Using Rational Protein Engineering, Analyze the Expansion of Substrate and Template Specificities in DNA Strands
Received: 02-Aug-2022 / Manuscript No. bcp-22-71259 / Editor assigned: 04-Aug-2022 / PreQC No. bcp-22-71259 / Reviewed: 10-Aug-2022 / QC No. bcp-22-71259 / Revised: 17-Aug-2022 / Manuscript No. bcp-22-71259 / Published Date: 24-Aug-2022 DOI: 10.4172/2168-9652.1000392 QI No. / bcp-22-71259
Abstract
The use of enzymes in industrial applications has been recognised for providing clean processes with minimal impact on the environment. Enzyme engineering is undergoing the most profound and exciting transformation in its history. It promises unprecedented expansion in the scope and applications of modified or improved enzymes with desired physical and catalytic properties. Two complementary strategies are currently available: rational redesign and directed evolution. Although both approaches have met with great success, each has limitations. In this article,the perspectives for these enzyme-engineering strategies are discussed briefly.
Introduction
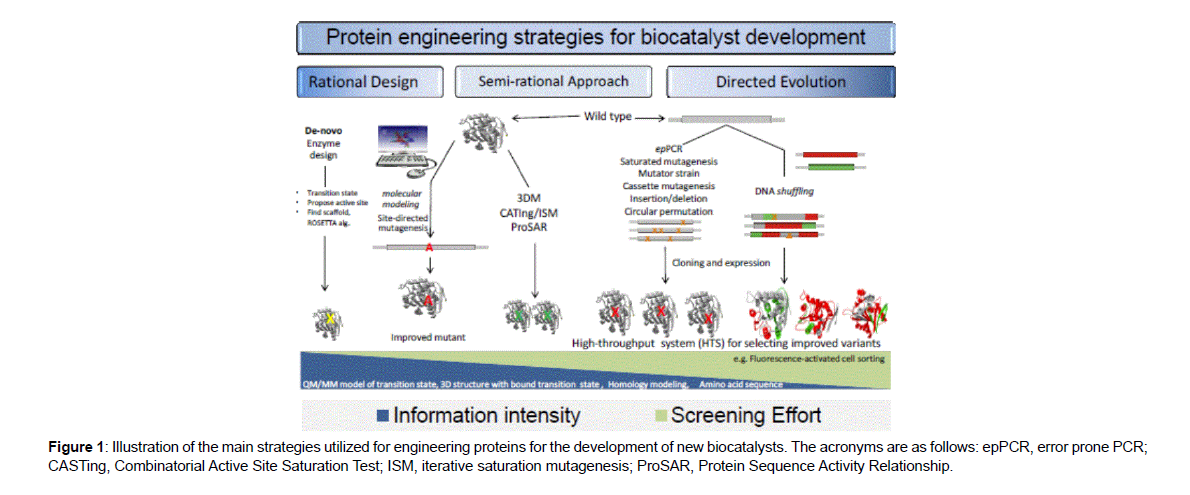
Enzymes in their native form suffer several limitations for use as industrial catalysts, such as instability at high temperature, narrow substrate spectrum or insufficient enantio selectivity. These limitations may be overcome by engineering the enzyme at the genetic level or by modification of the enzyme without structural changes e.g., immobilization to a support [1]. The former approach is commonly termed protein engineering, and involves introduction of changes in the gene coding for the enzyme, e.g., by rearrangement of the order of the amino acid sequence (circular permutations) or by introducing mutations either randomly or rationally. In general, protein engineering can be achieved via three main strategies: directed evolution, rational design and a hybrid of these two approaches known as semi-rational approach (Figure 1). The application of these tools has resulted in the successful engineering of biocatalysts from different enzyme families and has been reviewed elsewhere. (Figure 1 )
Figure 1: Illustration of the main strategies utilized for engineering proteins for the development of new biocatalysts. The acronyms are as follows: epPCR, error prone PCR; CASTing, Combinatorial Active Site Saturation Test; ISM, iterative saturation mutagenesis; ProSAR, Protein Sequence Activity Relationship.
Directed evolution does not require information about how enzyme structure relates to function. This technique employs a random process in which error-prone PCR is used to create a library of mutagenized genes. Genetic selection or high throughput screening subsequently identifies the mutants that possess improved properties. The sorted genes might be subjected to further cycles of mutation and screening to enhance the original beneficial mutation. Recently, directed evolution has been significantly improved using in vitro recombination or DNA shuffling [2]. These methods rapidly combine beneficial mutations that arise from random mutagenesis and significantly expand the sequence diversity derived from small pools of homologous genes. In the last few years, directed evolution has been widely adopted by industry and has proven extremely valuable for improving enzymes, as well as ‘evolving’ new metabolic pathways Although these techniques have been relatively successful in improving enzyme catalytic activity and physical properties, engineering substrate specificity appears to be more challenging. In the majority of cases, the enzymes had low catalytic activity and modest substrate specificity, and enzymes with new functions were rarely demonstrated. Evolutionary analysis of enzyme families suggests that drastic changes in enzyme function might require considerable changes in polypeptide backbones.
Enzymes are nature’s biocatalysts catalyzing chemical reactions at high velocity, with great specificity, under mild temperatures, with water as solvent. These reaction conditions are regarded as energy efficient and environmentally friendly. The number of commercial enzyme applications is continuously growing, despite the suboptimal performance of many natural enzymes under industrial process conditions. The current limitations of applications of enzymes in industry are poor stability, low reaction rates, product inhibition, and limited substrate conversion. In addition, there are many reactions for which no enzymes are known today. Protein engineers are therefore focusing on the identification of enzymes with new reaction specificities and are improving the performance of existing enzymes [3]. Also, DNA databases and environmental DNA libraries are screened for better performing enzymes, even though newly identified enzymes are likely to require additional optimization via protein engineering. Since the 1980s, site-directed mutagenesis (rational design) has been used to improve the properties of enzymes. Often, this approach has met with limited success, mainly because of a general lack of understanding of how protein structure relates to enzyme function. Nature, in contrast, applies Darwinian selection, for example, survival of the fittest, to alter the properties of enzymes. Since the 1990s, the Darwinian selection strategy has been applied in laboratory evolution of proteins [4]. This approach, called directed evolution, has quickly proven to be much more effective in enzyme engineering than rational design. Directed evolution involves the generation of random genetic diversity followed by highthroughput screening for desirable variants (Figure 2) and requires no structural knowledge of the protein. Where structural information is available, rational design and directed evolution are often combined to create ‘‘smart libraries,’’ introducing genetic variations at functional sites, such as the active site region of an enzyme.
Enzymes are biocatalysts evolved in nature to achieve the speed and coordination of nearly all the chemical reactions that define cellular metabolism necessary to develop and maintain life. The application of biocatalysis is growing rapidly, since enzymes offer potential for many exciting applications in industry. The advent of whole genome sequencing projects enabled new approaches for biocatalyst development, based on specialised methods for enzyme heterologous expression and engineering. The engineering of enzymes with altered activity, specificity and stability, using site directed mutagenesis and directed evolution techniques are now well established.
The use of enzymes in industrial applications has been recognised for providing clean processes with minimal impact on the environment. Enzyme engineering is undergoing the most profound and exciting transformation in its history. It promises unprecedented expansion in the scope and applications of modified or improved enzymes with desired physical and catalytic properties [5]. Two complementary strategies are currently available: rational redesign and directed evolution. Although both approaches have met with great success, each has limitations. In this article, the perspectives for these enzymeengineering strategies are discussed briefly.
Directed evolution has become the preferred engineering approach to generate tailor-made enzymes. The method follows the design guidelines of nature: Darwinian selection of genetic variants. This review discusses the different stages of directed evolution experiments with the focus on developments in screening and selection procedures.
Enzymes are increasingly being used in an industrial setting as a cheap and environmentallyfriendly alternative to chemical catalysts [6]. In order to produce the ideal biocatalyst, natural enzymes often require optimization to increase their catalytic efficiencies and specificities under a particular range of reaction conditions. A number of enzyme engineering strategies are currently employed to modify biocatalysts, improving their suitability for large-scale industrial applications. These include various directed evolution techniques, semi-rational design techniques, and more recently, the de novo design of novel enzymes. (Figure 2)
Enzyme Engineering
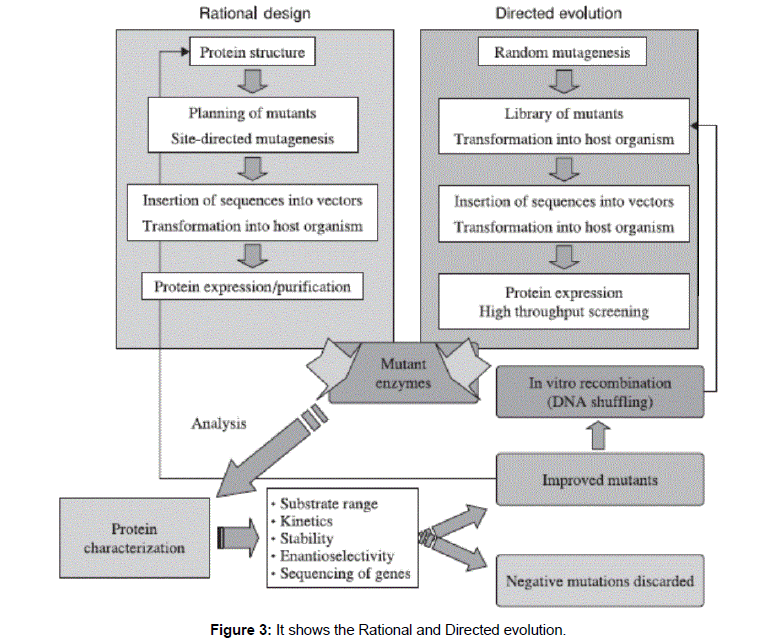
New enzyme structures may be designed and produced in order to improve existing ones or create new activities [7]. Over the past two decades, with the advent of protein engineering, molecular biotechnology has permitted not only the improvement of the properties of these isolated proteins, but also the construction of ‘altered versions’ of these ‘naturally occurring’ proteins with novel or ‘tailor-made’ properties (Figure 3).
Rational Enzyme
The rational protein design approach is mainly used for the identification and evaluation of functionally important residues or sites in proteins. Although the protein sequence contains all the information required for protein folding and functions, today’s state of technology does not allow for efficient protein design by simple knowledge of the amino acid sequence alone. For example, there are 10325 ways of rearranging amino acids in a 250-amino-acid-long protein, and prediction of the number of changes required to achieve a desired effect is an obstacle that initially appears impossible [8]. For this reason, a successful rational design cycle requires substantial planning and could be repeated several times before the desired result is achieved.
Rational Design
Molecular Modeling Docking
Homology modelling: The advances of molecular modelling software, new generation of databases and computational tools allow non-experts to perform rational design for protein engineering. The approach of rational design was successfully applied in many cases to engineer enzyme selectivity and activity. Rational design has reached advanced levels with the help of quantum mechanical calculations and information about the reaction mechanism [9-11]. For example, Baker and co-workers have designed “de-novo” enzymes for reactions without known natural enzymatic catalyst.
Conclusion
This has mainly been thanks to the introduction of a number of excellent computer algorithms, and to the exponential growth in the number of three dimensional structures and protein sequences which are available to the researcher. Smaller, high quality mutant libraries are now commonplace and a range of techniques have been introduced which permit the rapid and straightforward analysis of library members. We are now on the cusp of a new era of complete de novo design of biocatalysts, with successful reports of enzymes being designed to catalyze unnatural reactions already emerging.
Nowadays, directed evolution is the method of choice for enzyme engineering. Over the last few years, several selection and screening methods have been reported allowing screening of over 107 variants per day. The more traditional microtiter plate screenings are mediumthroughput, but are compatible with most analytical tools. In an ever-evolving climate of laboratory enzyme evolution, it is difficult to predict which of the screening technologies will dominate in the future. However, to expand the scope of ultra-high-throughput screenings, these methods should move outside the limitations of fluorescence detection only. A promising development in this field is the recent report on alkaline phosphatase expressed from single cells in droplets using microfluidics, as this technology has the potential to integrate alternative analytical tools.
References
- White HB, Benore M, Sumter T, Caldwell B, Bell E (2013)What skills should students of undergraduate biochemistry and molecular biology programs have upon graduation?Biochemistry and Molecular Biology Education41(5): 297–301.
- Wilson C D, Anderson C, W Heidemann M (2006).Assessing students’ ability to trace matter in dynamic systems in cell biology.CBE—Life Sciences Education5(4): 323–331.
- Wright L, Fisk J, N Newman D (2014).DNA→ RNA: What do students think the arrow means?CBE—Life Sciences Education13(2): 338–348.
- St Johnston D (March 2002)"The art and design of genetic screens:Drosophila melanogaster".Nat. Rev Genet 3(3): 176-188.
- Bastock R, St Johnston D (December 2008)"Drosophilaoogenesis".Curr Biol18(23): 1082-1087.
- Nusslein-Volhard C, Lohs-Schardin M, Sander K, Cremer C (1980) "A dorso-ventral shift of embryonic primordia in a new maternal-effect mutant ofDrosophila".Nature 283(5746): 474-476.
- Wesp LM, Deutsch MB (March 2017) Hormonal and Surgical Treatment Options for Transgender Women and Transfeminine Spectrum Persons.Psychiatr Clin North Am 99-111.
- Dahl, M, Feldman, JL, Goldberg, J,Jaberi, A (2015) Endocrine Therapy for Transgender Adults in British Columbia: Suggested Guidelines.Vancouver Coastal Health Endocrine therapy 120-153.
- Bourns Amy (2015)Guidelines and Protocols for Comprehensive Primary Care for Trans Clients. Sherbourne Health Centre 425-521.
- Murad Mohammad Hassan, Elamin Mohamed B (2010) Hormonal therapy and sex reassignment: A systematic review and meta-analysis of quality of life and psychosocial outcomes.Clinical Endocrinology 72(2): 214-231.
- White Hughto, Jaclyn M, Reisner, Sari L (2016) A Systematic Review of the Effects of Hormone Therapy on Psychological Functioning and Quality of Life in Transgender Individuals.Transgender Health1(1): 21-31.
Indexed at,Google Scholar,Crossref
Indexed at,Google Scholar,Crossref
Indexed at,Google Scholar,Crossref
Indexed at,Google Scholar,Crossref
Indexed at,Google Scholar,Crossref
Indexed at,Google Scholar,Crossref
Indexed at,Google Scholar,Crossref
Citation: Sharma SV, Hooda S (2022) Using Rational Protein Engineering, Analyze the Expansion of Substrate and Template Specificities in DNA Strands. Biochem Physiol 11: 392. DOI: 10.4172/2168-9652.1000392
Copyright: © 2022 Sharma SV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1272
- [From(publication date): 0-2022 - Jan 29, 2025]
- Breakdown by view type
- HTML page views: 1037
- PDF downloads: 235