Utilizing UL97 Resistant Codon Specificities to Guide Pharmacotherapy Treatment Decisions in Resistant Cytomegalovirus Infections
Received: 28-Feb-2018 / Accepted Date: 22-Mar-2018 / Published Date: 26-Mar-2018 DOI: 10.4172/2475-7640.1000119
Abstract
The development of cytomegalovirus (CMV) drug resistance has become an emerging clinical challenge. In kidney transplant recipients (KTR), CMV infection is a risk factor for acute rejection, has a detrimental effect on patient survival, and predisposes patients to secondary opportunistic infections. We report here the case of a kidney transplant patient on valganciclovir prophylaxis who developed recurrent CMV infections, complicated by UL97 ganciclovir (GCV) resistance. Our patient underwent unsuccessful treatment courses with high dose GCV, foscarnet and cidofovir. Compassionate use maribavir was initiated and resulted in full recovery. Through this case report and literature review, we highlight potential strategies for the successful management of CMV resistance.
Keywords: cytomegalovirus, transplantation, transplant recipient, bone marrow suppression, immunosuppression.
Introduction
Cytomegalovirus (CMV) infections are a common cause of morbidity and mortality in solid organ transplant recipients [1,2]. Currently, ganciclovir (GCV), valganciclovir (VGCV), cidofovir (CDV), and foscarnet (FOS) have Food and Drug Administration (FDA) marketing approval for the systemic treatment of CMV infection [3] Unfortunately, viral resistance is a potential problem with the current drug armamentarium [4]. Herein, we present a CMV multidrug-resistant case report and discuss how specific mutations and adverse effect profiles are important in the selection of alternative therapies.
Case Report
A 34-year-old African-American male received a deceased donor renal transplant secondary to sickle cell disease at Vidant Medical Center in Greenville, North Carolina. He received anti-thymocyte globulin induction along with a standard steroid taper and was discharged on mycophenolate sodium, tacrolimus, and prednisone for maintenance immunosuppression. Tacrolimus trough goals were 8-10 ng/mL for the first 3 months, followed by 5-8 ng/mL thereafter. CMV serology was donor-positive, recipient negative (D+/R-). Patient was discharged on post-operative day 4 on VGCV prophylaxis at a dose of 450 mg twice weekly according to his renal function (Table 1) with an anticipated course of 6 months. Approximately 3 months after transplantation, the patient had asymptomatic CMV viremia with a quantitative real-time polymerase chain reaction (PCR) assay viral load of 15,034 IU/mL upon routine screening (Artus CMV RGQ MDx Kit US, Qiagen, Germantown, MD, LLOQ 159 IU/mL). At the time, he had been maintained on VGCV 450 mg daily but had been a candidate for a dose increase to 900 mg daily 2 months prior due to improved renal function. Given inadequate dosing, codons 457-630 of the UL97 gene and codons 393-1000 of the UL54 gene were sequenced and mutations associated with GCV, CDV, and FOS were assessed (CMV Antiviral Drug Resistance PCR/Sequencing, ARUP Laboratories, Salt Lake City, Utah), (Sample 1), and the patient was initiated on treatment dose VGCV 900 mg twice daily with a concurrent dose reduction of immunosuppression.
| CrCl (mL/min) Cockcroft-Gault | Treatment dose | Prophylaxis dose |
|---|---|---|
| 60 or greater | 900 mg twice daily | 900 mg once daily |
| 40 to 59 | 450 mg twice daily | 450 mg once daily |
| 25 to 39 | 450 mg once daily | 450 mg every 2 days |
| 10 to 24 | 450 mg every 2 days | 450 mg twice weekly |
| less than 10 (on hemodialysis) | not recommended | not recommended |
Table 1: VGCV Prophylaxis and treatment dosing per creatinine clearance.
Initial episode
The patient was admitted two weeks after initial positive screening due to leukopenia, diarrhea and increasing viral loads despite optimal VGCV treatment. Resistance testing (UL97 mutation at the C607F codon) resulted in a change from VGCV therapy to high dose intravenous GCV therapy at 10 mg/kg twice daily with adjunctive 100 mg/kg CMV intravenous immune globulin (CMVIG). Immunosuppression was further reduced with cessation of mycophenolic acid, and TAC trough goal reduction to 3-5 ng/mL. The patient was discharged with home infusion IV GCV for the following 4 weeks. Due to leukopenia severity, the use of granulocyte colony stimulating factor (G-CSF) to maintain an absolute neutrophil count (ANC)>500 was necessary. However, this exacerbated the patient’s sickle cell disease causing hip and bone pain. A transition to VGCV 900 mg twice daily for the maintenance phase of treatment was initiated for one month.
Second episode
With the resolution of viral replication, VGCV was decreased to prophylaxis dosing at 900 mg daily to mitigate bone marrow suppression. Unfortunately, this resulted in reoccurrence of CMV viremia. Despite increasing VGCV back to treatment dose 900 mg twice daily, CMV titers continued to rise and the patient was reinitiated on high dose IV GCV. Repeat resistance testing (Sample 2) indicated unchanged UL97 mutation. Due to the antiviral potential of mammalian target of rapamycin inhibitors (mTOR-I), the patient was converted from TAC to everolimus. Despite successful conversion, the patient experienced mTOR-I induced anemia with no improvement in viral loads thus was re-initiated on TAC. Due to low quality of life from the continued need for G-CSF and periodic transfusions due to GCV bone marrow suppression, foscarnet (90 mg/kg IV every 12 hours) was initiated in an attempt to avoid GCV side effects, while acknowledging the risk of nephrotoxicity with foscarnet therapy. The patient completed 28 days of FOS therapy with complete resolution of CMV viremia 5 days into therapy. This was at the cost of serum creatinine increase from a baseline of 1.0 mg/dL to a peak of 2.67 mg/dL and subsequent decline to a new baseline of 1.5 mg/dL and he was to continue VGCV treatment dose indefinitely.
Third episode
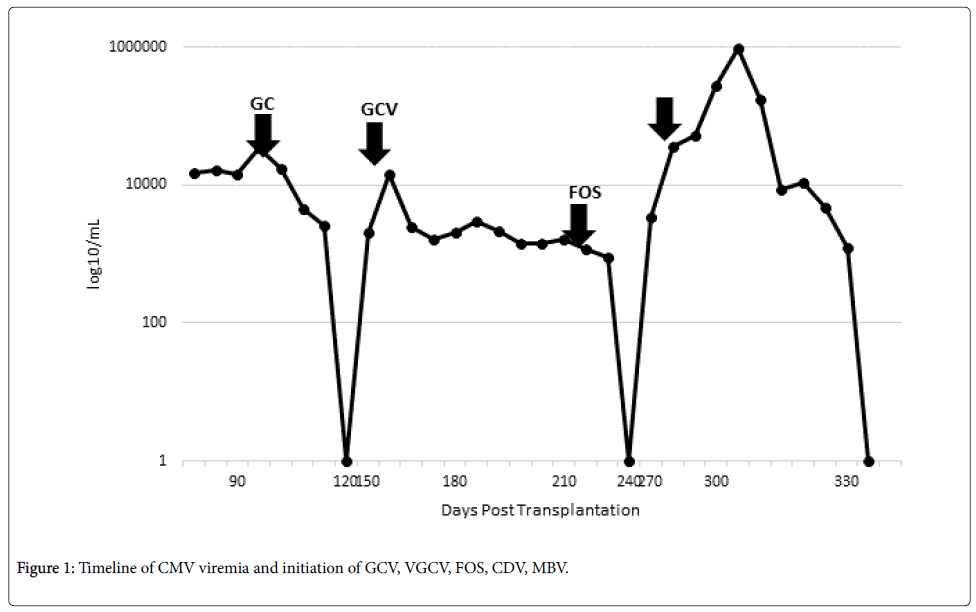
The patient became viremic 44 days after viral clearance from the second episode. Repeat resistance panel (Sample 3) results were unchanged. Given the patient’s complicated history with ganciclovir and severe renal impairment demonstrated with foscarnet, the decision was made to proceed with compassionate use maribavir (SHP620, Shire Pharmaceuticals). The patient did not qualify for current phase 2 studies due to severe neutropenia, current sickle cell crisis, and acute kidney injury. CMV viral load went from 3,423 IU/mL on admission to a peak of 948,321 IU/mL and a single dose of cidofovir 5 mg/kg was given while awaiting maribavir (MBV) approval. The patient became profoundly pancytopenic but was otherwise asymptomatic. MBV 400 mg orally twice daily every 12 hours was initiated with a decrease in the viral load to 174,546 IU/mL 4 days later. The viral load continued to decrease with sustained viral response 21 days after treatment initiation. The patient completed 8 weeks of MBV therapy and has not relapsed as of the time of this report (Figure 1).
Discussion
The therapeutic management of drug-resistant CMV infection remains a challenge. Ganciclovir resistance is associated with significantly increased morbidity and mortality compared to GCVsusceptible CMV [5,6]. In the absence of randomized trials evaluating treatment options, guideline recommendations are based on expert opinion [7]. Selection of anti-CMV therapy involves an understanding of the mechanism of action of the various agents and the levels of resistance conferred by specific CMV mutations. In our patient, GCV resistance was associated with an initial high viral load that could not be cleared with high dose ganciclovir and demonstrated marked adverse reactions to cidofovir and foscarnet. GCV, CDV, and FOS are all potent inhibitors of CMV DNA polymerase. These agents however differ in their cellular activation, which lends itself to different levels of activity against resistant CMV strains [8].
Point mutations in gene UL54 confer CMV resistance to GCV, CDV, and FOS. In addition, resistance to GCV has been mapped to mutations in gene UL97 [3,9]. Studies suggest that mutations M460V/I, H520Q, C592G, A594V, L595S, C603W, L595F/W represent the most frequent UL97 mutations present in GCV-resistant strains [7,9-11]. Marker transfer studies suggest that M460V/I, H520Q, A594V, L595S, and C603W mutations result in moderate resistance given a 5 to 15-fold increase in GCV IC50, which makes clinical doses of GCV inadequate to achieve sufficient concentrations [7,12]. In contrast, a point mutation in the 607 codon may yield various degrees of GCV resistance. For example, C607Y confers a 5 to 15-fold increase in IC50, while a 2 to 5-fold increase in IC50 is seen with a C607F mutation [7,9]. Given these findings, the UL97 C607F mutation observed in our patient was believed to be associated with low-level resistance and amenable to high dose IV GCV (10 mg/kg BID). Few reports describing rare codon mutations including codon 607 have been published. Baldanti and colleagues demonstrate that a cysteine to tyrosine substitution in position 607 reduces GCV kinase activity resulting in GCV resistance. The authors hypothesize that positions 590 to 607 on UL97 phosphotransferase may be involved in substrate recognition [12].
With limited options for our patient after dose limiting toxicity to GCV and FOS, and continued relapse on VGCV, MBV was obtained under compassionate use. MBV is a potent anti-CMV drug that inhibits UL97 protein kinase resulting in a downstream interference with viral nucleocapsid egress from the nucleus [4]. Despite promising early clinical trial data, MBV remains investigational after phase III clinical trials showed inadequate antiviral efficacy at doses tested [13]. Avery et al. describe use of MBV as a salvage treatment in 6 immunocompromised patients with drug-resistant CMV infection. Four of 6 patients had no detectable CMV DNAemia within 6 weeks of starting MBV therapy. Notably, one patient with a high initial viral load developed MBV resistance mutations. In addition, the authors note that 2 of the patients who originally cleared CMV DNA on MBV had recurrences after discontinuation of MBV for 2-3 months and required re-treatment with MBV [14]. A phase II randomized study assessing MBV 400–1200 mg BID for resistant CMV infections was effective for treatment of CMV infection resistant/refractory to standard therapy among transplant recipients. Among all MBV doses, 67% of the patients had undetectable plasma CMV viral loads within 6 weeks; however, 78% experienced treatment emergent related adverse events and 35% had recurrent CMV infection [15].
At our center, the decision to initiate CMV resistance testing is primarily ad hoc and predicated on two factors: exposure of the patient to subtherapeutic oral VGCV dosing per renal function (Table 1), or failure to elicit a decrease in viral loads or symptomatic improvement within 7 days of antiviral therapy (two quantitative CMR PCR values separated by at least 72 hours) although current consensus guidelines recommend evaluating for resistance if there is no improvement after 2 weeks of treatment, or relapses in either the clinical or virologic response [7]. In addition to a patient’s D+/R-status, severe immunosuppression is a significant host risk factor for drug resistance [7]. As such; our patient’s immunosuppressive regimen was modified by either the cessation of mycophenolic acid, decrease in tacrolimus dose or substitution of tacrolimus with everolimus. Given the patient’s severe CMV disease, changes in immunosuppressive therapies were accompanied with the use of anti-CMV therapies and the impact of a reduction in the level of immunosuppression cannot be assessed in isolation.
Conclusion
Decisions on GCV-resistance management in our patient were based on genotypic analysis of CMV genes, disease severity and adverse event profiles. This patient was successfully treated with MBV, and highlights the need for a concerted effort in developing new anti- CMV agents and optimizing current treatments for GCV-resistant infections.
Conflict of Interest
The Authors declares no Conflicts of Interest regarding the publication of this article.
References
- Hakimi Z, Aballéa S, Ferchichi S (2017) Burden of cytomegalovirus disease in solid organ transplant recipients: A national matched cohort study in an inpatient setting. Transpl Infect Dis: 19.
- Hamouda M, Kahloun R, Jaballah L, (2016) Cytomegalovirus ocular involvement in a kidney transplant recipient. Exp Clin Transplant.
- Biron KK (2006) Antiviral drugs for cytomegalovirus diseases. Antiviral Res 71: 154-63.
- Rolling KE, Jorgenson MR, Descourouez JL, Mandelbrot DA, Redfield RR, et al. (2017) Ganciclovir-resistant cytomegalovirus infection in abdominal solid organ transplant recipients: Case series and review of the literature. Pharmacotherapy. 37: 1258-1271.
- Myhre HA, Haug Dorenberg D, Kristiansen KI. (2011) Incidence and outcomes of ganciclovir-resistant cytomegalovirus infections in 1244 kidney transplant recipients. Transplantation 92: 217-23.
- Fisher CE, Knudsen JL, Lease ED, Jerome KR, Rakita RM, et al. (2017) Risk factors and outcomes of ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients. Clin Infect Dis 6: 57-63.
- Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, et al. (2013) Transplantation Society International CMV Consensus Group. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 96: 333-60.
- Gilbert C, Boivin G (2005) Human cytomegalovirus resistance to antiviral drugs. Antimicrob Agents Chemother 49: 873-83.
- Chou S, Waldemer RH, Senters AE, Michels KS, Kemble GS, et al. (2002) Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J Infect Dis 185: 162-9.
- Chou S, Ercolani RJ, Marousek G, Bowlin TL (2013) Cytomegalovirus UL97 kinase catalytic domain mutations that confer multidrug resistance. Antimicrob Agents Chemother 57: 3375-9.
- Kleiboeker S, Nutt J, Schindel B, Dannehl J, Hester J (2014) Cytomegalovirus antiviral resistance: characterization of results from clinical specimens. Transpl Infect Dis 16: 561-7.
- Baldanti F, Underwood MR, Talarico CL, Simoncini L, Sarasini A, et al. (1999). The Cys607Tyr change in the UL97 phosphotransferase confers ganciclovir resistance to two human cytomegalovirus strains recovered from two immunocompromised patients. Antimicrob Agents Chemother 42: 444-6.
- Marty FM, Boeckh M (2011) Maribavir and human cytomegalovirus-what happened in the clinical trials and why might the drug have failed? Curr Opin Virol 1: 555-62.
- Avery RK, Marty FM, Strasfeld L, Lee I, Arrieta A, et al. (2010) Oral maribavir for treatment of refractory or resistant cytomegalovirus infections in transplant recipients. Transpl Infect Dis 12: 489-96.
- Papanicolaou G, Silveira FP, Langston A, Avery R, Wijatyk A, et al. (2017) Maribavir for treatment of cytomegalovirus infections resistant or refractory to ganciclovir or foscarnet in hematopoietic stem cell transplant or solid organ transplant recipients: A randomized, dose-ranging, double-blind, phase 2 study. Biol Blood Marrow Transplant 23: S18-S51-S52.
Citation: Maldonado AQ, Leeser DB, Asempa TE (2018) Utilizing UL97 Resistant Codon Specificities to Guide Pharmacotherapy Treatment Decisions in Resistant Cytomegalovirus Infections. J Clin Exp Transplant 3: 119. DOI: 10.4172/2475-7640.1000119
Copyright: © 2018 Maldonado AQ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4298
- [From(publication date): 0-2018 - Dec 22, 2024]
- Breakdown by view type
- HTML page views: 3662
- PDF downloads: 636