Use of the BonebridgeTM for the Percutaneous BAHA user with Skin Complications: A Case Report
Received: 29-May-2020 / Accepted Date: 12-Jun-2020 / Published Date: 19-Jun-2020 DOI: 10.4172/2161-119X.1000396
Abstract
We performed BonebridgeTM (BB) implantation as a clinical trial. Transcutaneous bone conduction implants (BCIs) have not been approved for medical use in Japan, and the use of BB has been reported in few cases in Asia. The BB was used for a 16-year-old male who complained of bilateral mixed hearing loss due to bilateral congenital external auditory meatus atresia and microtia. Although he had already undergone percutaneous BAHA surgery, skin disorders inhibited the continued use of a sound processor. The BB was implanted at the opposite side without any intra- or postoperative complications. Transcutaneous BCI provides advantages over percutaneous BCI with respect to postoperative skin care and cosmetic outcomes for patients who experienced difficulty in using a conventional hearing aid and percutaneous BCIs. Patient satisfaction with BCIs differed between BB and Baha.
Keywords: Bone conduction implant; Mixed hearing loss; Auditory meatus atresia; APHAB
Abbreviation
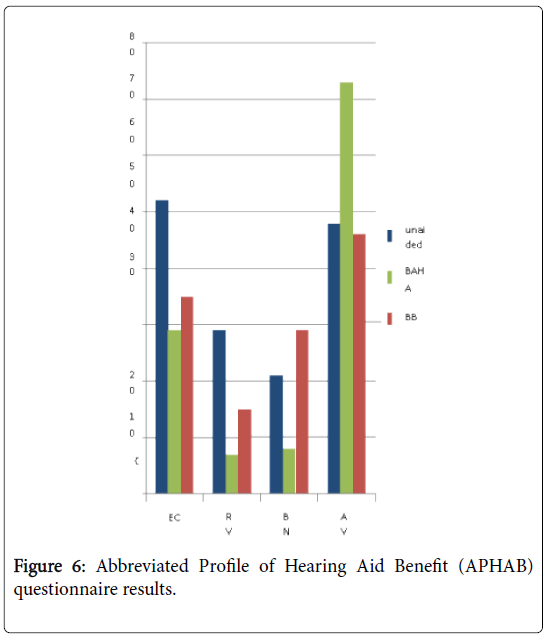
EC: Ease of Communication; RV: Reverberation; BN: Background Noise; AV: Aversiveness
Introduction
Bone conduction hearing implants (BCIs) are used for patients with conductive or mixed hearing loss who have difficulty in using conventional hearing aids. Bone-anchored hearing aid (BAHA®) is the most commonly implanted BCI in many countries and comprises an implant and a sound processor. The implant is connected to the sound processor by a percutaneous abutment. Direct transmission of the vibration from the sound processor to the bone through the abutment and implant provides high-quality sound for BAHA users.
Although percutaneous BCI is widely used for improving hearing, there are some disadvantages compared with conventional nonimplantable bone conduction devices. The first disadvantage is the necessity of daily maintenance of skin hygiene to prevent skin disorders, and the other disadvantage is the low cosmetic appeal associated with these devices. Transcutaneous BCI has recently been approved in several countries and may have the potential to reduce these problems because it does not penetrate the skin. The use of the Bonebridge™ (BB, MED-EL Innsbruck, Austria) has been reported in a few cases in Asia [1,2].
We conducted clinical trials with two patients using the following transcutaneous BCIs: the BAHA attract (Cochlear Inc. Australia) and the BB both devices have not been approved for medical use in Japan. Patients whohad mixed hearing loss and difficulty in using conventional hearing aids were selected and provided the option of participating in the clinical trial of transcutaneous BCI between 2014 and 2015. This study was conducted under the approval of the research ethics committee of the University of Miyazaki Hospital (2014-110) in accordance with the declaration of Helsinki (ethical principles for medical research involving human subjects created by the World Medical Association) and the participants had given their informed consent prior to inclusion in the study. In this clinical trial, we experienced BB surgery on a patient who suffered from skin trouble with previously implanted conventional BAHA.
Case Presentation
History of present illness and preoperative examinations
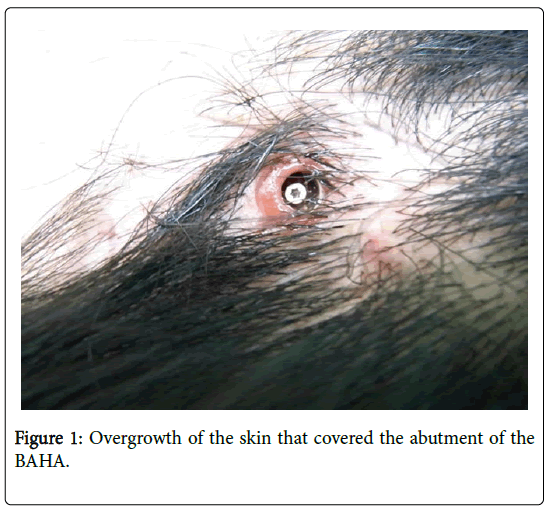
A 16-year-old male complained of bilateral mixed hearing loss. He had previously undergone otoplasty and tympanomeatoplasty for bilateral congenital external auditory meatus atresia and microtia in his early teens. Mixed hearing loss did not reduce postoperatively, and conventional hearing aids were not a feasible option owing to the insufficiently enlarged external auditory meatus. A conventional BAHA was implanted in his left ear when he was 14 years old. Although postoperative auditory evaluation showed agood hearing outcome, recurrent skin overgrowth around the implant occurred. Conservative therapy including topical injection of steroid merely exerted a temporal effect on the lesion. Recurrent overgrowth contraindicated the continued usage of BAHA (Figure 1).
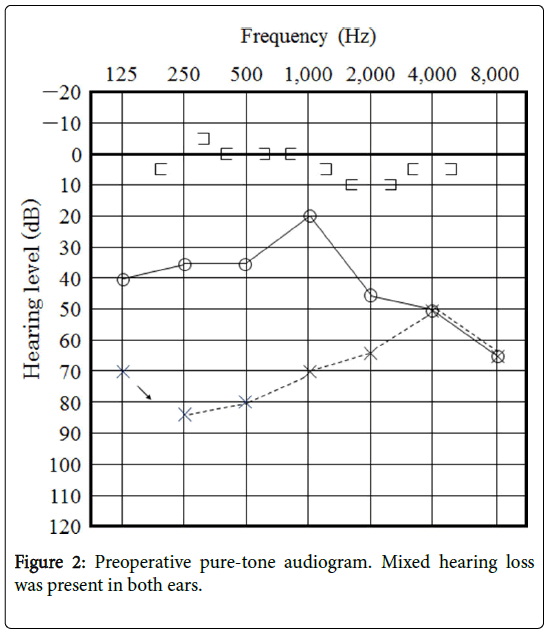
Preoperative PTA showed bilateral mixed hearing loss (Figure 2).
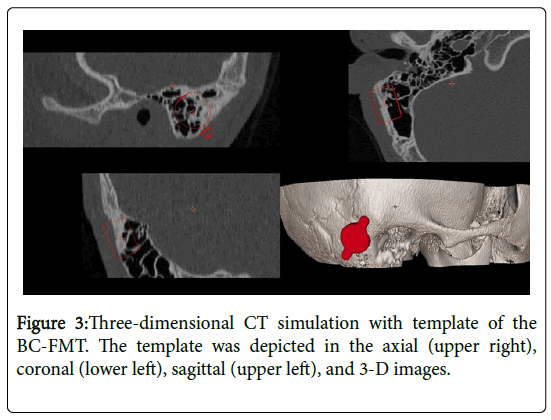
Temporal bone computed tomography (CT) revealed a wellpneumatized mastoid cavity. CT images were evaluated using 3- dimensional (3-D) simulation software (BB Fast View 1.0, CEIT, Spain) to estimate the site of placement of the bone conduction floating mass transducer (BC-FMT) of BB (Figure 3).
Surgical procedure and hearing results
The surgery was performed under general anesthesia. A retroauricular skin incision was made (Figure 4a), and the anteroinferiorly-based pericranialfascial flap was elevated. According to the preoperative positioning determined by CT, the bone bed for the BC-FMT was created in the sinodural angle. Because the bottom of the bed was partly in contact with dura mater (Figure 4b), BCI lifts (1 mm) were attached to the wings of the BC-FMT to avoid further drilling of the bed. After secure fixation was achieved with screws tightened using a wrench (Figure 4c), the wound was closed. There were no perioperative complications such as hematoma orinfection or late complications of the skin flap at least 1 year postoperatively.
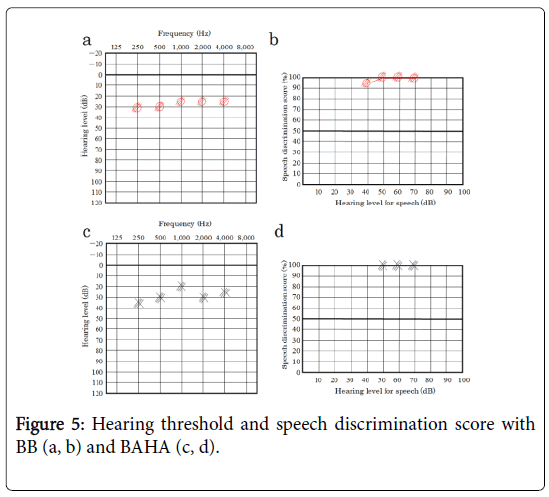
The hearing threshold and speech discrimination score of BB were approximately 30 dB and 100% in 55 dBSPL, respectively (Figure 5a, b), which were almost same as the results of BAHA in the left ear (Figure 5c, d).
Abbreviated Profile of Hearing Aid Benefit (APHAB) questionnaire was administered for evaluating the efficacy of the devices. The improvement was observed with both devices in the two following subscales; ease of communication (EC) and reverberation (RV). In background noise (BN) subscale, the score of BAHA was better. In aversiveness (AV) subscale the score of BB was lower than that of BAHA, which meant the result of BB was better in AV (Figure 6).
Discussion
For the patients with mild to moderate conductive or mixed hearing loss who were unable to wear conventional hearing aids, BCI is an effective procedure to improve hearing. Among the several clinically available devices, the BAHA has been most frequently used in many countries including Japan. This device is placed percutaneously on the temporal bone. Direct transmission of sound vibrations to the temporal bone supplies high-quality sound that provides sufficient satisfaction for BAHA users. The surgical technique is simple and straightforward, as that in short-stay surgery.
Although percutaneous BAHA is effective and is used worldwide, it has certain disadvantages owing to the nature of the percutaneous system [3]. Daily maintenance of skin hygiene to prevent soft tissue complications around the implant is necessary. Further, the abutment exposed at the skin might be cosmetically unsatisfactory. In the present case, recurrent skin complication prevented the patient from comfortably using the sound processor. Both surgical resection and the topical injection of steroid to the lesion merely brought a temporal effect. Reimplantation of percutaneous BAHA to the opposite side may be one of the choices of treatment, but such intervention might have resulted in similar skin disorders.
Recently, transcutaneous BCI has been used wherein the implant with a magnet is fixed on the skull bone and is completely covered with the skin flap. A sound processor was attached to the magnet through the skin. Transcutaneous BCI may have a lower incidence of postoperative skin complications than percutaneous BAHA. Among newly developed transcutaneous BCIs, none of which have been approved in Japan, we used the BB as a clinical trial. This has already been approved in Europe and the United states and the efficacy has been reported [4,5]. There are some case reports present in Asia [1,2]. The transducer of the BB is implanted under the skin; thus, the vibrations are transmitted directly to the skull bone, which indicates more powerful output potential than other transcutaneousBCIs. The sound processor does not transmit the vibrations but transmits electromagnetic signals through the skin. With respect to the implant size, BAHA needs drilling of the skull bone to a depth of 4 mm, whereas BB requires a sufficient area of the bone bed to place the BCFMT, which has a cylindrical shape with 15.8 mm diameter and 8.7 mm depth. Drilling the mastoid to create a same-sized bed is sometimes dangerous because of the presence of adjacent structures such as the dura and sigmoid sinus. Computed tomography is indispensable for pre- and postoperative evaluations [6]. Moreover, 3- D image simulation using conventional temporal bone CT was also useful for planning the operation. The dura was nearly exposed, as expected from the simulation, and the BC-FMT was placed safely. If a more precise preoperative determination such as a small temporal bone is required, the template-assisted method might be valuable [7,8].
Although both BB and BAHA exhibited similar excellent hearing outcomes in aided hearing levels and speech discrimination scores, patient satisfaction evaluated using APHAB showed some difference between the devices. Outcomes with BCIs show improvement in the EC, RV, BN subscales, and the deterioration of AV subscale [9,10]. EC, RV, and BN indicate speech understanding in various everyday environments, and AV doesnegative reactions to environmental sounds. In this study, the result of BAHA wassimilar to that in the literature, and that of BB seemed less effective in EC, RV, and BG subscales and more effective in AV subscales compared with percutaneous BAHA. The reason supposed to result in the power of the devices. Percutaneous system can vibrate the skull more powerfully than transcutaneous system. The score of APHAB can be influenced by the degree of output from the device. Regarding the AV, the sounds of BB proved to be more comfortable than that of BAHA in a loud environment [10].
Conclusion
We used BB on the percutaneous BAHA user with recurrent skin complications under a clinical trial. Postoperative complications were not observed and hearing outcomes were satisfactory. It is true that transcutaneous BCIs have a disadvantage in output, but audiological results might be enough to contribute to patient ’ s satisfaction. Moreover, they could reduce the frequency of postoperative complications and improve the patient’s quality of life. This case seems valuable in that the results of patient ’ s satisfaction between percutaneous and transcutaneous BCI was compared in the same individual. Although there are lack of evidence in re-implantation of BCI, that might be one of the effective choice of strategy for improvement in QOL as well as treatment for postoperative complications of percutaneous BCI user.
Acknowledgements
We are grateful to Mrs. Kawabata (MED-EL Japan Co., Ltd.) for providing technical advice regarding BB implantation.The authors declare that there is no conflict of interest regarding the publication of this article.
References
- Tsang WS, Yu JK, Bhatia KS, Wong TK, Tong MC. (2013) The Bonebridge semi-implantable bone conduction hearing device: experience in an Asian patient. J LaryngolOtol 127: 1214-1221.
- Nozomu M, Byunghyun C, Hidenori O, Kentaro M, Keita T, Aya I, Hidekane Y, Satoshi I, Shizuo K, Shin-ichi U.
- Hobson JC, Roper AJ, Andrew R, Rothera MP, Hill P, et al. (2010) Complications of bone-anchored hearing aid implantation. J LaryngolOtol124:132-136.
- Riss D, Arnoldner C, Baumgertner WD, Blineder M, Flak S, et al. (2014) Indication criteria and outcomes with the Bonebridge transcutaneous bone-conduction implant. Laryngoscope 124: 2802-2806.
- Rahne T, Seiwerth I, Goetze G, Heider C, Radetzki F, et al. (2015) Functional results after Bonebridge implantation in adults and children with conductive and mixed hearing loss. Eur Arch Otorhinolaryngol. 272: 3263-3269.
- Güldner C, Heinrichs J, Weiß R, Zimmermann AP, Dassinger B, et al. (2013) Visualization of the Bonebridge by means of CT and CBCT. Eur J Med Res 18:30.
- Matsumoto N, Takumi Y, Cho B, Mori K, Usami S, et al. (2015) Template-guided implantation of the Bonebridge: clinical experience. Eur Arch Otorhinolaryngol 272: 3669-3675.
- Cho B, Matsumoto N, Mori M, Komune S, Hashizume M. (2014) Image-guided placement of the BonebridgeTM without surgical navigation equipment. Int J Comput Assist RadiolSurg 9: 845-855.
- Gawęcki W, Stieler OM, Balcerowiak A, Komar D, Gibasiewicz R, et al. (2016) Surgical, functional and audiological evaluation of new Baha® Attract system implantations. Eur Arch Otorhinolaryngol 273: 3123-3130.
- Gerdes T, Salcher RB, Schwab B, Lenarz T, Maier H. (2016) Comparison of audiological results between a transcutaneous and apercutaneous bone conduction instrument in conductive hearing loss. OtolNeurotol37: 685-691.
Citation: Nakashima T, Matsuda K, Hirahara SY, Tono T (2020) Use of the BonebridgeTM for the Percutaneous BAHA user with Skin Complications: A Case Report . Otolaryngol 10:396. DOI: 10.4172/2161-119X.1000396
Copyright: © 2020 Nakashima T, et al. This is an open-access article distributed under the terms of the Creative Use of the BonebridgeTM for the percutaneous BAHA user with skin complications.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2153
- [From(publication date): 0-2020 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 1440
- PDF downloads: 713