Using Glass Waste to Prepare a Type of Medical Ceramic
Received: 01-Mar-2024 / Manuscript No. ico-24-129155 / Editor assigned: 04-Mar-2024 / PreQC No. ico-24-129155(PQ) / Reviewed: 18-Mar-2024 / QC No. ico-24-129155(QC) / Revised: 25-Mar-2024 / Manuscript No. ico-24-129155(R) / Accepted Date: 30-Mar-2024 / Published Date: 30-Mar-2024 DOI: 10.4172/2469-9764.1000269
Abstract
Promoting sustainable living in the future involves efficiently managing waste to reclaim resources and convert them into new raw materials. There is a global focus on accelerating the utilization and recycling of waste to create valuable biomaterials, driving significant research efforts in this area. In this research, pure silica was isolated from glass waste and used in the manufacture of a type of bioceramic for medical applications. Several sequential stages were followed, starting with collecting samples of crushed glass and treating them physically (grinding, granular sorting), mixing the ground glass with NaOH in the following proportions (1 :1), (2:1) (3:1), then heat treatment was carried out at different temperatures (1000-900-800), then the silica was isolated by treating the sample with HCl solution, followed by heat treatment at 900 degrees to obtain SiO2 with a yield of 68% and purity reaches 99.4%. Isolated pure silica was used to prepare standard bioglass 45S5.
Specifications of prepared bioglass was determined using XRD, IR, and DTA, then the bioglass was converted into bioceramic through heat treatment according to DTA to ultimately obtain a product whose properties, composition, and susceptibility to biodegradation were studied, results indicate that it was usable in medical applications.
Keywords
Biodegradation; Bio-glass; Glass waste; Medical Ceramic; Silica
Introduction
Biocompatible materials are specifically designed to interact with the human body, aiding in the assessment, support, and replacement of specific tissues or organs. These materials are intended for implantation and seamless integration into the body, with their performance evaluated based on their functionality and compatibility within the biological environment post-implantation. The biological function of these materials pertains to their ability to fulfill a specific role from a physical or mechanical standpoint, while biological compatibility refers to their capacity to maintain this function without causing harm or rejection by the surrounding tissues [1-3].
Medical ceramics are highly valued as biologically active materials due to their excellent biological compatibility, resistance to corrosion, low porosity, and minimal thermal and electrical conductivity. Bio ceramics form a bond with bone tissue by developing a biologically active hydroxycarbonate apatite (HCA) layer that mimics the structural and chemical composition of natural bone mineral, facilitating interfacial bonding. One of the most extensively studied bone bonding materials is the 45S5 bioactive glass system, composed of 45% SiO2, 24.5 % CaO, 24.5 % Na2O, and 6 % P2O5. This bioactive glass exhibits a highly reactive surface that interacts vigorously when exposed to human plasma or similar solutions, leading to the formation of a silicarich gel layer and subsequent precipitation of a calcium phosphate layer on its surface [4-8].
However, the primary limitation of bioactive glasses is their inadequate mechanical strength, which hinders their widespread application. To address this issue, methods such as transforming bioactive glasses into bioactive ceramics are employed to enhance their mechanical properties. Through heat treatments, these glasses undergo structural modifications that not only impact their microstructure but also improve their mechanical and biological activity characteristics [9,10].
Many agricultural and industrial wastes, especially rice straw, sodalime- silica waste glass, and egg shells, have been used as a source of silica in order to manufacture bioactive ceramic materials.
The aim of the current study is to isolate pure silica from various glass wastes, as glass waste varies from one source to another depending on the type of glass that is manufactured and the field of its application. Therefore, it is not possible to use various glass wastes to produce specific types of biomedical ceramics. Therefore, it is necessary to isolating pure silica from glass waste, regardless of its quality and composition, first then using it as a raw material in the preparation of various types of biomedical ceramics. Thus, we achieve two goals. The first is to reduce environmental pollution with glass waste, second goal is to obtain a source of pure silica in order to develop the manufacture of bioactive products.
Materials and methods
Collect and analyze of glass waste
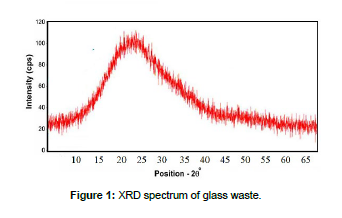
Collected samples of glass waste from a variety of sources, washed for dust and dirt disposal, milled by a porcelain mill with grinding balls of different sizes for granular size less than 100 microns by standard sieve with an electric vibrator made by Retch Model (AS.200), chemical composition of ground glass was determine using X-ray (XRF) as in [Table 1], A Bruker D8 Advance X-ray diffractometer equipped with X'Pert HighScore software was used to determine the crystalline phases through XRD [Figure 1].
| Component | W% |
|---|---|
| SiO2 | 72.45 |
| Al2O3 | 1.17 |
| Fe2O3 | 0.05 |
| CaO | 8.41 |
| MgO | 3.28 |
| SO3 | 0.14 |
| K2O | 0.12 |
| Na2O | 14.38 |
| L.I.O | 0.03 |
Table: 1 Chemical analysis using XRF of glass waste
Extraction of pure silica from glass waste
The grinded glass was blended with sodium hydroxide which is a dissolved melting material of the glass net, blended with several weight ratios (glass : NaOH) (1:1) (2:1) (3:1) which is an optional blending ratio in this research, then put the mixtures in a porcelain crucible for thermal treatment in an electric oven at different temperatures (1000-900-800)˚C at a constant heating rate of ten degrees per minute until the required heat reaches where the processing was installed for an hour and left to cool in the oven, the next step, sample was treated with a hydrochloric acid (diluted 1:1) with heating to 60 ̊C to isolate silica (as H2SiO3) for the rest of the accompanying compounds that form decadent compounds with water chlorine acid filtered the sample and washed with distilled water so as to ensure that the leaf is free of chloride ions by using silver nitrate as a qualitative detector for chloride ions, sample was dried then treated thermally at 900˚C for 30min to form white SiO2 particles according to the equation: H2SiO3 → H2O↑ + SiO2
silica (SiO2) yield was determine by equation: Sp% = (Ws/Wg)x100
Where: Sp%: yield of silica%.
Ws: weight of silica produced.
Wg: weight of glass waste.
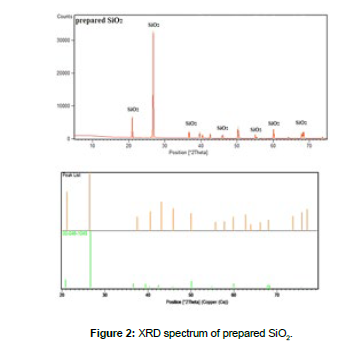
Chemical analysis using XRF of prepared silica in [Table 2], Comparison between the XRD spectrum of the prepared silica with the reference No: 00-046-1045 was showed in Figure 2. The distance between the crystal planes determined by Miller's clues (hkl) is determined based on Bragg's law as follows: nλ=2dsinθ, Where: d: the distance between parallel crystal planes according to the direction hkl, θ: is the diffraction angle, n: diffraction order, λ wavelength of the X-rays (λ=1.540A˚), distance between parallel planes was calculated in terms of the crystal lattice constants of the tetragonal structure and the lattice constants according to the following equation: 1/d2 =(h2+k2/a2) +(l2/c2). Determine both the density of dislocations and the stress or tension coefficient (crystal lattice strain ε) based on the Debye-Scherer relationship according to the following: D = Kλ / βCosθ, Where: βhkl mid-intensity width of the diffraction peak corresponding to the plane hkl, K: constant equal to 0.94 in the case of spherical particles, D: size of the crystalline grains, θ is the angle of diffraction in radians corresponding to the plane hkl. Density of dislocations δ was determine as: δ = n/D2, where n: is a constant equivalent to one in order to obtain the lowest value for the density of dislocations. Also stress or tension coefficient (crystal lattice strain ε) determine by: ε = β cosθ / 4 .
| Component | W% |
|---|---|
| SiO2 | 99.42 |
| Al2O3 | 0.12 |
| Fe2O3 | 0.05 |
| CaO | 0.09 |
| MgO | 0.07 |
| SO3 | 0.01 |
| K2O | 0.04 |
| Na2O | 0.09 |
| L.I.O | 0.02 |
Table: 2 Chemical analysis using XRF of prepared silica
Crystallinity size, crystal lattice strain, and dislocation density of the prepared silica it’s consistent with reference values [Table 3].
| Sample | 2θ | hkl | d (Aº) | D(nm) | δ 1015 ( lines.m^(-2)) |
ε (10-4 line s^(-2).m^(-4)) |
βohkl |
|---|---|---|---|---|---|---|---|
| SiO2 | 26.452 | 101 | 3.369 | 9.8 | 10.4 | 35.78 | 0.8423 |
Table: 3 Crystallinity size, crystal lattice strain, and dislocation density of the prepared silica
Bioglass preparation
Based on the pure silica prepared in this research, 45S5 glass was prepared as in [Table 4], which is considered one of the glass systems that have biological properties and belongs to the glass system (CaOP 2O5-SiO2). An identical glass mixture was prepared. For preparation 45S5 glass high-purity chemicals from Sigma-Aldrich were used: calcium carbonate CaCO3 (99%), sodium carbonate Na2CO3 (98%), calcium phosphate Ca3(PO4)2 (99%) were used as in [Table 5].
W% |
(%)Σ | |||
|---|---|---|---|---|
| SiO2 | CaO | P2O5 | Na2O | 100 |
| 45 | 12.25 | 6 | 24.5 | |
Table: 4 Chemical composition of bio glass 45S5
W(gr) |
(gr)Σ | |||
|---|---|---|---|---|
| SiO2 | CaCO3 | Ca3(PO4)2 | Na2CO3 | 132.12 |
| 45.28 | 31.39 | 13.36 | 42.09 | |
Table: 5 materials for preparing 45S5 glass using pure prepared silica
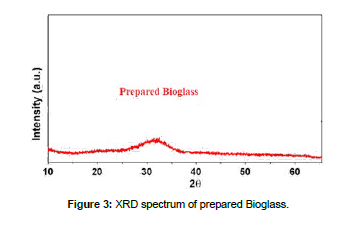
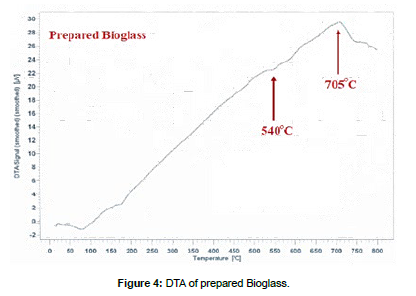
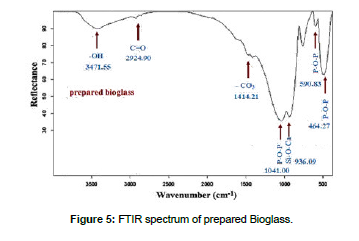
Mixture was processed thermally by electric oven running until the temperature 1700˚C equipped with several heating systems, produced by Carbolite, so mixture was poured into a carbon crucible after being mixed, crucible was transferred electric oven, temperature was slowly raised to 200°C at a rate of 10°C per minute, while maintaining this temperature for 30 minutes to remove moisture. The temperature was gradually raised to 1100° C at a heating speed of 15˚C/min to avoid scattering of the mixture components as a result of the release of CO2 resulting from the dissolution of carbonates. This temperature was held constant for 60min, then the temperature was raised to 1400˚C at a heating speed of 20˚C/min while monitoring the melting of the sample by holding it steady Temperature for 30 minutes every 100˚C. After the temperature reached 1000˚C, the sample completely melted after 10 minutes after the temperature reached 1300˚C. The formed glass melt was poured onto a chrome metal mold designed for the casting process. Keep the result in a container away from moisture for future tests after it has cooled to a temperature in the room. [Figure 3] showed XRD spectrum of prepared bioglass, DTA/TG (PLSTA 1640) was used to determine the thermal behavior of the prepared samples, and a heating rate of 10 °C/min was used up to 1000°C [Figure 4]. A Bruker Vector 22 FTIR spectrometer was used to determine the IR spectra of the prepared samples in the range of 4000–400 cm-1 [Figure 5].
Preparation of medical ceramic (bioceramic)
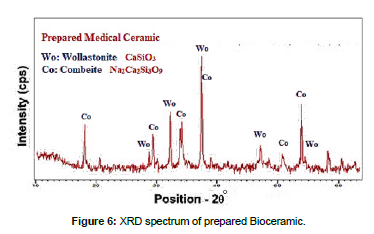
Based on the data of the differential thermal analysis (DTA) curves, a thermal program was developed to convert bioglass into medical ceramic (bioceramic) by raising the temperature to the nuclei formation temperature TN with heating rate 20˚C/min with stabilization for 20min, then raised to the crystallization temperature TC at a rate of heating 10˚C/min with stabilization for 20min, sample was left in the oven to cool slowly until the oven temperature is reached to prevent any stresses from appearing in them. [Figure 6] show XRD of prepared medical ceramic (bioceramic).
Biodegradability of prepared medical ceramic (bioceramic)
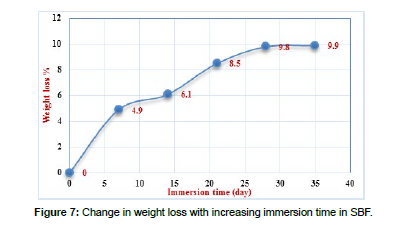
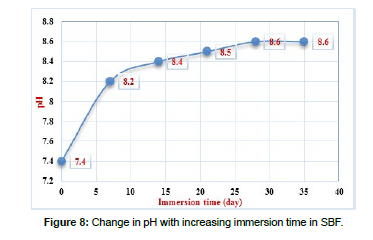
Biodegradation is crucial for synthetic biomaterials, as the degradation process should align with tissue regeneration. The presence of Ca2+, P5+, and Si4+ ions is vital for tissue function. Bio medical ceramics primarily degrade through dissolution by surrounding media. To study of biodegradability in the laboratory, prepared bioceramic was grinded as powder, soaked in the “Simulated Body Fluid” solution SBF for 7, 14, 21, 28, and 35 days and put in an incubator at 37°C, preparation of the SBF solution (compassion) was based on the studies mentioned in [Table 6]. SBF solution to powder ratio was taken by 50:1, The pH level was measured on various days, to calculate the biodegradability equation was used: %WL =W0 – W1/ W0 , Where: %WL Percent of weight loss, W0: weight of the sample before sinking in SBF, and W1: dry weight of the sample after dipping in SBF.
| Weight | Substances |
|---|---|
| 7.92 gr | NaCl |
| 0.32 gr | NaHCO3 |
| 0.23 gr | K2HPO4.3H2O |
| 0.24 gr | KCl |
| 0.31 gr | MgCl2.6H2O |
| 0.28 gr | CaCl2 |
| 0.07 gr | Na2SO4 |
| 40 ml | HCl 1M |
| 7.86 gr | NH2C(CH2OH)3 |
Table: 6 chemical composition of SBF solution
Figure 7 and Figure 8 showed Change in weight loss, pH with increasing immersion time in SBF.
Results and discussion
XRF chemical analysis of glass waste Table.1 showed that it contains a high percentage of silicon dioxide (SiO2), 72.45%, and this is due to the fact that it represents one of the main acidic oxides of the glass mesh, while sodium oxide, Na2O, whose presence reached 14.38%, is considered one of the alkaline oxides that aid in melting. As for calcium oxide, CaO, and magnesium oxide, MgO, they are among the materials fixed to the glass mesh, and the percentage of loss on ignition (L.O.I) was almost non-existent because the glass is a group of oxides bonded to each other as a result of applying high temperatures to form an amorphous glass phase, and this is confirmed by the X-ray diffraction spectrum (XRD) in Figure 1. It did not contain any peaks that indicated the presence of a crystalline compound. By comparing the chemical composition of the glass used with the chemical composition of international types of glass, it can be concluded that the glass waste sample used is of the type of flat glass distinguished by high mechanical durability and high transparency.
The yield of the prepared silica reached 68%, with a purity of up to 99.4%, when using a mixture of ground glass: sodium hydroxide with mixing ratios corresponding to (2:1) and (3:1) and heat treatment at temperatures (900 and 1000)˚C. This indicates that the method employed in this research to produce silica is effective, as for the mixing ratio (1:1), it was excluded because it gave heterogeneous agglomerates, and the silica yield was low, not exceeding 25%, which indicates that the complete disintegration of the glass network did not occur, so treatment was also excluded. Thermal processing of the mixtures: glass: sodium hydroxide at a temperature of 800˚C because it also gave heterogeneous agglomerates (and not dissolved with hydrochloric acid) and complete disintegration of the glass network did not occur. Therefore, dismantling the glass network of the studied glass waste and liberating the silica is sufficient with a mixing ratio of 2:1, then heat treatment at 900˚C.
The results of the chemical analysis of the prepared silica indicated the efficiency of the method used to isolate silica because SiO2 is distinguished from the rest of the other oxides included in the composition of glass in that it does not dissolve in concentrated hydrochloric acid or other acids, when comparing the XRD spectrum of the prepared silica and the reference XRD spectrum in the Figure 2, it noted that there was a perfect agreement between the peaks of the prepared silica spectrum and the reference code No. \1045-046-00\, this also indicates the purity of the prepared silica and agrees with the chemical analysis and the percentage of purity obtained, also there were perfect agreement in value of crystallinity size, crystal lattice strain, and dislocation density of the prepared silica with references.
XRD spectrum of prepared bioglass Figure 3 did not contain any peaks that indicated the presence of a crystalline compound, this confirms the formation of a glassy phase and thus the success of melting process, results IR spectra of the prepared bioglass Figure 5 were consistent with previous work.
According to DTA/TG in Figure 4 there was an endothermic reaction that occurred at 540˚C resulting from the beginning of the formation of crystallization nuclei, so this temperature was called the nuclei formation temperature TN, there was another endothermic reaction that occurs at 705˚C resulting from the crystallization process, so this temperature was called the crystallization temperature TC. Therefore, it Bioglass was treated at these two temperatures with a stabilization period to ensure the formation of nuclei and crystallization well, which was proven by XRD analysis Fig.6, According to XRD patterns, the most apparen crystalline phases were in prepared ceramic were combeite Na2Ca2Si3O9 and wollastonite CaSiO3, Combeite and wollastonite is known as a biologically active ceramic.
The results in Fig.7 and Fig.8 showed that the percentage of weight loss increased as the period of SBF immersion increased to 28 days, where the percentage reached to 9.8%, then it almost stabilized at 9.9%, this increase is attributed to the ionic interaction between the ceramic and the components of the SBF solution. Therefore, the pH of the SBF solution rises during the first 14 days to 8.4, before stabilizing with longer soaking times, according to the previous report, the alkaline pH enhanced the proliferation of osteoblast cells. The presence of phosphate and calcium in these exact molar ratios when preparing the bioceramic material leads to the formation of a layer of hydroxyapatite (HA), Once HA was formed, the pH remains relatively constant as the immersion time continues to increase.
Conclusion
Pure silica can be prepared from glass waste of different type and composition with a yield of up to 68% by grinding the glass waste and mixing it with sodium hydroxide in a ratio of 2:1, then melting the mixture at 900˚C, followed by treating the result with hydrochloric acid to isolate the silicic acid, which is treated thermally to obtain SiO2 with a purity of 99.4%.
Silica isolated from glass waste can be used to prepare bioactive glass according to the system (CaO-P2O5-SiO2).
Bioactive glass can be converted into medical ceramic by heat treatment at 540˚C to form crystallization nuclei and then raising the temperature to 705˚C to complete the crystallization process.
The properties of the prepared ceramics and their ability to biodegrade in SBF solution indicated the possibility of using it in medical applications.
Authors’ Declaration
Conflicts of Interest: None.
I/We hereby confirm that all the Figures and Tables in the manuscript are mine/ours. Furthermore, any Figures and images, that are not mine/ours, have been included with the necessary permission for re-publication, which is attached to the manuscript.
Authors’ Contribution Statement
Abdulrazzaq Hammal: Preparing samples, testing them, and writing the research.
Nawzat Nabgali: Discuss the results.
References
- Silver IA, Deas J, Erecińska M (2001) Interactions of bioactive glasses with osteoblasts in vitro: effects of 45S5 Bioglass®, and 58S and 77S bioactive glasses on metabolism, intracellular ion concentrations and cell viability. Biomaterials 22: 175-85.
- Negut I, Ristoscu C (2023) Bioactive Glasses for Soft and Hard Tissue Healing Applications—A Short Review. Applied sciences 13: 6151-1.
- Miguez-Pacheco V, Hench LL, Boccaccini AR (2015) Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomaterialia 13: 1-15.
- Mubina MSK, Shailajha S, Sankaranarayanan R, Smily ST (2021) Enriched biological and mechanical properties of boron doped SiO2-CaO-Na2O-P2O5 bioactive glass ceramics (BGC). Journal of Non-Crystalline Solids 570: 121007.
- Shan Z, Zhang Y, Liu S, Tao H, Yue Y (2020) Mixed-alkali effect on hardness and indentation-loading behavior of a borate glass system. Journal of Non-Crystalline Solids 548: 120314-4.
- Crovace MC, Soares VO, Rodrigues ACM, Peitl O, Raucci LMSC et al. (2021) Understanding the mixed alkali effect on the sinterability and in vitro performance of bioactive glasses. Journal of the European Ceramic Society 41: 4391-405.
- Dimitriadis K, Tulyaganov DU, Vasilopoulos KC, Karakassides MA, Agathopoulos S (2021) Influence of K and Mg substitutions on the synthesis and the properties of CaO-MgO-SiO2/Na2O, P2O5, CaF2 bioactive glasses. Journal of Non-Crystalline Solids 573: 121140.
- Wetzel R, Blochberger M, Scheffler F, Hupa L, Brauer DS (2020) Mg or Zn for Ca substitution improves the sintering of bioglass 45S5. Scientific Reports 29: 10.
- Lefebvre L, Chevalier J, Gremillard L, Zenati R, Thollet G et al. (2007) Structural transformations of bioactive glass 45S5 with thermal treatments. Acta Materialia 55: 3305-13.
- Abbasi M, Hashemi B (2014) Fabrication and characterization of bioactive glass-ceramic using soda–lime–silica waste glass. Materials Science and Engineering: C 37: 399-404.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Hammal A, Nabgali N (2024) Using Glass Waste to Prepare a Type of Medical Ceramic. Ind Chem, 10: 269. DOI: 10.4172/2469-9764.1000269
Copyright: © 2024 Hammal A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 633
- [From(publication date): 0-2024 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 448
- PDF downloads: 185