecium and Enterococcus faecalisbacteremia: acquisition and outcome. Clin Infect Dis 20:296-301.
- Caballero-Granado FJ, Becerril B, Cisneros JM, Cuberos L, Moreno I, et al. (2001) Case-control study of risk factors for the development of enterococcalbacteremia. EurJ ClinMicrobiol Infect Dis 20:83-90.
- Eykyn S (1982) The microbiology of postoperative bacteremia. World J Surg268-272.
- Brown DFJ, Brown NM, Cookson BD, Duckworth G, Farrington M, et al. (2006) National glycopeptide-resistant enterococcal bacteraemia surveillance working group report to the Department of Health - August 2004. J Hosp Infect 62.
- Woodford N, Reynolds R, Turton J, Scott F, Sinclair A, et al. (2003) Two widely disseminated strains of Enterococcus faecalis highly resistant to gentamicin and ciprofloxacin from bacteraemias in the UK and Ireland. J AntimicrobChemother711-714.
- Togo Y, Tanaka S, Kanematsu A, Ogawa O, Miyazato M, et al. (2013) Antimicrobial prophylaxis to prevent perioperative infection in urological surgery: a multicenter study. J Infect Chemother 19:1093-1101.
Citation: Hansen KG, Hertz FB, Rasmussen SC, Frimodt-Møller N (2015) UTI-Related Bacteremia Due to Enterococcus faecalis: A Retrospective Case Control Study of Potential Risk Factors. epidemilogy (sunnyvale) 5:188. DOI: 10.4172/2161-1165.1000188
Copyright: © 2015 Hansen KG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 26290
- [From(publication date): 6-2015 - Mar 30, 2025]
- Breakdown by view type
- HTML page views: 21584
- PDF downloads: 4706