Research Article Open Access
Using Chlorella vulgar is to Decrease the Environmental Effect of Garbage Dump Leachates
| Sarunporn Thongpinyochai and Raymond J Ritchie* | |
| Tropical Plant Biology, Faculty of Technology and Environment, Prince of Songkla University Phuket Campus, Kathu, Phuket 83120, Thailand | |
| Corresponding Author : | Raymond J Ritchie Tropical Plant Biology, Faculty of Technology and Environment Prince of Songkla University Phuket Campus Kathu, Phuket 83120, Thailand Tel: +6676 276130 Fax: +66 76 276102 E-mail: raymond.r@phuket.psu.ac.th |
| Received May 28, 2014; Accepted July 25, 2014; Published July 29, 2014 | |
| Citation: Thongpinyochai S, Ritchie RJ (2014) Using Chlorella vulgar is to Decrease the Environmental Effect of Garbage Dump Leachates. J Bioremed Biodeg 5:239. doi:10.4172/2155-6199.1000239 | |
| Copyright: © 2014 Ritchie RJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Waste treatment in Phuket, Thailand produces two sources of leachate. The Landfill Leachate (LF) has low BOD (60-405 mg O2/L) and heavy metal levels are below legal limits. Garbage awaiting incineration, Garbage Pit Leachate (GPL), also produces a second leachate with a very high BOD (50-100 g O2/L) and NH3-N (763-2,045 mg/L). Zn and Cr exceed standards but Cu and Pb are low. Chlorella vulgaris was tested for its ability to decrease NH3-N, NO3--N, Total-P, BOD and COD and to remove and/or immobilize heavy metal at varying dilutions of leachate. The objectives of this study on Chlorella bioremediation were: 1) measure Chlorella growth in Landfill and Garbage Pit Leachate, 2) measure the effectiveness of Chlorella to decrease the physico-chemical parameters NH3- N, NO3-N, Total-P, BOD, COD 3) measure heavy metal removal from leachates. The minimum inoculum of Chlorella biomass which can grow in the LF was Chlorophyll a 0.259 μg/mL (A750=0.075). Chlorella significantly lowered its BOD and COD: Cr and Ni, already low in landfill leachate, were decreased by 70% and 66%. Chlorella grew well in LF diluted 30 % with tapwater: % removal of NH3-N (53.91%), BOD (52.78%) and COD (51.05%). The GPL was very toxic: only 10-20% dilutions of GPL were tolerated by Chlorella and cultures required continuous aeration by shaking to grow. The minimum necessary inoculum of Chlorella biomass in the GPL was Chlorophyll a=0.92 μg/mL (A750=0.19). In 20% dilution of GPL, Cr and Zn were decreased by 33% and 90% respectively to legal levels. % removal of NH3-N (41.5%), NO3--N (32.4%), Total-P (55.1%), BOD (49.2%) and COD (50.8%). Chlorella inoculations with a biomass of 1.17 μg/mL Chlorophyll a (A750=0.202) removed 90% of the Zn.
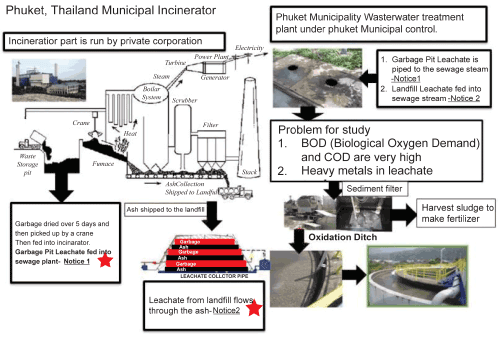
In detail there are two main parts of the garbage management process. First, the incineration phase is run under a private corporation. The second phase is a water quality improvement (sewage treatment) plant in Phuket under Phuket Municipal control. The Garbage Pit Leachate (GPL) arises directly from decomposition of raw garbage. The Landfill leachate (LF) arises from the landfill which has a sandwich arrangement of layers of garbage and burnt garbage (ash). The leachate moves slowly down through all layers of the garbage (Figure 1) and is collected at the base of the landfill. Hospitals in Phuket have separate incineration facilities and their ash is also dumped in the landfill. The content of their ash waste is well documented. Leachate in the landfill passes through ash and garbage layers picking up dissolved organic compounds, toxic organic compounds and heavy metals along the way. There are two types of leachate to deal with: the direct garbage leachate from the holding bays of the incinerator (GPL) and the leachate from the landfill (LF). Currently all leachate is fed into the water quality improvement plant mixed along with the domestic sewage from the municipality. The BOD of the GPL is much too high for safe disposal in rivers or for recycling by spreading on agricultural land. The BOD load from the GPL has become a pressing problem ever since the incinerator had to be expanded to two input feed-heads because it overloads the sewage treatment plant and is very toxic.
The other major problem with the leachate arises from its potentially high heavy metal content. In the LF, the water leaching through the layers of garbage and garbage ash would be expected to mobilize sand dissolve the heavy metals. Early stages of the present study showed that the LF had relatively low heavy metal content but the GPL had very high levels of heavy metals, in particular Zinc and Chromium [3]. The Zinc and Chromium levels far exceeded environmentally permitted levels in the case of the GPL but did not in the case of the LF. The leachate from the holding bays of the incinerator also has very high BOD and COD [3].
Several methods are currently being used for the removal of heavy metal ions from aqueous wastes. One of the alternative methods to remove heavy metals and nutrients in leachate is using some form of bioremediation. In the present study, growth of microalgae and nutrient absorption by microalgae from leachate was used to attempt to reduce the toxic effects of leachate.
Microalgae are widely employed as a tertiary treatment process to remove nitrogen and phosphorus from wastewater since they require nitrogen, phosphorus, CO2, and light for their photoautotrophic metabolic growth. Many species of microalgae have been used as a bioremediation agent when combined with wastewater treatment, but the most commonly used are various species and strains of Chlorella such as Chlorella pyrenoidosa, and Chlorella vulgaris. Chlorella is a very hardy alga able to tolerate variable salinities, a wide range of pH and is not hypersensitive to metal poisoning. Chlorella species predominate in the sewage ponds at the Saphanhin Klongkopee sewage plant and also in prawn farm ponds in the region. Chlorella is able to use many organic compounds photoheterotrophically and so can use many organic compounds in wastewater and leachates [4].
Our study focused on 3 major topics 1) To investigate and record heavy metal contamination of leachates from the GPL and LF, 2) To investigate using green algae (Chlorella vulgaris) to reduce BOD in the leachates before feeding the effluent into the recycling process, 3) To investigate heavy metal reduction in leachates by using bioassay based on Chlorella vulgaris.
Two types of leachate samples were collected (a) Leachate from the base of the Landfill (LF) and (b) Leachate from the Garbage Pit (GPL).
Growth measurement: Absorbance (optical density, OD) of cell cultures was measured at 750 nm. For chlorophyll determinations, 5 mL samples were centrifuged at 5,000 rpm for 5 minutes. After centrifugation, the liquid was decanted off as much as possible. The pellet was then mobilized by vortexing before adding 3 mL of ethanol. The 3 mL of ethanol extract was then left in the refrigerator for about 1 hr, then centrifuged at 5,000 rpm for 5 minutes and the supernatant was used for chlorophyll determination using a spectrophotometer (Shimadzu UV-1601) at 649 and 665 nm using A750 nm as the absorbance blank. The equations of Ritchie [5] were used to estimate Chlorophyll a [Chl a (μg/mL)=11.867 × (A665 nm-A750 nm) – 5.201 × (A649 nm-A750 nm)].
The effect of different concentrations of leachate on algal growth: Chlorella vulgaris was grown at a different concentration of LF and GPL. Leachate samples for experiment were centrifuged at 5,000 rpm. All experiments were set up in temperature range 25-27°C under 24 hours light using cool-white fluorescent lamps with light intensities=200 μmol (quanta) m-2 s-1 (PAR,400-700 nm). Experiment set up in two sets (1) LF diluted with tap water to following concentrations: 10%, 30%, 50%, 100% (undiluted leachate) and (2) GPL diluted with tap water to following concentrations: 10%, 20%, 30%, 50% and 100% (undiluted leachate) with two conditions: shaking [120 rpm-WiseShake (SHO-2D)] and non-shaking. The non-shaken cultures were given a manual gentle stir each day.
The optimum biomasses of Chlorella in terms Chl a to remove heavy metals:GPL was very toxic and had to be diluted to 20% GPL/ 80% tap water for Chlorella to grow. Tap water was used as the diluent because in realistic field situations deionised water or distilled water would not be used. The optimal GPL inoculum was 20% of GPL and 80% of tap water based on the results from experiments however Chlorella would grow successfully even in undiluted LF and besides the heavy metals in LF did not exceed the environmental standard values. For the determination of minimum Chlorella vulgaris cell concentrations which efficiently removes metals, cell densities of 11.7, 16.0, 25.0, 30.5 μg Chl a /mL were used as starting inocula.
Standard relationship between absorbances 750 nm and Chlorophylla: The relationship between A750 nm and biomass as measured by Chl a (μg) of Chlorella vulgaris was determined spectrophotometrically. For Chlorella vulgaris the equation y=7.7809x - 0.2047 (n=18, ±95% conf. lim.), with a correlation r=0.9787 can be used to describe the relationship between A750 nm (x) and μg of Chl a (y).
Standard relationship between Absorbances 750 nm and cell number: The relationship between A750 nm and cell number count by the hemocytometer method was determined. Using the A750/cell number relationship, the amount of Chlorella used as a starting inoculum for experiments could be calculated. In the range of absorbances measured (up to A750=1.4) the absorbance was directly proportional to cell numbers. For Chlorella vulgaris cells in log phase an A750 of 0.00738 ± 0.00060 was equivalent to a cell density of 106 cells/mL (n=23, ±95% conf. lim.), Pearson’s r=0.9354.
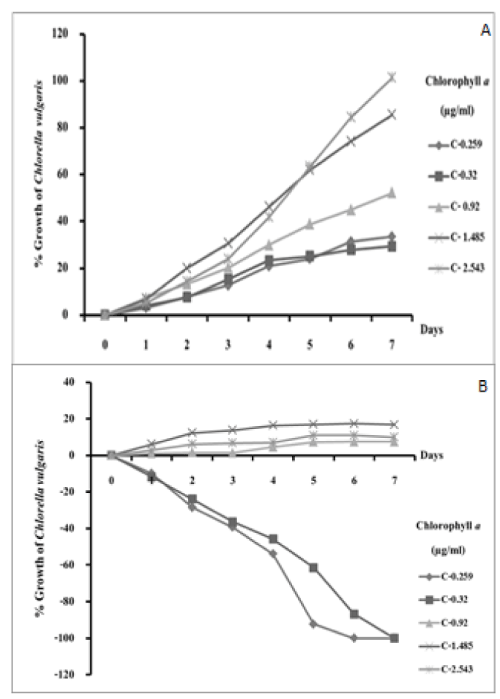
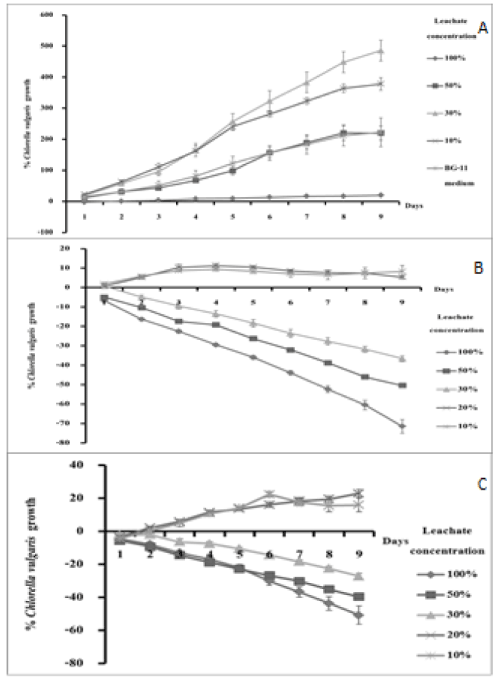
Chlorella established itself very well in the LF and grew exponentially even with the minimum inoculum (Figure 4a). The results showed that in LF a Chlorella inoculum as low as 0.259 μg/mL Chl a (A750 nm=0.075) grew well. GPL on the other hand was highly toxic (Figure 4b). Low-level inoculations of Chlorella slowly died off (0.259 and 0.32 μg/mL Chl a). The heavier inoculations did not die but only grew marginally. Only the heaviest inoculation showed substantial growth (2.543 μg/mL Chl a). The minimum inoculation which survived and grew in GPL was 0.92 μg/mL. It was concluded that a heavy inoculation of Chlorella was needed for the alga to grow in the GPL. We used the same biomass of Chl a (0.92 μg/mL) to cultivate Chlorella in the LF and GPL.
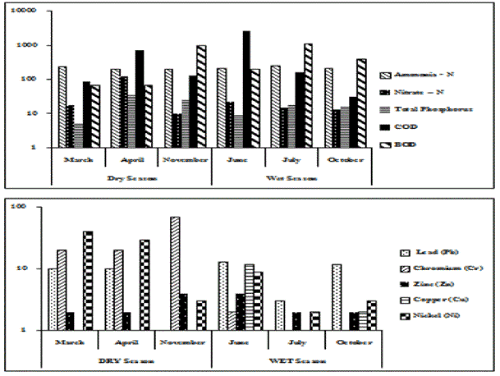
Growth was followed over a 9 day experimental period. Chlorella grew in all the dilutions of LF (in terms of Chl a). The Chlorella grew the best in media containing 30% LF (Analyzed using two ways ANOVA with replication). The growth experiment was run on each monthly sample (over 6 months) with 3 replicates per concentration (the data points shown in Figure 5a are means ± SE error bars from the 6 months of data).
Growth experiment on GPL (not shaken but under light conditions): The GPL was much more toxic than the LF [3]. Experiments to test the toxicity of GPL followed a similar protocol to those described above for experiments with LF. Chlorella would not grow in 100%, 50% or 30% leachate and slowly died off over the course of the experiment (Figure 5b). Chlorella grew well in the undiluted tap water and grew marginally in GPL diluted to 20% and 10% in tap water (20% leachate grew +11% and 10% leachate grew +9%). The data was analyzed using two-way ANOVA with replication. The experiment was repeated each month over 6 months and each growth experiment was run in 3 replicates per concentration. The data points shown in Figure 5b are means ± SE error bars from the 6 months of data.
Growth experiment on GPL (well aerated conditions): Chlorella was grown over a 9 day cultivation period with shaking on an orbital shaker (120 rpm) in a range of diluted GPL: 100%, 50%, 30%, 20%, 10% leachate diluted with tap water. Growth in 100% BG-11 medium was used as the blank control. Growth in undiluted leachate was very poor and the alga died off with time (Figure 5c) even though some marginal growth might have occurred during the early stages of the incubation. Positive growth occurred in 20% and 10% leachate (Analyzed with two way ANOVA with 5-fold replication). In terms of Chl a, the alga was able to grow continuously over the course of the experiment in 10 and 20% GPL but at all higher concentrations gradually died off with time. Growth of Chlorella in GPL was much better under well-aerated shaking conditions (Figure 5c) than cultures kept under non shaking conditions (Figure 5b), Nevertheless GPL at any of the dilutions tested was severely inhibitory compared to the blank control grown in tap water. Comparison with the results for the LF experiments clearly shows that the GPL was much more highly toxic. The data points shown in Figure 5b and c are means ± SE error bars from the 6 months of data based on 5 replicates. Some error bars are smaller than the symbols used for the mean values.
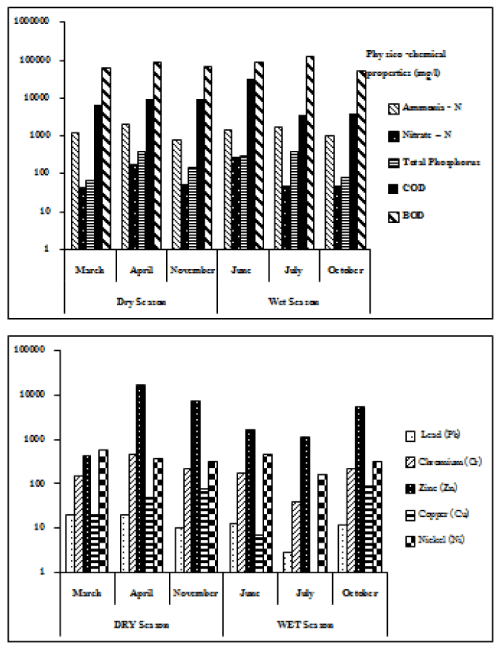
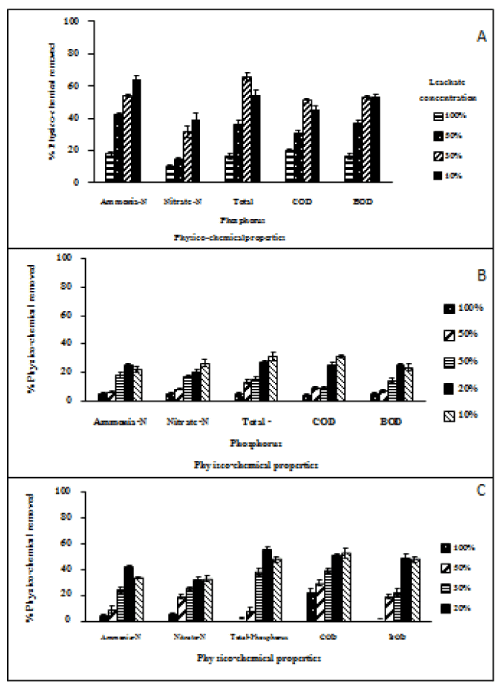
Nutrient removal on garbage pit Leachate (no shaking and under light conditions): GPL-Percentage removal of ammonia-N, total-phosphorus BOD and COD by Chlorella cultures were measured after 9 days incubation in a range of dilutions of GPL. Percentage removal of ammonia-N, Total-P, BOD and COD were calculated using dilutions of leachate that were not inoculated with Chlorella. The ammonia-N, total-phosphorus, BOD and COD were also measured in the GPL at the start of the experiment. The 20% GPL diluted with tap water showed the highest percent removal rates than the other concentrations of leachate (Figure 6b). The percentage removed compared to the control blank were: ammonia-N (24.67 ± 1.45%), nitrate-N (20.17 ± 1.90%), total phosphorus (27.32 ± 0.96%), COD (25.21 ± 1.50%) and BOD (24.6 ± 1.22%). Removal of the pollutants from the GPL was much poorer than in the case of the similar incubation experiments run on LF (Figure 6a-6c). The experiment was repeated on monthly collections of leachate over 6 months and each experiment was run in 3 replicates (Figure 6b). The data points shown in Figure 6b are means ± SE error bars.
Nutrient removal on garbage pit Leachate (well aerated conditions): Removal of pollutants from the GPL by Chlorella was better under aerated and shaken conditions. During the 9 day cultivation period, the 20% GPL diluted with tap water had the highest percentage removal compared to the initial condition (GPL at t=0): percentage removal compared to the t=0 control were: ammonia-N (41.5 ± 1.22%), nitrate-N (32.4 ± 1.45%), total phosphorus (55.1 ± 2.56%), BOD (49.2 ± 1.74%) and COD (50.8 ± 3.20%) (Figure 6b and 6c). The data points shown in Figure 6b and 6c are means ± SE error bars based on 5 replicates.
Garbage Pit Leachate (GPL) (non-shaking condition): The results of heavy metals analysis (Table 3) shows that Chlorella was not successful in removing heavy metals at any of the dilutions of GPL tested. This can be attributed to the lack of growth of the Chlorella resulting in no binding up of the toxic metals in insoluble form [7] or that the GPL was so overloaded with Zn, Cr and Nickel that binding of these metals to the cellular material was not significant because the cells failed to grow well.
Garbage Pit Leachate (well aerated conditions): Chlorella grown in 20% GPL diluted with tap water removed 89.7% of the Zn and 33.3% of the Chromium. The errors in these standard chemical analyses by the ISO-certified laboratory in Hat Yai would be about ± 2% relative error. Chlorella in 30% leachate removed 41% of the Zinc and 22% of the Nickel and 60% of the Chromium. Chlorella in 10% GPL removed nearly all the Zinc (99.4%) and most of the Nickel (85%).
The physico-chemical composition of LF in Phuket was comparable to the range of values found in Effluent standards in Thailand and typical of values found in developing countries [9]. The physico-chemical properties of leachate from the LF and the GPL compared with Thai and International standards values are provided as a Supplementary table. The analyses for Phuket are analyses based on 6 separate collection trips spread over 6 months that included collections made in both the wet and dry season (Table 1 and Figures 2, 3 and 5). The BOD level of LF and GPL were both higher than the Thai standard, however BOD level of LF was in the range of international standards. The COD levels were on the lower side of values found internationally but the COD of Phuket GPL was well over the Thai standard. Levels of metals were very low by both Thai and International standards with the notable exception of Zinc in the GPL which sometimes exceeded both Thai and international standards. Phuket does not have a large amount of heavy industry. The source of so much Zn in GPL from a non-industrial city is not clear. Ammonia levels of the Phuket LF are high but the GPL has extremely high BOD (5 days) levels and very high COD. Nitrate levels in both the Phuket LF and the GPL were much higher than . The BOD of the GPL is exceptionally high. In combination with the very high ammonia content this results in BOD > COD. The GPL would have had many ammonia oxidizing bacteria which resulted in BOD > COD as a result of microbial oxidation of the ammonia (ultimately to NO3) [10]. This interpretation is supported by the high observed nitrate-N. Total phosphorus of the GPL was also exceptionally high. The levels of heavy metals in the Phuket LF were generally low by international standards but the levels of heavy metals in the Phuket GPL were high by international standards in particular Zinc.
Garbage Pit Leachate (GPL): The GPL was much more toxic than the LF [3]. Chlorella would not grow in 100%, 50% or 30% leachate and slowly died off over the course of the experiment in light under non-shaking conditions (Figure 5b). Chlorella grew well in the undiluted tap water and grew marginally in GPL diluted to 20% and 10% in tap water (20% leachate grew +11% and 10% leachate grew +9%). Other growth experiments were set up in the light with shaking conditions to maintain aeration. Chlorella was grown over a 9 day cultivation period with shaking on an orbital shaker in a range of diluted GPL: 100%, 50%, 30%, 20%, 10% leachate diluted with tap water. Growth in 100% BG-11 medium was used as the blank control. Growth in undiluted GPL was very poor and the alga died off with time (Figure 5b). Positive growth occurred in 10 and 20% leachate (Analyzed with ANOVA two ways with 5-fold replication). In terms of Chl a, the alga was able to grow continuously over the course of the experiment in aerated 10 and 20% GPL but at all higher concentrations gradually died off with time. Growth of Chlorella in GPL was much better under well-aerated shaking conditions (Figure 5c) than cultures kept under non shaking conditions (Figure 5b), nevertheless GPL at any of the dilutions tested was severely inhibitory compared to the blank control grown in tap water. Comparison with the results for the LF experiments clearly shows that the GPL was much more highly toxic.
Garbage Pit Leachate
•Chlorella incubated without shaking: The results of heavy metals analysis (Table 3) shows that Chlorella was not successful in removing heavy metals at any of the dilutions of GPL tested. This can be attributed to the lack of growth of the Chlorella resulting in no binding up of the toxic metals in insoluble form [7]. This is consistent with the very high toxicity shown in Figure 5b and 5c and the poor removal of ammonia-N, nitrate-N, total-phosphorus and BOD and COD (Figure 6b and 6c). In the dilution treatments where there was some growth of Chlorella (10% and 20% GPL diluted with tap water) there was some increase in available Zn probably due to the breakdown of organic matter. Chlorella slowly died in 100%, 50% and 30% GPL. The pH of GPL started at pH 5.05 and the end of incubation pH was 4.9. This pH change is small but Starodub et al. [15] and Rai et al. [16] found increasing metal toxicity with decreasing pH to be due to the predominance of the free metal ion at low pH [16].
The Chlorella vulgaris growth in GPL (non-shaking conditions): Chlorella in light under non-shaking conditions grew the best in 20% of GPL. Compared to measurements made at the beginning of the incubation, the overall rates of removal based on all six replicate runs of the experiment were (mean % ± SE%): ammonia-N 24.67 ± 1.45%, nitrate-N 20.17 ± 1.90%, total-phosphorus 27.32 ± 0.96%, COD 25.21 ± 1.50% and BOD 24.65 ± 1.22%. Heavy metals: Chlorella vulgaris was not successful in removing heavy metals at any of dilutions of GPL tested (Table 3).
•Chlorella incubated under aerated conditions: Chlorella grown in 20% GPL diluted with tap water removed 89.7% of the Zn and 33.3% of the Chromium (Table 4 and 5). The errors in these standard chemical analyses by the ISO-certified laboratory in Hat Yai would be about ± 2% relative error. Chlorella in 30% leachate removed 41% of the Zinc and 22% of the Nickel and 60% of the Chromium. Chlorella in 10% GPL removed nearly all the Zinc (99.4%) and most of the Nickel (85%). During the course of the experiment the pH in 30%, 20%, 10% leachate increased on average from pH 5.5 to pH 7.56. This would have made metals less soluble as the pH increased and soluble heavy metal would decrease. It was also observed that surface-bound metal concentrations increased when pH was varied over the range pH 6.0 – 8.0 and growth of Chlorella sp. improved as the pH increased. This can be explained in terms of cation binding to fixed negative changes in the cell walls of the alga increasing as the pH increased. Parent and Campbell [17] and Franklin et al. [18] proposed that the apparent protective effect of increasing pH on metal toxicity may be due to reduced competition between H+ and metal biding site at the surface of the cell membrane of the alga. Crist et al. [19] and Macfie et al. [20] showed that as the pH increased from 4 to 7 there was an increase in the number of negative charge sites on the cell wall surface. This is what would be predicted from the cation exchanger properties of cells walls and the proton concentration may also alter plasma membrane permeability to metals, thereby affecting metal binding and uptake. Many transport systems for metal ions are secondary active transport mechanisms using the proton motive force (electrochemical potential for protons), given a relatively constant membrane potential and intracellular pH (pHi) an increase in external pH tends to decrease the proton motive force and hence metal uptake would be expected to decrease as outside pH increased [21]. The major effect though of external pH is to decrease the solubility of metals because most heavy metal hydroxides are extremely insoluble [22].
The LP was not toxic and Chlorella grew in diluted LF. The BOD and ammonia-N were very high. The heavy metal in GPL exceeded the legal limit. Chlorella grown in 20% GPL diluted with tap water removed 41.5 of the ammonia-N, 49.2% of the BOD, 90% of the Zinc (90%) and 33% of the Chromium.
References
- Bull PS, Evans JV, Wechler RM, Cleland KJ (1983) Biological technology of the treatment of leachate from sanitary landfills. Water Res 17: 1473-1419.
- Lin L, Chan GY, Jiang BL, Lan CY (2007) Use of ammoniacal nitrogen tolerant microalgae in landfill leachate treatment. Waste Manag 27: 1376-1382.
- Thongpinyochai S, Ritchie RJ (2013) Bioremediation of Landfill and Garbage Pit Leachates using Growth of the Green Alga Chlorella vulgaris. The International Conference on Waste Management and Environment (ICWME) 2013, Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia, 41-45.
- Chen CY, Yeh KL, Aisayah R, Lee DL, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. BioresourceTechnol 102: 78-81.
- Ritchie RJ (2006) Consistent sets of spectrophotometric equations for acetone, methanol and ethanol solvents. Photosynth Res 89: 27-41.
- Zar JH (2010) Biostatistical Analysis, 5th ed. Prentice Hall, New Jersey, USA.
- Rosko JT, Rachlin JW (1977) The effect of Cadmium, Copper, Mercury, Zinc and Lead on Cell Division, Growth and Chlorophyll a content of the Chlorophyte Chlorella vulgaris. Bull Torrey Bot Club 104: 226-233.
- Chain ESK, De Walle FB (1975) Characterisation and treatment of leachate generated from landfills. American Institute of Chemical Engineering Symposium Series No. 145 71:319.
- Thailand Government Pollution Control Department (2548 BE, 2005 CE).
- Attiogbe FK, Glover-Amengor M, Nyadziehe KT (2007) Correlating Biochemical and Chemical Oxygen Demand of Effluents – A Case Study of Selected Industries in Kumasi, Ghana. WAJAE 11: 110-118.
- CheSa SN, Surif M, Ibrahim MIM, Omar WMW (2011) Study on The feasibility of Using Landfill Leachate as a Low Coast media For Mass Culturing of Microalgae. Universiti Malaysia Teregganu Annual Symposium (UMTAS), Kuala Tereggannu, Malaysia, 111-117.
- Ritchie RJ, Trautman DA, Larkum AWD (2001) Phosphate-limited cultures of the cyanobacteriumSynechococcus R-2 (PCC 7942) are capable of very rapid, opportunistic uptake of phosphate. New Phytol 152: 189-202.
- Silva AC1, Dezotti M, Sant'Anna GL Jr (2004) Treatment and detoxification of a sanitary landfill leachate. Chemosphere 55: 207-214.
- Adamsson M, Dave G, Forsberg L, Guterstam B (1998) Toxicity identification evaluation of ammonia, nitrite and heavy metals at the Stensund wastewater aquaculture plant, Sweden. Water SciTechnol 38: 151-157.
- Starodub ME, Wong PTS, Mayfield CI, Chau YK (1987) Influence of complexation and pH on individual and combined heavy metal toxicity to a freshwater green alga. Can J Fish AquatSci 44: 1173-1180.
- Rai LC, Rai PK, Mallick N (1996) Regulation of heavy metal toxicity in acid-tolerant Chlorella: physiological and biochemical approaches. Environ Exp Bot 36: 99-109.
- Parent L, Campbell PGC (1994) Aluminiumbioavailablity to the green algae Chlorella pyrenoidosa in acidified synthetic soft water. Environ ToxicolChem 13: 597-598.
- Franklin NM, Stauber JL, Markich SJ, Lim RP (2000) pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.). AquatToxicol 48: 275-289.
- Crist RH, Oberholser K, Schwartz D, Marzoff J, Ryder D, et al. (1988) Interactions of metals and protons with algae. Environ SciTechnol 22: 755-760.
- Macfie SM, Tarmohamed Y, Welbourn PM (1994) Effect of cadmium, cobalt, copper and nickel on growth of the green algae Chlamydomonasreinhardtii: The influences of the cell wall and pH. Arch Environ ContamToxicol l27: 454-458.
- Ritchie RJ (1998) Bioenergetics of membrane transport in Synechococcus R-2 (Anacystis nidulans, S. leopoldiensis) PCC 7942. Can J Bot 76: 1127-1145.
- Atwell BJ, Kreidermann PE, Turnbull CGN (1999) Plants in Action: adaptation in nature, performance in cultivation. Macmillan Education Publ, South Yarra, Australia.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
| Table 4 | Table 5 | Table 6 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15277
- [From(publication date):
September-2014 - Jul 18, 2025] - Breakdown by view type
- HTML page views : 10557
- PDF downloads : 4720
