Utilization of Solar Energy as a Fuel in Production of Energy Intensive Industrial Products
Received: 17-Apr-2019 / Accepted Date: 30-Apr-2019 / Published Date: 05-May-2019
Abstract
Fossil fuels such as petrol, diesel, coal and natural gas are used as energy source in the production of domestic, commercial and industrial products such as hydrogen, nitrogen, oxygen, ammonia, urea and clean water. There are environmental degradation problems associated with the burning of these fuels due to combustion process associated. It is evaluated whether it is possible to utilize an alternative renewable energy source and process that can be used in the production of these industrial products. In this paper, an alternative energy fuel and processes are devised for the production of oxygen, hydrogen (2H2O→2H2+O2), nitrogen, ammonia (N2+3H2→2NH3) and urea (NH3+CO2→(NH2)2CO+H2O) and clean water using water electrolysis, air separation, ammonia, urea synthesis and oxy fuel combustion process. The amounts of these products that can be produced are calculated on basis of energy and mass of ingredient products required at all stages in these processes. The results reveal that solar energy powered alternative production processes can be utilized in the production of nitrogen, hydrogen, oxygen, ammonia, urea and clean water in an environmentally sustainable manner. It is calculated that for 56.6×103 kg of ammonia, 0.77×103 kg of carbon dioxide would be required to produce 1.06 ×103 kg of urea and 0.31 m3 water.
Keywords: Solar electrical energy; Urea synthesis production; Environmental sustainability
Introduction
Optimization of conventional production process of energy intensive domestic, commercial and industrial products is important for gradual switchover of traditional fossil fuels in domestic, commercial and industrial sector. Fossil fuels are limited and are not environmental friendly [1,2]. Application of alternative energy fuel and process in these sectors of economy could provide a source for minimization the role of fossil fuels and considerable reduction in environmental pollution problems. It is believed that if alternative energy source and processes are applied in production sector it can yield positive results. Therefore, the need of optimization of production process is felt by energy experts generally and urea industry specifically [3]. Such energy and process alternatives can facilitate the objective of utilization of an environmentally sustainable and energy efficient energy source in energy intensive commodities sector.
Renewable energy sources utilization varies accordingly. If solar energy is utilized an alternative electrical energy source. It can attain an objective of energy accessibility, reliability, higher energy conversion efficiency and environmental sustainability. According to a study, solar energy based electrical power generation is more efficient than electrical power generated from fossil fuels, because fossil-fuel energy to electricity conversion efficiency is 40%, which is less in comparison to the Rankine cycle, which can attain solar power conversion beyond 40% [4].
Both thermal and photovoltaic solar energy utilization methods can be used reliably on small, medium and large scale as an energy fuel in the production of useful commodities in the solar-rich countries. However, concentrating solar photovoltaic power generation systems are favored over thermal systems on account of increasing the efficiency of the cells, high efficiency, low system cost and a low capital investment as well as use of less expensive semiconducting PV material to achieve a specified electrical output [5].
Positive impacts of solar energy utilization in terms of improvement in standard of living in solar-rich countries can be served. Due to the increase in installed capacity of solar energy utilization, the standard of living has been raised considerably in developing countries [6]. These projects have contributed considerably towards accessibility to electrical power. Therefore, solar energy seems to be a viable option for supply of basic amenities to people living in solar-rich countries [7].
The role of solar energy in production of clean water is also considerable. High radiation solar energy can be utilized to convert underground aquifer brackish water o portable clean water in an environmentally sustainable manner with an objective to address the problems of lack of not only potable water but also of dryness and land infertility [8,9]. Solar energy can be utilized for pumping underground water to the land surface for irrigation of fertile land, which is helpful for countries having agrarian economy. Therefore solar energy can be used as a reliable substitute to fossil fuels, on account of its capability to meet energy needs in present and in future in an environmentally sustainable manner [10].
Solar energy based electrical power generation and freshwater production processes are used on small, medium and large scale in domestic, commercial and industrial sectors in solar-rich areas [11- 15].Utilization scale depends on user needs and level of conventional electrical power and water supply systems. Solar energy can be utilized in the production of domestic, commercial and industrial products such as; hydrogen, nitrogen, oxygen, using from water electrolysis, air separation, ammonia synthesis and urea synthesis processes. These products can be obtained by chemical reaction/processing of these substances. Hydrogen can be obtained from water using electrolysis process and nitrogen can be obtained from air through air separation process; nitrogen can be synthesized with hydrogen to get ammonia in the ammonia synthesis process.
CO2 and ammonia can be utilized to produce urea by use of the urea synthesis process. In urea synthesis process, ammonia and carbon dioxide are fed into the reactors at high pressure (240 barg), and the urea solution is then concentrated to give 99.6% molten urea, and granulated for use as fertilizer and chemical feedstock [11].The major impurities from the urea production reaction, including water and unconsumed reactants such as; ammonia, carbon dioxide and ammonium carbamate can be removed in three stages. Firstly, the pressure is reduced from 240 to 17 barg and the solution is heated, which causes the ammonium carbamade to decompose into ammonia and carbon dioxide. The pressure is then reduced to 2.0 barg and finally to -0.35 barg, with more ammonia and carbon dioxide being lost at each stage, and a solution of urea is dissolved in water, free of all impurities [11]. The drain water obtained from the urea synthesis process can be treated further to convert it to a pure form, which could be utilized for drinking and for external use.
Oxygen obtained at electrolysis and air separation process can be utilized in oxy-fuel combustion process. Oxygen, can be utilized in the oxy-fuel combustion process [11].The techno-economic studies reveal that oxy-fuel combustion is a cost-effective method of CO2 capture [13].
Solar photovoltaic electrical power generation, electrolysis, air separation, ammonia formation, urea synthesis, coal-fired oxy-fuel power generation process and water treatment processes are illustrated in the energy model. The amounts of these substances that would be required in electrolysis, air separation, urea synthesis and oxy acetylene processes can be calculated on basis of energy mass balance on basis of molar masse values of these substances, as provided in the published literature.
Methodology
Following methods are used for utilization of indigenous resources to produce alternative energy generation methods or production of energy intensive products.
Energy model
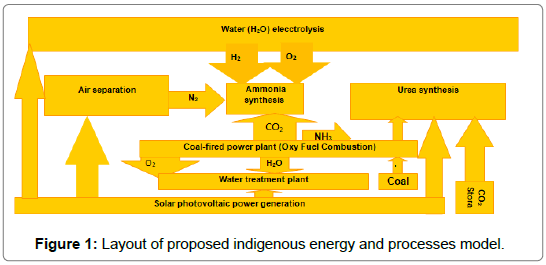
In this model, a solar photovoltaic electrical power generation method is used for electrical power to ensure an uninterrupted electrical power supply from the solar energy during the day. In the first stage, as proposed, hydrogen is obtained from water using electrolysis process. In the second stage, oxygen and nitrogen are obtained from air using separation process and in the third stage, nitrogen generated in the air separation process is synthesized with H2 and in the fourth stage oxygen, a coal fired oxy-fuel combustion process is carried out for generation of electricity, water and CO2. In the last stage, water so obtained from oxy-fuel combustion process can be treated in a water treatment plant to make it portable. The layout the process is shown in Figure 1.
Energy and mass balancing
Energy and mass of hydrogen, oxygen, nitrogen, ammonia and water are balanced for at all stages of processes for determination of the resultant amounts of these substances in 103 kg. The total amount of output commodities and total energy equivalent are maintained in order to avoid the problems of energy generation gaps in energy demanded and generated. Details of energy and masses of substances used in all mentioned processes are illustrated below in the result section.
Results
Molar mases of hydrogen and oxygen, at first stage in watereelectrolysis process are calculated by substiting their mass values, as is illustrated in Eqns. (1) and (2).
2H2O → 2H2 + O2 (1)
2H2O(1) → O2(0.11)+0.88O2 (2)
On basis of the equation balanced above it can be said that for every 1 m3 of water; 0.11×103 kg of hydrogen, and 0.88×103 kg of oxygen can be produced.
In order to determine the amount of nitrogen and hydrogen obtained already for getting ammonia, their mass values are substituted in respective chemical process equations, which are shown in Eqns. (3) and (4). The purpose of this process is to produce an ingredient for urea production.
N2+3H2→2NH3 (3)
(46.6)N2+(0.6)H2→(56.6)NH3 (4)
The energy and mass balance reveal that for each 0.6×103 kg of hydrogen, as obtained at water electrolysis, 46.6×103 kg of nitrogen would be needed, to produce 56.6×103 kg of ammonia.
Carbon dioxide would be required to be chemically processed with ammonia for urea synthesis process. Mass values of ammonia and carbon di oxide are substituted in urea synthesis process chemical equation to determine amounts of ammonium carbamade that can be obtained, which is illustrated in Eqns. (5) and (6).
NH3+CO2→(NH2)2CO+H2O (5)
(56.6)NH3+(0.77)CO2→(1.06)(NH2)2CO+(0.31)H2O (6)
It is calculated that for 56.6×103 kg of ammonia, 0.77×103 kg of carbon dioxide would be required to produce 1.06×103 kg of urea and 0.31 m3 water.
Discussion and Conclusion
It can be said that for 5 6.6×103 kg of ammonia, 0.77×103 kg of carbon dioxide would be required to produce 1.06×103 kg of urea and 0.31 m3 water. Energy intensive commercial products such as clean water, Nitrogen, ammonia, oxygen, hydrogen and ammonium carbamade can be produced mainly from solar energy, air, water and to some extent from coal, available locally at small and medium scale to cater electrical power, portable water and urea in a self-contained manner. It could serve the purpose of meeting basic amenities of electrical power and potable water and commercial and industrial needs such as urea production for soil fertilization.
References
- https://www.worldenergy.org/wp-content/uploads/2016/10/World-Energy-Resources-Fullreport-2016.10.03.pdf
- Shah SA, Zhang Y, Edwards R (2011) Hybrid energy based and CO2 sequestration capable desert potential development. NUST J Eng Sci 3: 39-45.
- Zhang YU (2010) Design and realization of optimization system of urea production process based on BP neural network. International Conference on Computer Applications and System Modelling.
- MacKay DJC (2008) Sustainable energy-without the hot air. UIT Cambridge, England 3: 1-15.
- Hertog S, Luciani G (2009) Energy and sustainability policies in the GCC.pp: 1-4.
- Raja IA, Dougar MG, Abro RS (1999) Solar energy applications in Pakistan. Renew Energ 9: 1128-1131.
- Harijan K, Uqaili MA, Memon M (2007) Renewable energy for managing energy crisis in Pakistan. International Multi Topic Conference pp: 449-455.
- Zaigham NA (2001) Strategic sustainable development of groundwater in Thar desert of Pakistan. Water Resources in the South: Present Scenario and Future Prospects 56: 61-74.
- Pimentel D, Rodrigues G, Wane T, Abrams R, Goldberg K, et al. (1994) Renewable energy: Economic and environmental issues. Bioscience 44: 1-6.
- www.fertilizer.org/ifacontent/download/5427/85520/version/1/file/117.pdf
- Buhre BJP, Elliot LK, Sheng CD, Gupta RP, Wall TF (2005) Oxy-fuel combustion technology for coal-fired power generation. Progress in energy and combustion Science pp: 283-307.
- Modak JM (2011) Haber process for ammonia synthesis. Resonance 7: 1159-1167.
- Shah SA, Jumani MS, Rafique S (2014) Analyses on utilization of solar energy as a sustainable source of clean water production. Sind Univ Res J 46.
- Shah SA, Jumani MS, Rafique S (2014) Prospective study of solar energy as a sustainable energy source of electrical power generation. Sind Univ Res J 4.
Citation: Shah SA, Edwards R, Sanjrani AN (2019) Utilization of Solar Energy as a Fuel in Production of Energy Intensive Industrial Products. Innov Ener Res 8:228.
Copyright: © 2019 Shah SA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4110
- [From(publication date): 0-2019 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 3233
- PDF downloads: 877