Research Article Open Access
Utility of Ultrasound vs. Gene Expression Classifier in Thyroid Nodules with Atypia of Undetermined Significance
Carmen V Villabona1*, Vineeth Mohan1, Karla M Arce2, Julia Diacovo3, Alisha Aggarwal1, Jessica Betancourt4, Hassan Amer1, Tessey Jose1, Pascual DeSantis1 and Jose Cabral1
1Department of Endocrinology, Cleveland Clinic Florida, 2950 Cleveland Clinic Blvd., Weston, Florida, USA
2Department of Endocrinology, Cleveland Clinic Ohio, 9500 Euclid Avenue, Cleveland, Ohio, USA
3Department of Pathology, Cleveland Clinic Florida, 2950 Cleveland Clinic Blvd., Weston, Florida, USA
4Department of Internal Medicine, Lincoln Medical and Mental Health Center, NewYork, USA
- *Corresponding Author:
- Carmen V Villabona, MD
Department of Endocrinology
Cleveland Clinic Florida
2950 Cleveland Clinic Blvd
Weston, Florida, 33331, USA
Tel: 3123074488
E-mail: villabc@ccf.org
Received date: January 18, 2016 Accepted date: January 30, 2016 Published date: February 06, 2016
Citation: Villabona CV, Mohan V, Arce KM, Diacovo J, Aggarwal A, et al. (2016) Utility of Ultrasound vs. Gene Expression Classifier in Thyroid Nodules with Atypia of Undetermined Significance. J Cancer Diagn 1:103. doi: 10.4172/2476-2253.1000103
Copyright: © 2016 Villabona CV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Cancer Diagnosis
Abstract
Introduction: Thyroid nodules with fine needle aspiration (FNA) cytology categorized as atypia of undetermined significance (AUS) often undergo additional diagnostic analysis with the Afirma Gene Expression Classifier (GEC) which classifies these as either high probability of being benign (GEC-B) or suspicious for malignancy (GEC-S). Our goal was to assess the clinical validity and utility of GEC in the evaluation of AUS cytology and evaluate the performance of ultrasonography (USG) for predicting malignancy in this subset.
Methods: We conducted a study with a retrospective cohort of patients from January 2012- January 2014 who had FNA of thyroid nodules >1 cm in size with AUS cytology.
Results: Cleveland Clinic Florida has an overall incidence of AUS of 5%. 119 cases with nodules >1 cm in size were reported as AUS. 48 (40.3%) had a GEC performed after the first FNA (AUS-1) and 27 of these were GEC-S. Of those 27, 21 went for surgery and 14 (66.6%) had thyroid cancer on histopathology. The remaining 71 with AUS-1 were sent for a 2nd FNA:19 nodules were benign and did not undergo further evaluation while the remaining 52 were reported as AUS for the second consecutive time (AUS-2). AUS-2 samples were sent for GEC. Of these 52 AUS-2, 38 (73.1%) were reported as GEC-S. 35 went for surgery and 32 (91.4%) had confirmed malignancy on histopathology. Positive Predictive Value (PPV) was 91.4% for AUS-2 vs. 66.6% for AUS -1. Moreover, AUS-2 nodules that were hypoechoic and solid on USG showed a PPV of 92% for malignancy.
Conclusion: In our practice, the diagnostic accuracy to predict malignancy with GEC for AUS-1 nodules was poor (PPV 66.6%). The PPV of GEC testing was markedly higher at 91.4% performed after 2 consecutive AUS cytologies. AUS-2 nodules that were solid and hypoechoic on USG also had a high probability to be malignant (PPV 92%). We recommend repeat FNA on AUS-1 nodules rather than proceeding directly to GEC testing. Also, we suggest that among AUS-2 nodules, surgery can be recommended when USG shows solid and hypoechoic features with GEC testing reserved for the remainder.
Keywords
Thyroid nodules; Thyroid cancer- clinical; Pathologythyroid cytology; Radiology- imaging
Introduction
Thyroid nodules are one of the most frequent disorders referred to the endocrine practice. While 4%-7% of the general adult population has palpable nodules, more than 50% has thyroid nodules on ultrasonography (USG) [1].
Approximately 5% of all thyroid nodules are malignant [2], and the incidence of thyroid carcinoma has been increasing in recent years [3]. Therefore, the correct identification of malignant nodules and the avoidance of unnecessary procedures for benign nodules represent a major clinical and diagnostic challenge.
USG is an important diagnostic tool in predicting thyroid malignancy and highlighting thyroid nodules that should be assessed by FNA [4-6]. Suspicious USG features include marked hypo echogenicity, irregular borders, micro calcifications and a taller thanwide shape. A combination of these features is known to provide better diagnostic accuracy than a single feature alone [4,7-10].
Fine needle aspiration (FNA) biopsy is a widely used method for assessing the malignancy risk of thyroid nodules. In most instances, the FNA results are either benign or malignant, allowing for appropriate management whereas 15% to 30% of cases are considered indeterminate [11-13]. As per the Bethesda system for reporting thyroid cytopathology, indeterminate categories includes atypia of undetermined significance (AUS)/follicular lesion of undetermined significance (FLUS) and follicular neoplasm or suspicious for follicular neoplasm (FN). The risk of malignancy in indeterminate nodules varies greatly between institutions as does the subsequent approach after receiving such a cytological result [14-20].
The Afirma gene expression classifier is a proprietary diagnostic test developed by Veracyte Inc. (South San Francisco, California) for the preoperative identification of indeterminate thyroid nodules with a cytology diagnosis of AUS/FLUS and FN. The assay analyzes the mRNA expression of 167 genes to identify the signature of benign thyroid nodules. Based on this information, it reclassifies nodules as either “benign” (GEC-B) or “suspicious for malignancy” (GEC-S) [21-24].
This review focuses specifically on nodules that display atypia of undetermined significance (AUS) cytology. Our goal was to assess the clinical validity and utility on GEC in the evaluation of AUS cytology at our institution. We also evaluated the performance of ultrasound for predicting malignancy in the setting of AUS cytology.
Methods
A retrospective chart review was conducted from January 2012- January 2014 including patients who had FNA of thyroid nodules >1 cm in size with AUS cytology and GEC testing. Nodules were classified as benign based on GEC, cytology, or histopathology results. Nodules were classified as malignant based on final histopathology.
The study protocol was approved by investigational review board (IRB). The approving IRB found the study to be of minimal patient risk, and thus granted a waiver of informed consent from patients whose data was collected for study analysis.
Patients were selected for thyroid nodule FNA based on American Thyroid Association (ATA) 2009 guidelines criteria for thyroid nodules [11]. Patients 18 years or older with one or more thyroid nodules >1 cm or greater confirmed by USG with an AUS result on cytology were included. Patient demographics such as age, sex, FNA and GEC results, final recommendations by the endocrinologist, subsequent clinical and surgical follow up, USG characteristics of the biopsied nodule, including size, echogenicity, vascularity and the presence of calcifications were collected.
All FNAs were performed under ultrasound guidance by a radiologist and/or endocrinologist. Each nodule was sampled with 2-5, 25 gauge needle passes and slides were fixed in 95% ethanol and dried for transport. The initial three passes were evaluated on-site by a cytopathologist, who confirmed the presence of adequate material for diagnostic interpretation and rendered a preliminary diagnostic impression. The cytopathology was performed only by our institution. Up to 1-2 additional passes were obtained in any given case if it was believed that the original attempts had limited cellularity. In some cases GEC testing was recommended at the time of the initial FNA based on observations made by the on-site cytopathologist in collaboration with the endocrinologist or interventional radiologist. GEC testing was collected at the time of the second FNA of all thyroid nodules with a prior AUS cytology. When needed, the second FNA was performed after an average time interval of 4-8 weeks. Nodules that had only a single FNA consistent with AUS were defined as AUS-1. Nodules that had repeated cytology showing AUS were defined as AUS-2.
Thyroid surgery was performed on the basis of the clinical judgment of the treating physician. If the surgery was performed, surgery type (hemi thyroidectomy [HT] or total thyroidectomy [TT]) and the reason for recommending surgery were reported by physicians. Sensitivity, specificity and negative and positive predictive values were calculated with the use of established methods [25].
Results
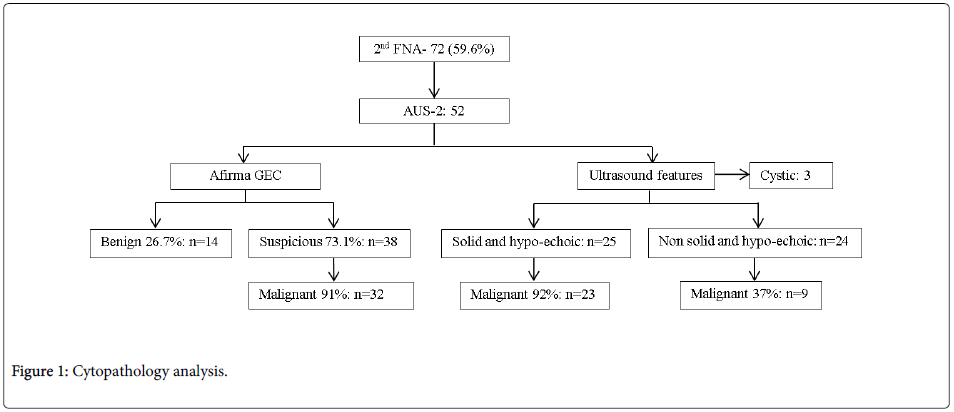
Cleveland Clinic Florida has an overall incidence of AUS cytopathology of thyroid nodules of 5%. We had 119 cases, each having just one nodule evaluated. Of these 119, 48 (40.3%) based on initial cytopathology were sent for GEC with 27 classified as GEC-S. 21 of these went for surgery and 14 (66.6%) had thyroid cancer on histopathology. 71 with AUS cytology on the initial FNA did not receive GEC and were sent for a second FNA: 19 (26.7%) were benign and 52 with sizes ranging from 1-4 cm were again reported as AUS (AUS-2, AUS on 2nd FNA) (Figure 1). The nodule size ranged from 1 cm to 4 cm. 29 (55.7%) nodules were located in the right lobe, 23 (44.3%) in the left lobe.
Afirma vs. surgery
Of these 52 cases with repeated AUS after second FNA, 14 (26.9%) cases were classified as GEC-B. 4 cases with GEC-B results had surgical follow-up with 3 (75%) of them confirmed benign and 1 (25%) being malignant on final histopathology. The other 38 (73.1%) cases were reported GEC-S. 35 went for surgery and 32 (91.4%) were confirmed malignant on histopathology.
The malignant diagnoses included 9 cases of follicular variant of papillary thyroid carcinoma (FV-PTC), 23 cases of papillary thyroid carcinoma (PTC), 2 cases of follicular carcinoma (FC), and 1 case of Hurthle cell carcinoma (HC).
GEC correctly identified 32 of the 35 malignant samples as GEC-S yielding a sensitivity of 96%; 13 of 14 non-malignant samples were correctly identified as GEC-B by the Gene Expression Classifier yielding a specificity of 81.2%. The negative predictive value was 92.8% (Table 1).
| Afirma GEC | U/S (solid and hypo-echoic) | |
|---|---|---|
| Sensitivity | 96% (n=33) | 71.8% (n=32) |
| Specificity | 81.2% (n=16) | 88.2% (n=17) |
| PPV | 91.4% (n=35) | 92% (n=25) |
| NPV | 92.8% (n=14) | 62.5% (n=24) |
Table 1: Afirma GEC vs. ultrasound on AUS-2.
Afirma vs. ultrasound
The ultrasound examination revealed nodular micro calcifications in 9 of the 52 cases (17.30%). Of these the Afirma result was suspicious in 8 and benign in 1 case. Surgical follow up was available in 6 of the 8 nodules with calcifications and suspicious Afirma result. Of these 6 (75%) were diagnosed as malignant (4 cases as papillary thyroid carcinoma, 1 case as follicular carcinoma and 1 case as Hurthle cell carcinoma).
In contrast 27 of 38 cases with suspicious Afirma results but without reported calcification by ultrasound were found to be malignant on final histopathology result.
Hyper vascularity was reported in 16 of the 52 cases (30.7%). Afirma diagnosed 14 cases as GEC-S and 2 as GEC-B. Surgical follow up was available in 12 of the 14 cases with hyper vascularity and suspicious Afirma results. Malignancy was reported in 10 cases (83.3%).
For nodules that were AUS-2 with solid and hypoechoic features on USG (n=25), 23 (92%) cases confirmed malignancy on histopathology and 2 (8%) cases were benign. Of the AUS-2, nodules that were neither solid nor hypoechoic on US (n=24), only 9 (37.5%) of cases confirmed malignancy on histopathology and 15 (62.5%) were benign. Nodules (n=3) that showed cystic features on USG were excluded. Nodules that were AUS-2, solid and hypoechoic on ultrasound had a 92% positive predictive value for being malignant. 19 GEC-benign cases underwent clinical follow up for one year with 16 showing no change in size or ultrasonography characteristics. 3 cases underwent surgery due to compressive symptoms with benign final histopathology.
Discussion
This study describes the validity and utility of gene expression classifier in the evaluation of AUS cytology at Cleveland Clinic Florida. Additionally, we also aimed to evaluate the performance of ultrasound for predicting malignancy in the setting of AUS cytology.
Well defined criteria have been established for the interpretation of thyroid FNA specimens by The Bethesda System for reporting cytopathology. The indeterminate category includes AUS with an associated risk of malignancy of 5% to 15%. The traditional approach for management of AUS included a repeat FNA followed by surgical lobectomy if a definitively benign cytology was not obtained. More recently, attempts to develop gene expression classifiers to aid in the preoperative characterization of thyroid nodules with indeterminate cytology have been made. The Afirma GEC uses an algorithm based on the expression of 167 genes to classify thyroid FNA specimens as either benign or suspicious. Of these 167 genes, 142 are involved in the main classifier and the remaining 25 genes filter out rare neoplasm [26]. The NPV for the gene expression classifier for first FNA aspirates classified as AUS was 95% in previous reports [4,26]. We had 92.8% of NPV after two consecutive FNA with AUS results, implying that thyroid nodules with these cytological abnormalities and benign gene expression classifier results have a post-test probability for nodules with cytological benign features on fine-needle aspiration [7,27].
The overall positive predictive value (PPV) for malignancy of Afirma in cases diagnosed as AUS was 71.4% in the current study which was considerably higher than that noted in the published literature (38% to 48%)[26,29]. This discrepancy appears due to the use of GEC only after 2 consecutive AUS cytologies. It is evident from our data that the diagnostic accuracy of GEC testing was markedly higher at 91.4% when it was done after 2 consecutive AUS cytologies compared to its performance after a single such biopsy which was associated with a low positive predictive value of 66.6%.
With regards to ultrasonography findings, we found that nodules that were AUS-2 with solid and hypoechoic features on ultrasound have a high probability of malignancy with a 92% PPV. The PPV of these two ultrasound features is equivalent to that of a “suspicious” GEC on these selected AUS-2 nodules. On the other hand, malignancy could not be excluded in those nodules with two separate AUS cytologies that are neither solid nor hypoechoic.
One limitation of our study is that we did not have histopathology reports on all nodules considered benign by biopsy and/or GEC. However, given the general acceptance of the performance of benign FNA and GEC test results we consider our calculations to still be valid. Although our study population and methods were homogeneous, the results might not apply to the use of these tests in the community since geographic variations, institution-specific prevalence of malignancy, and variations in cytopathology determination of AUS cytology results affect performance of the test [27,29]. There was a potential selection bias in categorizing some AUS nodules to get selected for GEC initially while others had repeat cytology.
Our data suggests that proceeding directly to surgery after two AUS cytologies for nodules that are solid and hypoechoic is a reasonable practice given a high yield for malignancy. Additional assessment with GEC would be helpful in nodules not having these ultrasound characteristics to better exclude malignancy. This recommendation would align with the current guidelines from the ATA [30]. Prospective multi-center studies with long term follow are required to more precisely define the best practices in the evaluation and appropriate management of thyroid nodules with indeterminate cytology.
Disclosures
No potential conflicts of interest relevant to this article were reported.
References
- KimDL, SongKH, and KimSK(2008)High prevalence of carcinoma in ultrasonography-guided fine needle aspiration cytology of thyroid nodules.� Endocr J 55: 135-142.
- Kim EK, Park CS, Chung WY, Oh KK, Kim DI, et al. (2002) New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol; 178: 687-691.
- Peccin S, de Castsro JA, Furlanetto TW, Furtado AP, Brasil BA, et al. (2002) Ultrasonography: is it useful in the diagnosis of cancer in thyroid nodules? J Endocrinol Invest 25: 39-43.
- Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB Jr. (2003) Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med 22: 1083-1090
- Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK (2010) Can vascularity at power Doppler US help predict thyroid malignancy? Radiology 255: 260-269.
- Moon WJ1, Jung SL, Lee JH, Na DG, Baek JH, et al. (2008) Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study.Radiology 247: 762-770.
- Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, et al. (2006) AACE/AME task force on thyroid nodules. American association of clinical endocrinologist guidelines for clinical practice for the diagnosis and management of thyroid nodules. EndocrPract 12:63-102.
- Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA 295: 2164-2167.
- Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, et al. (2002) Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J ClinEndocrinolMetab 87: 1941-1946.
- Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, et al. (2009) An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J ClinEndocrinolMetab 94: 1748-1751.
- Cooper DS, Doherty GM, et al. (2009)American Thyroid Association (ATA) Guidelines taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167-1214.
- Baloch ZW, LiVolsi VA, Asa SL, et al.(2008) Diagnostic terminology and morphologic criteria for cytologic diagnostic of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference DiagnCytopathol36: 425-437
- Cibas ES, Ali SZ (2009) The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 19: 1159-1165.
- Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference (2009) The Bethesda System For Reporting Thyroid Cytopathology. Am J ClinPathol 132: 658-665.
- Lee YH, Kim BH, Suh SI, Seo HS, Seo BK,et al. (2009) Comparison of cytological results obtained by repeated US-guided fine-needle aspiration biopsies of thyroid nodules: focus on the rate of malignancy and diagnostic concordance. Diagncytopathol37: 492-497.
- Sahin M, Gursoy A, Tutuncu NB, Guvener DN (2006) Prevalence and prediction of malignancy in cytologically indeterminate thyroid nodules.ClinEndocrinol (Oxf) 65: 514-518.
- Pang T, Ihre-Lundgren C, Gill A, McMullen T, Sywak M, et al. (2010) Correlation between indeterminate aspiration cytology and final histopathology of thyroid neoplasms. Surgery 148: 532-537.
- Yoon JH, Kwak JY, Kim EK, Moon HJ, Kim MJ, et al. (2010) How to approach thyroid nodules with indeterminate cytology. Ann SurgOncol 17: 2147-2155.
- Miller B, Burkey S, Lindberg G, Snyder WH 3rd, Nwariaku FE (2004) Prevalence of malignancy within cytologically indeterminate thyroid nodules. Am J Surg 188: 459-462.
- Kim JW, Park IS, Kim BM, Kim YM, Chu YC, et al. (2007) The clinical significance of atypia in thyroid fine-needle aspiration.Eur Arch Otorhinolaryngol 264: 1053-1057.
- Chudova D, Wilde JI, Wang ET, Wang H, Rabbee N, et al. (2010) Molecular classification of thyroid nodules using high-dimensionality genomic data. J ClinEndocrinolMetab 95: 5296-5304.
- Haugen BR, Baloch ZW, Chudova D, Cibas E, Friedman L, et al. (2010) Development of a novel molecular classifier to accurately identify benign thyroid nodules in patients with indeterminate FNA cytology. Program of the 14th International Thyroid Congress, Paris, France, p 64. Abstract LB-06.
- Altman DG, Bland JM (1994) Diagnostic tests 2: Predictive values. BMJ 309: 102.
- Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, et al. (2012) Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med 367: 705-715.
- Wang CC, Friedman L, Kennedy GC, Wang H, Kebebew E, et al. (2011) A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid21:243-251.
- Lewis CM, Chang KP, Pitman M, Faquin WC, Randolph GW (2009) Thyroid fine-needle aspiration biopsy: variability in reporting. Thyroid 19: 717-723.
- Alexander EK, Schorr M, Klopper J, Kim C, Sipos J, et al. (2014) Multicenter clinical experience with the Afirma gene expression classifier. J ClinEndocrinolMetab 99: 119-125.
- http://press.endocrine.org/e-letters/10.1210/jc.2013-2482
- Ferris RL, Baloch Z, Bernet V, Chen A, Fahey TJ(2015) American Thyroid Association Statement on Surgical Application of Molecular Profiling for Thyroid Nodules: Current Impact on Perioperative Decision Making. Thyroid25:760-768
- Haugen BR, Alexander EK, Bible KC, et al. (2015) American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. Oct 14.
Relevant Topics
- Anal Cancer Diagnosis
- Biomarkers
- Bladder Cancer Diagnosis
- Blood Cancer Diagnosis
- Brain Cancer Diagnosis
- Breast Cancer Diagnosis
- Cancer Diagnosis
- Cervical Cancer Diagnosis
- Colon Cancer Diagnosis
- Diagnostic Algorithms
- Early Detection
- Esophageal Cancer Diagnosis
- Genetic Mutations
- Imaging Techniques
- Kidney Cancer Diagnosis
- Leukemia Diagnosis
- Liver Cancer Diagnosis
- Lung Cancer Diagnosis
- Lymphoma Diagnosis
- Mesothelioma Diagnosis
- Mouth Cancer Diagnosis
- Ovarian Cancer Diagnosis
- Pancreatic Cancer Diagnosis
- Prostate Cancer Diagnosis
- Rectal Cancer Diagnosis
- Skin Cancer Diagnosis
- Testicular Cancer Diagnosis
- Thyroid Cancer Diagnosis
- Uterine Cancer Diagnosis
Recommended Journals
- Journal of Lung Cancer Diagnosis & Treatment
- Cancer Prevention Journal
- Advances in Cancer Prevention
- Breast Cancer: Current Research
- Cancer Surgery Journal
- International Journal of Inflammation, Cancer and Integrative Therapy
- Cervical Cancer: Open Access
- Journal of Oncology Research and Treatment
- Journal of Prostate Cancer
Article Tools
Article Usage
- Total views: 13985
- [From(publication date):
December-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 13121
- PDF downloads : 864