Using Mesenchymal Stem Cells to Treat Female Infertility: An Update on Female Reproductive Diseases A Review Article
Received: 02-Jun-2022 / Manuscript No. JCPHN-22-66004 / Editor assigned: 04-Jun-2022 / PreQC No. JCPHN-22-66004 (PQ), / Reviewed: 13-Jun-2022 / QC No. JCPHN-22-66004 / Revised: 18-Jun-2022 / Manuscript No. JCPHN-22-66004 (R) / Accepted Date: 18-Jun-2022 / Published Date: 25-Jun-2022 DOI: 10.4172/2471-9846.1000349
Abstract
Female physiological state impacts the Female physiological state impacts the standard of life and well-being
of affected people and couples. Feminine procreative diseases, like primary female internal reproductive organ
insufficiency, polycystic ovary syndrome, adenomyosis, Fallopian tube obstruction, and as her man syndrome, will
induce physiological state. In recent years, translational drugs have developed quickly, and clinical researcher’s area
unit that specializes in the treatment of feminine physiological state exploitation novel approaches. Thanks to the
benefits of convenient samples, abounding sources, and avertible moral problems, mesenchymal stem cells (MSCs)
will be applied wide within the clinic. This paper reviews recent advances in exploitation four kinds of MSCs, bone
marrow stromal cells, adipose-derived stem cells, menstrual flow mesenchymal stem cells, and duct mesenchymal
stem cells. every of those are used for the treatment of female internal reproductive organ and female internal
reproductive organ diseases, and supply new approaches for the treatment of feminine physiological state’s quality
of life and well-being of affected people and couples. Feminine procreative diseases, like primary female internal
reproductive organ insufficiency, polycystic ovary syndrome, adenomyosis, Fallopian tube obstruction, and Asherman
syndrome, will induce physiological state. In recent years, a translational drug has developed quickly, and clinical
researcher’s area unit that specializes in the treatment of feminine physiological state exploitation novel approaches.
Thanks to the benefits of convenient samples, abounding sources, and avertible moral problems, mesenchymal stem
cells (MSCs) will be applied wide within the clinic. This paper reviews recent advances in exploitation four kinds of
MSCs, bone marrow stromal cells, adipose-derived stem cells, menstrual flow mesenchymal stem cells, and duct
mesenchymal stem cells. Every of those are used for the treatment of female internal reproductive organ and female
internal reproductive organ diseases, and supply new approaches for the treatment of feminine physiological state.
Keywords: Infertility; Mesenchymal; Reproductive disease; Stem cells
Keywords
Infertility; Mesenchymal; Reproductive disease; Stem Cells
Introduction
Infertility is outlined because the failure to attain any physiological state (including a miscarriage) for a minimum of twelve months. In 2002, 7.4% of married girls, or about 2.1 million girls, were sterilized within these. The causes of sterility are divided into 3 main classes that the prevalence is variable: feminine causes (33 to 41%), male causes (25 to 39%), and mixed causes (9 to 39%). These statistics highlight the spectacular numbers of ladies undergoing sterility.
There are units several factors inflicting feminine sterility, among that fruitful system-related diseases area unit the most causes. Etiologies for feminine sterility embrace biological process disorders (polycystic ovary syndrome, neural structure pathology, and first female internal reproductive organ insufficiency), bodily structure sterility, pathology, and female internal reproductive organ and cervical causes (cervical pathology, polyps, and tumors). Endocrine replacement medical aid is effective in some forms of sterility, however there's substantial proof from experimental studies that such medical aid will increase the chance of carcinoma. Biological process induction, super ovulation, or motor-assisted fruitful technologies have shown trends toward hyperbolic physiological state rates, although various factors about the hyperbolic risks for multiple pregnancies should be thought-about. These findings indicate shortcomings of existing treatment regimens.
Scientists have investigated alternative therapeutic measures, like vegetative cell medical aid, for physiological state. Stem cells area unit undifferentiated cells with the power to renew themselves for long periods while not vital changes in their general properties. They will differentiate into varied specialized cell sorts below sure physiological or experimental conditions [1]. Because of the restrictions of victimization embryonic and induced pluripotent stem cells within the clinic, there's nice interest in mesenchymal stem cells (MSCs), that area unit freed from each moral issues and tumor formation.
MSCs, additionally known as mesenchyme stromal cells, area unit a set of non-hematopoietic adult stem cells that originate from the germ layer. They possess self-renewal skills and multiline age differentiation into not solely germ layer lineages, like chondrocytes, osteocytes, and adipocytes, however additionally germ layer and endodermic cells [2]. MSCs may be harvested from many adult tissues, like bone marrow, menorrhea, animal tissue, the fetal membrane, and placenta.
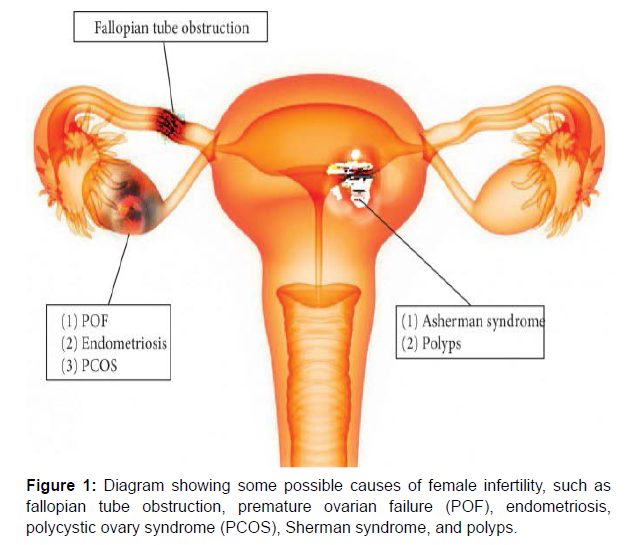
Causes of infertility in female reproductive organs
Causes of infertility in female reproductive organs include premature ovarian failure (POF), polycystic ovary syndrome, endometriosis, fallopian tube obstruction, Asherman syndrome, and other, less frequent anomalies of the reproductive tract (Figure 1 and Table 1).
| Disease | Etiologies | Definition |
|---|---|---|
| POF | Genetic defects, autoimmune processes, chemotherapy, radiation, and infections | Cessation of ovarian function after menarche but before the age of 40, without or with ovarian follicle depletion |
| PCOS | Maternal PCOS, intrauterine hyperandrogenism, inflammatory adipokines, aboriginal origin-Western diet | A complex disorder characterized by infertility, hirsutism, obesity, and various menstrual disturbances |
| Endometriosis | Oxidative stress, reactive oxygen species, antioxidants and inflammatory, genetic, and epigenetic factors | A condition in which functional endometrial tissue is present outside the uterus |
| Fallopian tube obstruction | Neoplasms, neoplasms, tuboovarian abscess | Tubal obstruction is caused by inflammation of the fallopian tube or pelvic peritoneum |
| AS | Trauma, infection, low level of estradiol, repeated or aggressive curettage, severe endometritis | Absence of a normal opening in the lumen of the female genital tract, from the fallopian tubes to the vagina |
Table 1: Causes of infertility in female reproductive organs.
| MSC types | Disease | Treatment | Model | Main results | References | |
|---|---|---|---|---|---|---|
| Bone marrow stromal cells | Ovarian dysfunction | CTX-induced ovarian failure | Intravenous injection | Rabbit | Ovarian function ↑ | [31] |
| CTX-induced ovarian failure | Local injection | Mice | Restore ovarian hormone production | [32] | ||
| Endometrial disorders | 24-gauge needle-induced AS | Labeled with SPIOs local/tail vein injection | Mice | Endometrial proliferation ↑ | [33] | |
| Refractory AS | Uterine artery injection | Human | Reconstruct the endometrium | [34] | ||
| Adipose-derived stem cells | Ovarian dysfunction | Cisplatin-induced ovarian failure | Local injection | Mice | Ovarian function ↑ | [35] |
| TG-induced ovarian damage | Collagen scaffold | Rat | Fertility ↑ | [36] | ||
| Endometrial disorders | Trichloroacetic acid-induced AS | Intraperitoneal injection | Rat | Fibrosis ↓, endometrial proliferation ↑ | [37] | |
| MB-MSCs | Ovarian dysfunction | CTX-induced POF | Local injection | Mice | Ovarian weight ↑, hormone secretion ↓ | [38] |
| Cisplatin-induced POF | Local injection | Mice | Ovarian function ↑, fibroblast growth factor 2 ↑ | [39] | ||
| Endometrial disorders | Severe AS | Deliver through the cervix to the fundus of the uterus | Human | Endometrial thickness ↑ | [40] | |
| Mechanical injured-induced intrauterine adhesion | Local injection | Rat | Pregnancy rate ↑ | |||
| UC-MSCs | Ovarian dysfunction | CTX-induced POF | Tail vein injection | Mice | Weight of the ovaries ↑, estradiol ↑, | |
| Paclitaxel-induced POF | Local injection | Rat | Follicle-stimulating hormone ↓, estradiol ↑, ovarian function ↑ | |||
| Perimenopausal ovary | Tail vein injection | Rat | Estradiol ↑, follicle-stimulating hormone ↓, follicle number ↑ | |||
| Busulfan CTX-induced premature ovarian insufficiency | Local injection | Mice | Fertility ↑, ovarian functions ↑ | |||
| Endometrial disorders | Uterine niche | Local intramuscular injection | Human | Uterine scar reconstruction ↑, uterine niche incidence ↓ | ||
| 95% ethanol-induced endometrial injury | Tail vein injection | Rat | Fertility ↑, endometrial fibrosis ↓, angiogenesis ↑ | |||
Table 2: Application of MSC therapy in the treatment of female reproductive dysfunction.
Mesenchymal stem cells
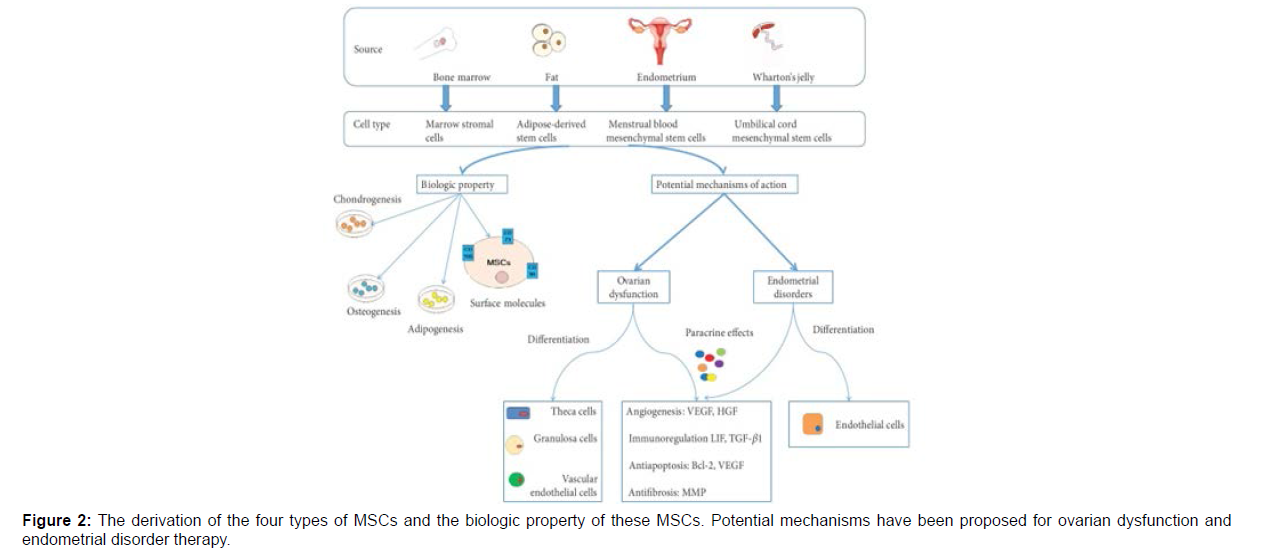
To begin to deal with the utilization of mesenchymal stem cells (MSCs), the Mesenchymal and Tissue somatic cell Committee of the International Society for Cellular medical care has planned to knish criteria to outline human MSCs. First, MSCs should be plastic-adherent once maintained in commonplace culture conditions. Second, MSCs should categorical CD105, CD73, and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR surface molecules. Third, MSCs should differentiate to osteoblasts, adipocytes, and chondroblasts in vitro. In 2016, the institute counseled adding Master of Science immunomodulation function-related issue detection as a supplementary take a look at commonplace [3]. The various MSCs are classified supported their supply (Figure 2).
The derivation of the four types of MSCs and the biologic property of these MSCs. Potential mechanisms has been proposed for ovarian dysfunction and endometrial disorder therapy. Vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), leukemia inhibitory factor (LIF), transforming growth factor (TGF), B-cell lymphoma 2 (Bcl-2), and matrix metalloproteinase (MMP). Laboratory experiments and clinical trials are now using MSCs, alone or in combination with other drugs, for potential application to ovarian dysfunction and endometrial disorders. Importantly, therapeutic interventions for numerous diseases in female reproductive organs are causing great excitement [4]. More importantly, these studies provide a desirable experimental model for elucidating the underlying mechanism of using MSCs for treating female infertility. This provides the theoretical basis for further studies and clinical therapy with MSCs.
For ovarian dysfunction, MSCs can directly and impulsively migrate to the injured ovary and survive there under the stimulation of multiple factors, which facilitates ovarian recovery. According to available studies, the number of differentiated and functionally integrated MSCs is too small to explain the observed improvements in ovarian function. Furthermore, whether MSCs differentiate into oocytes after migrating to injured tissue is still unknown [5]. Improved ovarian function in these studies might be driven by paracrine mechanisms. These mechanisms involve the secretion of certain cytokines, including vascular endothelial growth factor, insulin-like growth factor, and hepatocyte growth factor, which are helpful for angiogenesis, antiinflammation, immunore gulation, antiapoptosis, and antifibrosis to help ovarian restoration [6].
Further studies square measure required to explore whether or not MSCs differentiate into target cells like oocytes or supporting cells that improve sex gland functions and ultimately correct sex gland pathology [7]. Such differentiation would even be valuable for MSc transplantation applied as a clinical medical care. Similarly, MSCs improve the mucosa reserve that in the main depends on orientating and paracrine activities. In studies so far, it's wide accepted that the paracrine impact of MSCs is that the most significant, instead of differentiation [8]. Specifically, the regenerative properties of transplanted MSCs is attributed to mechanisms that involve cell-cell contact and secretion of bioactive molecules that promote ontogenesis and tissue repair, thereby inhibiting scarring, modulating inflammatory and immune reactions [9], and activating tissue-specific primogenitor cells. However, different analysis suggests that MSCs engraft the mucous membrane in rodents and humans, wherever they become animal tissue, stromal and epithelial tissue cells. Thus, MSCs would possibly promote mucosa regeneration and restore fertility by paracrine factors [10], however different mechanisms square measure plausible.
Bone marrow stromal cells
Initially described by Owen and Friedenstein in 1988, bone marrow stromal cells were separated from nucleated bone marrow cells on plastic culture dishes by density gradient centrifugation [11]. These cells had a longer replication cycle and premature senility, accounting for only 0.01–0.001% of nucleated bone marrow cells. Bone marrow stromal cells, which have been the main source of multipotent stem cells, serve as a standard for comparison with MSCs from other sources [12]. Bone marrow stromal cells not only commit to osteoblasts, adipocytes, and chondroblasts, but also differentiate into granulosa, endometrial, and endothelial cells in mammals [13]. Furthermore, bone marrow stromal cells have broad application prospects in the field of regenerative medicine, including reproductive dysfunction.
Application of bone marrow stromal cells to treat ovarian dysfunction
Several studies have shown beneficial effects of bone marrow stromal cell treatment in a chemotherapy-induced ovarian failure animal model [14]. Specifically, the results showed that ovarian structure and functions could be restored by bone marrow stromal cells. Although chemotherapy drugs can inhibit the growth of tumor cells, they can also lead to granulosa cell apoptosis, follicular atresia, ovarian function decline, and other manifestations of premature ovarian failure [15]. Granulosa cells, which are located on the lateral side of the oocyte zona pellucidum and secrete estrogen under the action of follicle-stimulating hormone and other paracrine factors, play a role in nutrition and support of oocytes [16]. Granulosa cell apoptosis thus leads to a decrease in estrogen levels in the body, affecting the normal development of oocytes.
Adipose-derived stem cells
Currently, adipose-derived stem cells, a new type of MSC, have been used primarily to repair tissues although these cells have the same biologic characteristics as bone marrow stromal cells, they are easier to isolate in large quantities (by minimally invasive liposuction) than bone marrow stromal cells [17]. Thus, compared with bone marrow stromal cells, adipose-derived stem cells represent a more practical option. Demonstrated that adipose-derived stem cell therapy improved ovarian graft quality by promoting an increase in vascular endothelial growth factor-A gene expression and the number of blood vessels in ovarian tissue to induce an earlier resumption of function in freshly grated ovaries of adult female rats [18]. In addition, adipose-derived stem cells ameliorated chemotherapy-induced ovarian dysfunction in mouse models and were capable of inducing angiogenesis and restoring the number of ovarian follicles and corpus luteum in damaged ovaries [19]. Another experiment, using a rat model of premature ovarian insufficiency, verified that adding a collagen scaffold enhanced the shortterm maintenance of adipose-derived stem cells in ovaries, compared with transplanting these cells alone[20]. In another experimental rat model, the use of estrogen in combination with adipose-derived stem cells efficiently induced regeneration of the endometrium in Asherman syndrome therapy.
Menstrual blood mesenchymal stem cells (mb-mscs)
Menstrual blood mesenchymal stem cells (MB-MSCs) can be isolated from menstrual blood. These cells have high proliferative, self-renewal, and multiple differentiation potentials [21]. In addition, they appear to possess numerous advantages over stem cells derived from other sources including ease of collection, safe and noninvasive proliferation, no ethical concerns, and no autoimmune rejection responses [23]. Some clinical trials have used MB-MSCs to treat neuronal diseases, diabetes mellitus and multiple sclerosis.
Application of mb-mscs to treat ovarian dysfunction
Several studies have shown that MB-MSCs reduce apoptosis in granulosa cells and fibrosis of the ovarian interstitial [24], thereby improving folliculogenesis and rescuing overall ovarian function in an animal model of POF, including restoring fertility [25]. In addition, demonstrated that MB-MSCs produced a high level of fibroblast growth factor 2, which enhanced cell survival, proliferation, and function to repair tissue damage. Furthermore [26],
Indicated that MB-MSCs reduced granulosa cell apoptosis and improved ovarian functions in mice by down regulating Gadd45b protein expression (a stress sensor whose effects are mediated via physical interactions with other cellular proteins implicated in cell cycle regulation) and up regulating cyclinB1 and CDC2 (regulators of the G2/M transition in mammalian cells) .
Application of MB-MSCs to Treat Endometrial Disorders
MB-MSCs isolated from ectopic endometriotic lesions contribute to the pathogenesis of endometriosis. A clinical study where autologous MB-MSCs were transplanted into seven patients with severe Asherman syndrome, followed by hormonal stimulation, showed that the thickness of the endometrium in five women reached 7 mm, one patient had a spontaneous pregnancy, and two of the remaining four patients undergoing embryo transfer became pregnant [27].
In rats with damaged endometrium (an Asherman syndrome model), transplanted MB-MSCs assembled into spheroids and significantly improved fertility by increasing the synthesis of antigenic and anti-inflammatory factors .The main properties of MB-MSCs were retained in the spheroids, except for the expression of CD146 that was negatively correlated with self-renewal ability . This seems to be a key to improve the therapeutic effect of MB-MSCs organized into spheroids [28]. Were the first to show that MB-MSCs could differentiate into endometrial cells in vitro and rebuild endometrial tissue in NOD SCID mice after administering estrogen and progesterone in vivo? As a transcription factor, OCT-4-positive cells can differentiate into three germ layers. Furthermore, the cloning efficiency and OCT-4 expression of MB-MSCs from patients with severe intrauterine adhesions were significantly decreased compared with controls.
Platelet-rich plasma (PRP), an autologous plasma product with platelet concentrations above baseline values, has been used to treat acute and chronic injuries. Compared placebo, MB-MSC transplantation, PRP transplantation [29], and combined MBMSC and PRP transplantation in the treatment of a rat model of intrauterine adhesion they found that combining MB-MSCs with PRP was more effective than either treatment alone in improving endometrial proliferation, angiogenesis, and morphological recovery. This treatment also reduced fibrosis and inflammation by changing the Hippo signaling pathway and regulating the downstream factors, connective tissue growth factor.
Umbilical cord mesenchymal stem cells
Umbilical cord mesenchymal stem cells (UC-MSCs), isolated directly from Wharton's jelly of the UC, are called human Wharton's jelly MSCs. They express the MSC markers CD29, CD44, CD73, CD90, and CD105, and do not express CD31, CD45, and HLA-DR85. Because they have lower oncogenicity and faster self-renewal abilities compared to other sources of MSCs, UC-MSCs are a new source of stem cells that can differentiate into several mesodermal cell types and be used for cell therapy.
Application of uc-mscs to treat ovarian dysfunction
UC-MSCs have been used in several animal models to successfully treat POF by reducing apoptosis of granulosa cells, decreasing folliclestimulating hormone serum levels, and increasing estrogen and anti- Mullerian hormone levels. Proposed that UC-MSCs could reverse paclitaxel-induced apoptosis of ovarian cells either by establishing a normal arrangement of the surface epithelium and tunica albuginea, or by up regulating cytokeratin 8/18, transforming growth factor-β, and proliferating cell nuclear antigen to suppress caspase-3 expression [30]. In another investigation, transplanted CM-Dill-labeled human UCMSCs into cyclophosphamide-injured ovaries in mice. They found that UC-MSCs were not distributed equally in different parts of the ovarian tissue. Specifically, the number of CM-Dill-labeled human UC-MSCs in the ovarian medulla was greater than those of the ovarian cortex and germinal epithelium.
UC-MSCs on a collagen scaffold have been transplanted into ovaries to treat POF. found that this technique activated primordial follicles in vitro via phosphorylation of FOXO3a, a major suppressor of primordial follicle activation, and FOXO1. Found that human UCMSCs used to treat peri menopausal rats secreted cytokines, such as hepatocyte growth factor, vascular endothelial growth factor, and insulin-like growth factor-1, resulting in improved ovarian reserve functions.
Application of uc-mscs to treat endometrial disorders
Wharton's jelly-derived MSCs have the ability to differentiate into endometrial cells. In a rat model, found that human UC-MSCs repaired injured endometrium, thereby improving fertility. These researchers also found that the number of implanted embryos was higher in groups with multiple UC-MSC transplantations compared to a single UC-MSC transplantation, by up regulating vascular and down regulating pro inflammatory factors. Furthermore, UC-MSCs in collagen scaffolds have been used to promote endometrial regeneration by up regulating matrix metalloproteinase-9 in rat uterine scars.
UC-MSCs can ameliorate damage to human endometrial stromal cells, and local intramuscular injection is effective for treating uterine niches after cesarean delivery. Additionally, UC-MSCs on collagen scaffolds have been used in a phase I clinical trial to treat patients with recurrent uterine adhesions. The results suggested that they can improve endometrial proliferation, differentiation, and neovascularization by up regulating estrogen receptor α, vimentin, Ki67, and von Willebrand factor expression levels, and down regulating the ΔNP63 expression level.
Conclusions and future perspectives
MSCs have incontestable nice potential and convenience for treating feminine physiological state in animal and human studies. Autologous adipose-derived stem cells are particularly helpful as a result of they're not solely simply obtained, however additionally avoid graft rejection once transplantation. In recent decades, autologous adipose-derived somatic cell transplantation or injection have shown positive effects on rat models of POF and Asherman syndrome and may increase fertilization rates. However, there are many main directions for exploitation MS to treat unfruitful girls caused by sex gland or female internal reproductive organ factors:
• Most studies are done on little animals, and there's a heavy lack of valuable analysis in massive animal models that a lot of closely mimic the sex gland or mucosa pathophysiology of human feminine physiological state. Moreover, an irregular controlled trial ought to be conducted to verify the therapeutic result of MSCs in fertility drugs.
• The mechanism of MSCs in treating disfunction of feminine fruitful organs remains unknown. Potentialities embody promoting development, differentiating into practical cells, and a paracrine mechanism. Among these, a paracrine mechanism can be the foremost vital for feminine physiological state treatment. However, helpful paracrine factors stay unknown and multiple mechanisms is also synergistic.
• Whereas MS medical aid is promising, the restricted survival and engraftment of bioactive agents thanks to hostile surroundings may be a bottleneck for malady treatment. Therefore, the way to maintain and enhance the survival and secretion of MSCs over an extended amount of your time needs a lot of in-depth analysis. One approach that maximizes the utility of MSCs for sex gland and mucosa disorders has been the event of assorted kinds of biomaterials. Collagen-based biomaterials have already been used as MS delivery vehicles to boost cell adhesion, retention, and engraftment. All the same, extra work is required to optimize this approach.
Declaration
This study was not supported by any other sources The authors have declared no conflicts of interest Consent to participate and ethics approval This evaluation does not require ethical approval because no patient data will be collected. Plagiarism, confidentiality, malfeasance, data falsification and/or falsification, double publishing and/or submission, and duplication are all ethical issues that the author has thoroughly examined in this work.
Acknowledgements First and foremost, I want to express my gratitude to God for allowing me the opportunity to undertake and complete this research project for my academic achievement. I'd want to thank and show thanks to my mentor for encouraging and guiding me during the process of writing this post. Who had devoted their valuable time to assisting me, either directly or indirectly, during the voyage? I'd want to express my heartfelt gratitude to all of my friends for their unwavering support and aid in completing this study. Finally, I'd want to express my gratitude to my family for their love, patience, and support during the study process.
References
- Hull MG, Glazener CM, Kelly NJ, Foster PA, Coulson C, et al. (1985) Population study of causes, treatment, and outcome of infertility. BMJ 291:1693–1697.
- Chandra A, Martinez GM, Mosher WD, Amba JC, Jones J(2005) Fertility, family planning, and reproductive health of U.S. women; data from the 2002 National Survey of Family Growth. Vital Health Stat 23:1–160.
- Deroux A, Dumestre PC, Dunand FC, Bouillet L, Hoffmann P (2017) Female infertility and serum auto-antibodies: a systematic review. Clin Rev Allergy Immunol 53:78–86.
- Holmberg L, Iversen OE, Rudenstam CM, Janusz J, Jacek J, et al.( 2008) Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst 100:475-482.
- Vermeulen RF , Korse CM, Kenter GG, Brood-van Zanten MM,van Beurden M( 2019) Safety of hormone replacement therapy following risk-reducing salpingo-oophorectomy: systematic review of literature and guidelines. Climacteric 22:352–360.
- Samantha P, Marc F, John Collins, Owen Davis, Glen S, (2012) multiple gestations associated with infertility therapy: an American Society for Reproductive Medicine Practice Committee opinion. Fertil Steril 97(4):825-834.
- Blum B, Benvenisty N (2008) The tumorigenicity of human embryonic stem cells. Adv Cancer Res 100:133-158.
- Dominici M, Le Blanc K, Mueller I, Marini Fc, Krause Ds, et al.( 2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317.
- Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M , et al.( 2005) Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 309:314–317.
- Salem HK, Thiemermann C (2010) Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 28(3):585–596.
- Körbling M, Anderlini P (2001) Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter?. Blood 98:2900–2908.
- Meng X, Ichim TE, Zhong J, Andrea R, Zhenglian Y, et al.( 2007) Endometrial regenerative cells: a novel stem cell population. J Transl Med 5(1):1–10.
- Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584.
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, et al.( 2004) Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 103:1669–1675.
- Anker PS, Scherjon SA, der Keur CK, Godelieve M , Frans H, et al.( 2004) Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22:1338–1345.
- Krampera M , Galipeau J, Shi Y, Tarte K, Sensebe L(2013) Immunological characterization of multipotent mesenchymal stromal cells-The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 15:1054–1061.
- Galipeau J, Krampera M, Barrett J, Francesco D, Robert JD, et al. (2016) International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 18:151–159.
- Naji A, Rouas-Freiss N, Durrbach A, Carosella ED, Sensébé L, et al. (2013) concise review: combining human leukocyte antigen G and mesenchymal stem cells for immunosuppressant biotherapy. Stem Cells 31:2296–2303.
- Squillaro T, Peluso G, Galderisi U (2016) Clinical trials with mesenchymal stem cells: an update. Cell Transplant 25:829–848.
- Galipeau J, Sensébé L (2018) Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22:824–833.
- Trounson A, McDonald C (2015) Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17:11–22.
- Owen M, Friedenstein AJ (1988) Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 136:42–60.
- Altaner C, Altanerova V, Cihova M(2013) Characterization of mesenchymal stem cells of “no-options” patients with critical limb ischemia treated by autologous bone marrow mononuclear cells. PLoS One 8:9.
- Yun Cheng H (2014) The impact of mesenchymal stem cell source on proliferation, differentiation, immunomodulation and therapeutic efficacy. Stem Cell Res Ther 4:1-8.
- Ullah, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 35:1–18.
- Besikcioglu HE, Sarıbas GS, Ozogul C (2019) Determination of the effects of bone marrow derived mesenchymal stem cells and ovarian stromal stem cells on follicular maturation in cyclophosphamide induced ovarian failure in rats. Taiwan J Obstet Gynecol 58:53–59.
- Gao L, Huang Z, Lin H, Tian Y, Li P, Lin S(2019) Bone marrow mesenchymal stem cells (BMSCs) restore functional endometrium in the rat model for severe Asherman syndrome. Reprod Sci 26:436–444.
- Liu Y, Tal R, Pluchino N, Mamillapalli R, Taylor HS(2018) Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J Cell Mol Med 22:67–76.
- Tepper OM, Sealove BA, Murayama T, Asahara T (2003) Newly emerging concepts in blood vessel growth: recent discovery of endothelial progenitor cells and their function in tissue regeneration. J Investig Med 51:353–359.
- Wei X, Yang X, Han ZP, Qu FF, Shao L, et al. (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacological Sinica 34:747–754.
- Abd-Allah SH, Shalaby SM, Pasha HF (2013) Mechanistic action of mesenchymal stem cell injection in the treatment of chemically induced ovarian failure in rabbits. Cytotherapy 15:64–75.
- Badawy A, Sobh MA, Ahdy M, Abdelhafez MS (2017) Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int J Women's Health 9:441–447.
- Fu X, He Y, Wang X (2017) Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res Ther 8:187.
- Mohamed SA, Shalaby SM, Abdelaziz M (2018) Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod Sci 25:51–63.
- Taylor HS (2004) endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 292(1):81–85.
- Panchal S, Patel H, Nagori C(2011) Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci 4:43–48.
- Singh N, Mohanty S, Seth T, Shankar M, Dharmendra S, et al. (2014) Autologous stem cell transplantation in refractory Asherman’s syndrome: a novel cell based therapy. J Hum Reprod Sci 7:93–98.
- Wang J, Ju B, Pan C(2016) Application of bone marrow-derived mesenchymal stem cells in the treatment of intrauterine adhesions in rats. Cell Physiol Biochem 39:1553–1560.
- Santamaria X, Cabanillas S, Cervelló I (2016) Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod 31:1087–1096.
- Cervelló I, Gil-Sanchis C, Santamaría X (2015) Human CD133+ bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil Steril 104:1552–1560.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1968
- [From(publication date): 0-2022 - Apr 06, 2025]
- Breakdown by view type
- HTML page views: 1564
- PDF downloads: 404