Case Report Open Access
Utilization of Industrial Microbe Side Streams for Biosorption of Heavy Metals from Wastewaters
| Sanna Taskila*, Tiina Leiviskä, Olli-Pekka Haapalainen and Juha Tanskanen | |
| Bioeconomy Research Community Oulu, Faculty of Technology, Chemical Process Engineering, University of Oulu, P.O. Box 4300, FI-90014, Finland | |
| Corresponding Author : | Sanna Taskila Bioeconomy Research Community Oulu Faculty of Technology, Chemical Process Engineering University of Oulu, P.O. Box 4300, FI-90014, Finland Tel: +358-294-482579 E-mail: sanna.taskila@oulu.fi |
| Received January 28, 2015; Accepted March 24, 2015; Published March 27, 2015 | |
| Citation: Taskila S, Leiviskä T, Haapalainen OP, Tanskanen J (2015) Utilization of Industrial Microbe Side Streams for Biosorption of Heavy Metals from Wastewaters. J Bioremed Biodeg 6:285. doi:10.4172/2155-6199.1000285 | |
| Copyright: © 2015 Taskila S, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The present report reviews the potential and applicability of industrial microbe side streams produced in Finnish bioprocess industries for biosorption of heavy metals from waste waters. Microbial side stream biomasses are formed worldwide in e.g. food, brewing, biofuel, pharmaceuticals, wood processing and enzyme manufacturing industries. Although these streams are typically used for low-valued animal feed or biogas production, they would also have potential for biosorbent materials to be used in in situ water treatment. Since major challenges have been recognized, especially regarding the availability of biomass, and to logistic and processing cost, biosorption is not the most economically attractive option for the use of these biomasses at the moment. However, in the future the situation may change due to new incentives or more cost-efficient biosorbent preparation technologies. This report will provide a knowledge basis for the consideration of biosorption applications for industrial microbe side streams.
| Keywords |
| Biosorption; Heavy metal; Side stream; Microbe; Yeast; Fungi; Bacteria; Saccharomyces; Aspergillus; Bacillus; Streptomyces; Trichoderma |
| Introduction |
| Heavy metals belong to the most problematic pollutants at various industrial sectors. They cause a wide variety of health problems due to their accumulation in the food-chain [1]. Heavy metals present in industrial wastewaters originate from various industries such as fuel processing, energy production, metallurgy, mining, smelting, surface processing etc. |
| Conventional methods for heavy metal removal such as chemical precipitation, electrochemical treatment, membrane separation, and adsorption on activated carbon have been proposed. Each of these methods introduces problems when treating large amounts of wastewaters with very low concentrations of polluting heavy metals. Problems such as ineffectiveness, production of large amounts of complexly treatable sludge, and high costs have been encountered. Biosorption is an attractive option for heavy metal removal from large quantities of industrial wastewaters containing small concentrations of pollutants [2]. It can be defined as a process where biomasses bind metal or metalloid species, compounds or colloids from solutions. Biosorption raw materials can be derived from e.g. industrial or agricultural side streams, forestry, marine or terrestrial plants or microbes. |
| Biosorption of heavy metals onto various microbial biomasses such as bacteria, fungi and algae have been widely studied. Microbial biomasses can be utilized either as viable or non-viable cells. Viable cells uptake metals in their metabolism and their binding is due to bioaccumulation. Non-viable cells on the other hand passively bind positively charged cations onto their cell surface due to negative net surface charge on their cell wall. Generally, bioaccumulation is considered less favorable option due to need for supporting the growth of living cells and limited reuse of biomass [3]. It is, however, more selective than biosorption [4]. Biosorption mechanisms of microbial cell walls are not fully understood, but they include at least diffusion, chelation, complexation, ion-exchange and attraction. Various functional groups are present in these biomasses and are likely involved in biosorption processes, i.e. hydroxyl, carboxyl, amino, ester, sulfhydryl, carbonyl and phosphate. |
| Various reports have shown clear potential of industrial microbe side streams, especially those originating from breweries, as lowcost biosorbent materials [5]. To allow the use of microbial biomass for this purpose it is essential to map the potential side streams and their properties. Thus, our objective was to examine the potential and applicability of microbial side streams produced in Finnish industries for biosorption applications. Based on the results microbial side streams are currently formed in food, biofuel and enzyme manufacturing industries and are usually further processed to low-valued animal feed or to biogas production. |
| Characteristics of Microbe Side Streams Produced In Finland |
| Mapping of potential biomasses |
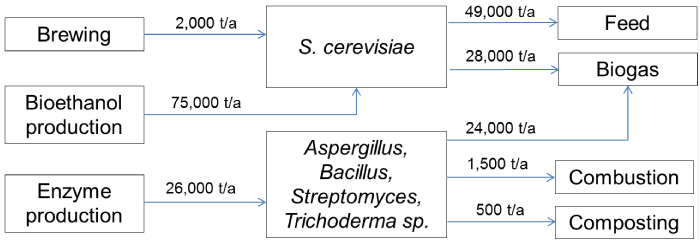
| In total ten companies were selected for investigation based on their current products or R&D activities. These included four breweries, two enzyme producing companies, three companies that produce biofuels by means of microbes or are developing processes for the purpose, and a baker’s yeast producer. All of the manufacturing sites are located in Southern and Middle part of Finland. Preliminary information on side streams and production capacities was acquired from personal communication with company representatives and was confirmed from public environmental permits appointed by the Finnish Centre of Economic Development, Transport and the Environment (ELY). The approximation of material balance for produced cell mass is presented in Figure 1. |
| Yeast fermentations |
| Ethanol fermentation process consists of several unit operations to convert carbohydrates into ethanol by S. cerevisiae. This technology is employed worldwide in production of alcoholic beverages and bioethanol. After brewing the yeast cells are collected from the bottom of tank or separated by centrifugation to form slurry of 20-50% solids [6]. Loose slurries are further concentrated by filtration after which they are thermally disrupted at around 80°C and optionally also with organic acids. The production capacity of major Finnish breweries is over 400 million l/a [7] and the respective quantity of formed yeast containing residues is approximately 2000 t/a. |
| Bioethanol is produced by St1 Biofuels Oy in quantity of 13,000 t/a in Lahti, Vantaa, Hamina, Jokioinen and Hämeenlinna. Ethanol is further concentrated at Hamina. These production sites produce altogether over 75,000 t/a of residues that contain also yeast cells. Hämeenlinna plant directs all organic residues to biogas production [8] while the other plants produce also animal feed. The amount of produced feed is over 45,000 t/a [9-12]. The company has also been appointed an environmental permit in 2013 for production of lignocellulose ethanol in Kajaani [13]. Yeast residues of the process together with other residues would be directed to energy production. |
| Enzyme production |
| The main steps in enzyme production processes are medium preparation, inoculum preparation, inoculation and fermentation, cell removal, product purification and concentration, and formulation. The nature of produced enzyme affects also properties of microbial residues. Extracellular enzymes are recovered from the fermentation broth after removal of cells which remains the cells intact. Cell removal is conducted using filter press together with filter aids and flocculants. On the contrary, intracellular products are recovered via disruption of cells by chemical or mechanical methods, after which cell debris are included in the residues. |
| Currently industrial enzymes are manufactured in Finland by two companies, Genencor and Roal Oy. Both companies occupy filamentous fungi and Gram-positive bacteria in their processes [14]. Genencor, owned by DuPont, has production sites at Jämsänkoski and Hanko. According to operative environmental permission appointed by authorities, 90% of the residues from Hanko plant comprise of microbial cell side stream which accounted approximately 16,000 t/a in 2002 [15]. Jämsä plant residues account to approximately 8,000 t/a microbial cell mass. Residues are currently used for biogas production and also composting has been considered. |
| Roal Oy, owned by Associated British Foods, has a production site at Rajamäki. The production capacity of the plant is 10,000 t/a, from which approximately half is in use. Microbial cell residues, among some diatomite, account to approximately 2,100 t/a in which solid content is some 40%. From this amount 1,500 t/a are directed to combustion in order to secure trade secrets related to microbial strain development, and the rest 600 t/a to composting [16]. |
| Other processes |
| Suomen Hiiva Oy, owned by Lallemand, produces approximately 8,000 t/a baker’s yeast in Lahti, the total production capacity being 12,000 t/a. Yeast is produced in batch processes by sequential scaling from 200 g inoculum to 200 t of culture within one week [17]. According to the interview and environmental permit, the plant does not produce significant amounts of cell waste [17]. |
| Finnish based company Neste Oil has been developing microbial oil production processes for the manufacturing of biodiesel. The company has operated a pilot plant for producing microbial oil from waste and residues at its site in Porvoo, Finland, since 2012 although the project is currently on hold. In principle, microbe oil is produced via accumulation of lipids to fungal and yeast cells utilizing plant biomasses as feedstocks. Also, genetically modified bacteria have been developed for the purpose [18]. |
| Cursor Oy, the Kotka-Hamina Regional Development Company, has developed a process that utilizes forest industry side streams as substrates for micro algae in order to produce a variety of products [19]. In this process the algal cells would be valorized comprehensively and thus their use for biosorption applications would not be possible. |
| Total biomass potential |
| The investigated companies produce altogether over 103,000 t/a of microbial side streams. This value represents all material in the streams and thus the actual quantity of cellular biomass is lower. Exact quantities of cell material cannot be determined without further characterization of each stream. However, some estimation can be made based on the environmental permit documentations. The majority of the side streams is formed in brewing and bioethanol production and accordingly, yeast Saccharomyces cerevisiae is the predominant organism in the streams. The produced side streams are typically directed to biogas plants and to feed production. Minor portion is combusted due to trade secrets. |
| Cell wall characteristics of microbes in side streams |
| The structure of cell walls is a major factor regarding the biosorption capacity of microbial biomass. The cellular composition tends to be rather similar between organisms within the same genus or order [20] while differences in structure can be found between eukaryotic and prokaryotic organisms, and between Gram-positive and Gramnegative bacteria. The general classification of microbes and respective cell wall characteristics are described in the following. |
| Fungi, higher plants, algae, protozoa and animals are eukaryotes, from which fungi, microalgae and most protozoa represent microbes. Fungi include both unicellular organisms, i.e. yeasts, and multicellular organisms, i.e. filamentous fungi and mushrooms. Eukaryotic cells have compartments for cell organelles and true nuclei, a membraneenclosed nucleus containing genome consisting of several linear DNA bundles referred to as chromosomes. Typical eukaryotic cell diameter varies between 2-200 μm, although also larger cells have been observed. |
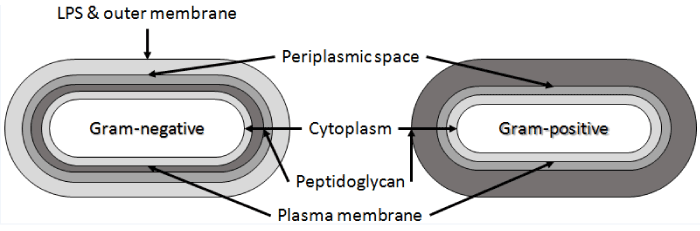
| Bacteria and Achaea are prokaryotic organisms. Prokaryotic cells do not have compartments for cell organelles. They include a free-existing genome, nucleoid, consisting of at least a single DNA molecule. Prokaryotic cell diameters vary between 0.5-1.0 μm, although diameters above 50 μm have been observed. Regarding the cell wall structure most bacteria can be divided into Gram-positive or Gramnegative cells based on their response to Gram-staining. Cell surface, structurally and chemically, is more complex in Gram-negative bacteria while the surface in Gram-positive bacteria is composed mostly from peptidoglycan (Figure 2) [21]. The most common representatives of Gram-positive bacteria include lactic acid bacteria, the main working horses of dairy industry, and Bacillus spp. that belong to the most occupied biocatalysts in global scale [22]. |
| Fungi and yeasts |
| The fungi present in the investigated side streams include yeast S. cerevisiae and filamentous fungi Aspergillus and Trichoderma. S. cerevisiae is used in brewing and biofuel production for ethanol fermentation. Aspergillus spp. are used e.g. in production of foods, citric acid production and enzyme production. Trichoderma reesei is a common host for the production of industrial enzymes. |
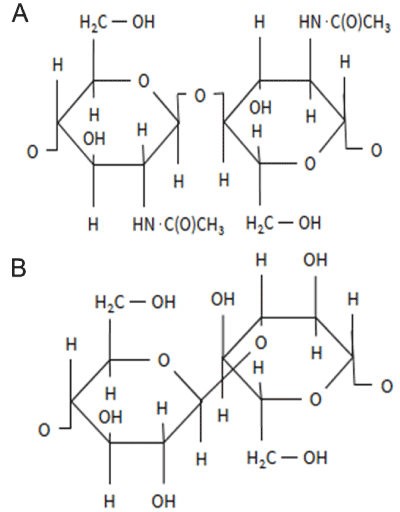
| The cell wall of fungi determines the morphology and integrity of the organism during growth and cell division. The cell wall is formed by three groups of polysaccharides: polymers of mannose (mannoproteins), polymers of glucose (β-glucan), and polymers of N-acetylglucosamine (chitin), accounting for approximately 40%, 60% and 2% of the cell wall dry mass, respectively [23]. The structures of β-glucan and chitin are presented in Figure 3. The fungal cell wall is a dynamic structure that can adapt to physiological and morphological changes [24], and respond to environmental stresses by restructuring. |
| The basic structure of fungal cell wall consists of fibrillar β-1,3- glucan and chitin components [25] embedded to an amorphous matrix of mannoproteins. Chitin is mostly located near to the plasma membrane while the β-glucans are present throughout the cell wall [26]. Fungal cell wall contains ca. 10–15% chitin, while yeast cell walls contain only 1-2%. The structure, i.e. crystalline or amorphous forms, and deacetylation degree of chitin vary largely between fungal species, Ascomycota having the least acetylated chitin due to presence of glucans [27]. |
| The matrix of yeast cell wall is composed most commonly of glycosylphosphatidylinositol proteins (GPI-CWP) which are linked to β-1,3- and β-1,6-glucans via glycosidic bond [28], or alkalisensitive linked cell wall proteins (ASL-CWP). The cell wall matrix of filamentous fungi composes of galactomannoproteins and α-glucans. The inner layer of the fungal cell wall is electron-transparent and mostly composed of chitin and 1,3-β-glucan while the outer layer is more electron-dense and composed of the glycosylated mannoproteins [28,29]. The negative charge of fungal cell wall is supposed to origin from the glycan side chains [28]. |
| Gram-positive bacteria |
| The bacterial genera within the present study include Bacillus and Streptomyces, both classified in the Gram-positive bacteria. Bacillus spp. are aerobic, endospore-forming, rod-shaped bacteria and an often referred representative of Gram-positive cells. In the investigated companies Bacillus are utilized for the production of α-amylases, cellulases, proteases, pullulanases and xylanases [14]. Streptomyces spp. are aerobic, spore-forming actinomycetes [30]. They are utilized for production of cellulases, glucose- and xylose-isomerases [14]. |
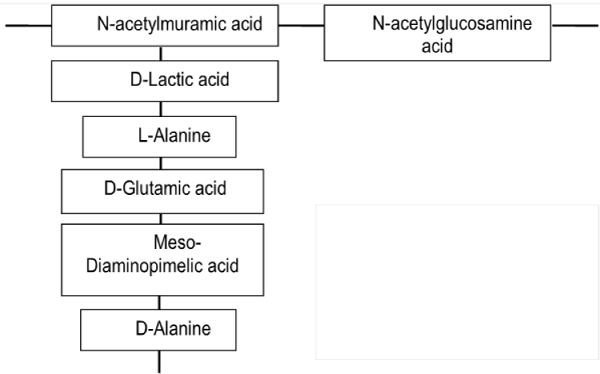
| The cell wall of Gram-positive bacteria is strong but also elastic structure with capability to respond to changes in the growth conditions. It is mostly composed of peptidoglycan, accounting typically 80–90% from their cell weight [21]. Peptidoglycan consists of linear chains of glycan, regularly alternating N-acetylglucosamine and N-acetylmuramic acid held together by β-(1-4)-glycosidic linkages (Figure 4). The β-(1-4)-glycosidic linkages are cross-linked together by the carboxyl groups of muramic acid residue. Peptides present in peptidoglycan are alternating D- and L-forms. |
| In Gram-positive cells peptidoglycan may be associated with a two-dimensional array of proteins or glycoproteins. Moreover, the peptidoglycan layer in Gram-positive bacteria includes also covalently bound teichuronic and teichoic acids, and diacetylglycerol anchored lipoteichoic acids. Teichuronic acids contain charged carboxyl groups, while teichoic acids contain charged phosphate groups and usually charged amino groups due to D-alanyl ester substituents. These anionic polymers have great effect on bacterial cell surface charge as well as to specific surface properties. |
| Biosorption capacities of side stream biomasses |
| Effect of side stream processing: During their cultivation and down-stream processing microbes are exposed to a variety of stress factors, inducing more or less detrimental changes in their metabolism and structure. Whether these changes affect the biosorption capacity of dead cells depends on the viability of cells during processing, i.e. their capacity to respond to stresses at that point, and the respective changes in the cell wall structure. Typical process phases and their reported effects on biosorption are presented in Table 1. In fungi environmental stresses induce changes in polysaccharide composition and variations in transcript levels of cell wall proteins [24,28]. In case of S. cerevisiae the polysaccharides content of cell wall varied by more than 50% depending on the carbon source, nitrogen availability, pH, temperature and dissolved oxygen tension in the cultivation [23]. |
| Ethanol-grown S. cerevisiae was rather resistant to mechanical and enzymatic disruption despite its reduced cell wall dry mass. The increased strength is supposedly related to increased β-1,6-glucosidic cross-linking between β-1,3-glucan, mannoproteins and chitin. This may associate with weakened biosorption capacity of the cells due to lower specific surface area and reduced concentration of mannans in the cell wall [31]. Thickening of the external protein layer and higher expression of 1,3-α-d-glucan synthase-encoding gene were observed in Aspergillus upon cell wall stress [32-34]. On the other hand, improved metal affinity was observed in ethanol and heat treated S. cerevisiae cells compared to non-treated cells [35,36]. This was supposedly due to denaturation of soluble cell wall proteins that form complexes with metal ions and inhibit their binding by the cell. |
| Chitin of the fungal cell walls complexes metal ions effectively [37] and it is thus a significant factor for biosorption efficiency of fungal cells. Chitin is traditionally obtained from exoskeleton of crustaceans. Nwe et al. [38], however, stated several advantages of chitosan derived from fungal mycelia over crustacean chitosan: e.g. availability of identical batches, lower molecular weight, and more uniform and stable charge distribution. Chitin can be also further processed to chitosan by N-deacetylation. Chitosan has a high affinity for metal ions due to its high content of amine groups [5] whereas the protonated amine groups attract anionic organics [39]. The stress induced changes in polysaccharide composition of fungal cell wall may lead to chitin proportion of up to 20% from cell dry weight [24]. Also increased nitrogen supply for cells may increase the chitin content in mycelial cell walls [27]. Thereby, the influence of altered cell wall structure on biosorption may be either negative, in case that the cell wall structures are used as such, or positive, in case that isolation of chitin is pursued. |
| Similar to fungi also bacteria possess strong stress response mechanisms that affect their cell wall structure and functionality. In Bacillus subtilis this is related to dramatic induction of general stress proteins (GSP) regulated by supposedly similar mechanisms also in many other Gram-positive bacteria [40]. GSPs are induced for instance by heat, ethanol, salt stress or by starvation for glucose, oxygen or phosphate [41]. Teichoic acids present in Gram-positive cell wall are mainly responsive for the negative charge of the cell surface [42], and their quantity is a major factor affecting the biosorption capacity of cells. Teichoic acids contain up to 30% phosphates and their synthesis is thereby limited under phosphate limitation [43,44]. Accordingly, Bacillus cells grown under phosphate limitation have weakened heavy metal affinity [45]. |
| Biomass pretreatment have a major impact to biosorption capacities, and thus detailed comparison of different studies is usually difficult to make. Several pretreatment and modification methods have been presented in literature including physical and chemical methods [46]. Homogeneity of the biomass should be increased via physical forces such as crushing by milling, and drying. Pretreatment will increase surface area of biosorbents and expose intracellular components. More surface binding sites will also be available due to destruction of cell membranes. The exposure of cells to high shear forces during separation from fermentation broth or cell disruption may lead to disintegration and leaking of cell wall polysaccharides to the medium, and thus perhaps to reduced biosorption capacity [47]. |
| Chemical treatment may include washing with different solutions, grafting of binding groups, and elimination of inhibitive groups or graft polymerization [46]. Various chemicals have been studied but the most commonly used chemicals are NaOH, HCl and ethanol. Treatment with alkali solution or ethanol increases the amount of negatively charged functional groups present in the microbial cell walls, while acidic treatments increases the amount of positively charged functional groups. Even though originally being waste material or produced very inexpensively, all the pretreatment stages will increase the prize of product. |
| Operation conditions: Biosorption is affected by many factors, which make optimization of the process challenging. Solution pH is a very important factor in the biosorption process. Microbial and plant biomasses have a zero-point-of-charge, which is a boundary value of pH where net negative surface charge is zero. At lower pH values, a positive charge is formed on the biomass surface. It results to lower adsorption rates of positively charged ions due to their competition of active binding sites with excess H+ ions. Higher pH values lead to increase in active sites and negative surface charge. This promotes positively charged ion adsorption due to increased electrostatic attraction forces. Besides biomass properties, pH affects the metal speciation and solubility. |
| Temperature of biosorption process does not affect the cell wall of non-viable micro-organisms, but rather on reaction rate of heavy metal adsorption. Since binding of heavy metals can be either exothermic or endothermic reaction depending on metal and biomass species, optimal temperature is usually determined between 10–50�?�?C. Exothermic reaction occurs more favorable at low temperatures and endothermic at higher temperatures. The biosorption of Pb2+, Ni2+, Cu2+ and Cr6+ onto S. cerevisiae cell wall was an exothermic reaction [48]. Dursun [49] concluded that Pb2+ and Cu2+ biosorption on A. niger cell wall was an endothermic reaction. The biosorption of Pb2+ on B. gibsonii S-2 [50], Cr3+ on B. subtilis [51] and As3+ on living cells of B. cereus [52] were also endothermic processes. |
| Since real effluents may consist of metals with both exothermic and endothermic binding reactions, a decision has to made to separate the most harmful contaminant. This is the case if process is operated in single reactor. Operation in multiple reactors allows separation of each metal in their optimal temperatures, thus increasing the biosorption rates, but also raising additional costs. |
| Adsorbent concentration in the solution should be optimized respective to metal concentration to avoid agglomeration, which reduces the availability of binding sites [53]. Ghorbani et al. [3] reported that the biosorption capacity of S. cerevisiae decreased when increasing amount of biomass from 1.6 to 6.0 g/l with optimum biosorption rate at 3.8 g/l. Similar results were observed in the study by Han et al. [54]. Increase in the amount of adsorbent respective to adsorbate resulted to unsaturation of adsorption sites in the reaction. |
| Increase in the metal ion concentration affects biosorption efficiency to the certain point. Metal ion uptake in cells increases due to fact, that metal ions surround active sites in biomass cell wall thus occupying them more effectively [3]. This suggests that removing ppb-to-ppm concentrations of polluting metals should be slower than removal of substantial amounts of pollutants. Solutions that include various metals simultaneously have been reported to decrease specific biosorption capacities of microbial species [53,55,56]. Therefore, studies should be conducted with effluent concentrations mimicking real wastewater effluents. |
| Biosorption has been studied in both batch and continuous fixedbed systems. Batch system is appropriate for studying kinetics and process conditions of biosorption. Fixed-bed system can achieve higher biosorption rates and will probably be utilized if biosorption process is commercialized as wastewater treatment method [57]. Sag [58] studied biosorption capacity of R. arrhizus by four different operating methods at exactly same conditions. Biosorption capacity decreased as follows: packed column (fixed-bed) reactor (PCR)>three batch stirred-tank reactors in series (BSTRS) >> continuous flow stirred-tank reactor (CFST)>batch stirred-tank reactor (BSTR). |
| For continuous processes biomasses should be immobilized in order to have higher particle size and rigid material. Immobilization techniques include cell immobilization on inert supports, entrapment within a polymeric matrix, and cross-linking [46]. Ross [59] presented a method for moldable building material production using filamentous growth of fungi. Similar approach could be useful for the immobilization of non-viable cells to achieve right size, porosity, rigidity, and mechanical strength without losing their uniform net negative surface charge. Utilization of such structures could also increase the availability of binding sites for heavy metal adsorption due to the binding sites available on fungal cell surface. |
| Wang and Chen [5] concluded that commercial utilization of biosorption technology is dependent on the efficiency of the regeneration of biosorbent after metal sorption. Study of nondamaging, environmental-friendly, effective and sufficiently cheap non-specific and metal-specific eluents are necessary in the near future to improve cost-effectiveness of biosorption. Chergui et al. [60] could not desorb and regenerate S. rimosus without significant decreases in biosorption capacities towards Cu2+ and Zn2+. Interestingly enough, no decrease in biosorption capacity towards Cr6+ was observed. Yu et al. [61] utilized EDTAD-modified baker’s yeast in biosorption of Pb2+ and Cu2+ and could effectively desorb used biomass by EDTA (ethylenediaminetetraacetic acid) with just little loss of the adsorption capacity. Kapoor et al. [62] were able to desorb at least 98.9% of adsorbed metals Cd2+, Cu2+, Ni2+, and Pb2+ from A. niger cell wall with 0.05N HNO3. Desorption of metal cations (Cd2+, Cu2+, and Zn2+) from formaldehyde cross-linked S. cerevisiae was effective with 0.1M HCl [63]. The capacities maintained the same level after regeneration (only Cd2+ and Cu2+ were studied). Zinc could be desorbed from Aspergillus flavus using 0.01M HCl with 83.3% elution efficiency and with 23.5% loss in biosorption capacity after five adsorption-desorption cycles [64]. Sodium hydroxide (5M) was able to desorb 81% of Cr3+ from B. subtilis biomass [51]. |
| Biosorption capacities: Tables 2-5 show examples of biosorption studies using the species discussed in previous sections. The yeast S. cerevisiae, obtained often from bakery or brewery, can remove toxic metals and recover precious metals in various extents from aqueous solutions (Table 2). Safety and abundance are the main advantages of S. cerevisiae. However, high variation can be observed in reported capacities as with other microbial species. For example the capacity of Pb2+ varied from 5.7 mg/g (HCl-treated waste brewer’s yeast, [54]) to 270.3 mg/g (dried, [65]). A review by Wang and Chen [66] can be referred for a detailed description of biosorption using S. cerevisiae [67- 73]. |
| Filamentous fungi, e.g. Aspergillus spp. and Trichoderma spp. are widely used at large-scale fermentation processes (Table 3). Kapoor and Viraraghavan [62] studied the removal of heavy metals from aqueous solutions using Aspergillus niger, which was immobilized in a polysulfone matrix. Before immobilization non-viable biomass was pretreated with NaOH, dried, powdered, and sieved to 75 μm particles for the experiment. Biosorption capacities were rather low (Table 3), however the adsorbed metal ions were easily eluted from the column with 0.05 N citric acid and no visible effects in physical properties were observed. Some higher capacities were reported for metals with NaOH-treated A. niger by Amini et al. [1] and Dursun [49]. The biomass pretreated with NaOH resulted in clean and porous surfaces but also in reduction of biomass by 40% [1]. However, 4.5 times higher ion-exchange capacity was achieved. Biosorption stuides using Trichoderma spp. has not been published much. However good capacities for several heavy metals were obtained by Kim et al. [74] and Ghaedi et al. [75]. |
| Table 4 presents selected examples of biosorption capacities of Bacillus spp. A polysaccharide from B. firmus showed a high affinity for binding Pb2+, Cu2+ and Zn2+ [55]. The biosorbent contained organic acids (uronic and pyruvic acids), glucose, fructose, mannose, and galactose. The water-soluble biosorbent was precipitated by ethanol from metal solution after reaching the equilibrium, which may not be feasible in actual applications [76,77]. |
| In comparative biosorption study for Pb2+ removal by Çolak et al. [78] two heavy metal resistant bacteria Bacillus strains, B. pumilus and B. cereus, were investigated in batch and fixed-bed operating systems. Maximum Pb2+ biosorption rates in batch system were 28.1 mg/g for B. pumilus and 22.1 mg/g for B. cereus. Higher capacities were achieved with fixed-bed system, namely 66.0 mg/g for B. pumilus and 59.2 mg/g for B. cereus. |
| Fang et al. [79] studied biosorption by comparing cell wall structures of Gram-positive bacterium Bacillus thuringiensis and Gram-negative bacterium Escherichia coli. The biosorption capacities of native cells, cells with esterified carboxylic acid groups (methanol treatment at acidic conditions) and cells with deprotonated carboxylic acid groups (NaOH treatment) were investigated. IR spectrum showed that functional groups responsible for adsorption of Cd2+ and Cu2+ on B. thuringiensis cell wall were amido and hydroxyl groups and possibly carboxylic acid groups. The functional groups on the E. coli cell wall were only amido and hydroxyl groups, but not carboxylic acid groups. For hydrolyzed cells, the maximum adsorption capacities of Cd2+ and Cu2+ for B. thuringiensis were 48.3 and 23.6 mg/g and for E. coli 39.4 and 33.7 mg/g, respectively [80-82]. |
| Table 5 presents some biosorption capacities for Streptomyces spp. Selatnia et al. [83] introduced dead, crushed and NaOH-treated bacterium Streptomyces rimosus for lead biosorption application. Str. rimosus was an attractive candidate due to its cell wall structure, which consists mainly from peptidoglycan linked with teichoic acids and variety of polysaccharides. Pb2+ was adsorbed by Str. rimosus up to 135 mg/g. |
| Cherqui et al. [60] studied the metal uptake by Str. rimosus waste biomass from an antibiotic production. About 30 mg/g capacities were obtained for Cu2+, Zn2+ and Cr6+ (Table 5). Metals could be eluted by acids at pH 2, however, the capacities were slightly decreased for Cu2+ and Zn2+ but not for Cr6+ [84-86]. |
| Regardless of intensive research on biosorption and good results achieved by many researchers, commercialization of microbial biomass based biosorbents and their use in actual applications is still a huge challenge. Demonstration with actual wastewater at pilot-scale and reducing the cost of treatment to minimum would enhance commercialization of products as well as their successful and continuous use in industrial applications. Actual wastewaters are typically complex mixtures of cations, anions, inorganic and organic components, all of which may have effect on sorption efficiency. Only a few reports are available on actual wastewater biosorption cases by species discussed in this review. For example Dai et al. [87] observed good lead biosorption performance of spent mushroom Tricholoma lobayense using wastewaters of sewage treatment plant and a storage battery manufacturer. Aftab et al. [64] investigated the use of Aspergillus flavus NA9 for zinc removal from paint industry effluents, achieving a biosorption capacity of 16.2 mg/g. |
| Applicability of Microbial Side Streams for Biosorption |
| The applicability of microbial side streams for heavy metal biosorption is generally dependent on the availability and characteristics of the material. The related challenges recognized in the present research and the weights of their probable impacts on biosorption applications are summarized in the Table 6. |
| In the present research the majority of the produced side streams originated from yeast fermentations. Principally, S. cerevisiae has beneficial properties regarding biosorption applications. It is however notable that the availability of the material relays essentially from the interests of the side stream producer which should be motivated to ensure a steady supply of the side stream throughout the year [88]. As the yeast residues are currently sold in open market for feed manufacturers, more economically attractive alternative would be required to motivate suppliers. This fact together with the unavoidable requirements for the processing and transportation of the biomass can be expected to cause intolerable increase of biosorbent cost. The transportation costs are especially notable in the Northern Europe where the distances are relatively long and the production sites of biomass residues are located in south while the major heavy metal emission originate from the mining industry in the north. |
| The microbial side streams formed in the enzyme production account to a minor and thus far less realistic biosorbent candidate. The use of these biomasses is limited partly due to strict procedures to keep the record of genetically engineered strains as a trade secret. For this purpose some 5% of the enzyme manufacturing residues are directed to combustion. Main characteristics of the other residues are their heterogeneity with respect to microbial strains and other material and, in case of intracellular enzyme production, also the presence of disrupted instead of intact cells. These factors would however not necessarily hinder biosorption applications. First of all the presence of mixed microbial consortium could actually be an advantage for the heavy metal binding [89]. The presence of filtration aids on the other hand is not likely to affect biosorption capacity of the biomass [90]. |
| The effect of cell disruption depends on the occupied disruption technique. Generally, thermal treatment may even increase the heavy metal affinity of cells by removal of soluble cell wall proteins that can reduce the metal sorption capacity of cell wall polysaccharides [35,36]. Mechanical disruption, on the contrary, may have opposite effects due via causing disintegration of cell wall components and their leaking to the medium [47]. |
| Conclusion |
| Based on the present research microbial side stream biomasses are formed in quantities of over 100 kilotons per annum in Finland, Saccharomyces cerevisiae being the predominant microbe in these streams. In principle dead S. cerevisiae cells could provide a low-cost material for biosorption applications. However, their use for this purpose would only be feasible if their price would exceed current price of animal feeds. Further challenges are related to preprocessing and logistic costs. The emerging of biosorption applications using microbial side streams is thus not expected in the near future. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14372
- [From(publication date):
May-2015 - Jul 18, 2024] - Breakdown by view type
- HTML page views : 9934
- PDF downloads : 4438
