Use of GLP-1 receptor agonists and subsequent risk of alcohol-related events. A nationwide register-based cohort and self-controlled case series study
Received: 22-Apr-2022 / Manuscript No. jart-22-61579 / Editor assigned: 25-Apr-2022 / PreQC No. jart-22-61579 (PQ) / Reviewed: 09-May-2022 / QC No. jart-22-61579 / Revised: 12-May-2022 / Manuscript No. jart-22-61579 (R) / Accepted Date: 14-May-2022 / Published Date: 19-May-2022 DOI: 10.4172/2155-6105.100466
Keywords: Addiction, Addcition Research, Addiction Therapy, Alcohol, GLP-1, Treatment, Cohort study, Case-series study, Epidemiology, Register study
Introduction
Alcohol use disorders are some of the most prevalent mental disorders worldwide affecting 8.6% of men and 1.7% of women [1] and with a lifetime prevalence of around 30% [2,3]. Alcohol use disorders are associated with markedly reduced life expectancy [4], a higher risk for concomitant chronic diseases and injuries as well as social deprivation including family disruption, unemployment and criminal convictions [5].
The treatment goal for most patients with alcohol use disorders is abstinence, and the way to achieve this often includes a combination of counselling (including motivational interviewing and encouraging patients to take responsibility for change) and medication [6]. To date, only few drugs such as disulfiram, acamprosate, nalmefene, and naltrexone have been approved as treatment of alcohol use disorders. However, the effect of the drugs varies and a recent population-based study found that disulfiram did not change risk of hospitalization for alcohol use disorder, and patients using acamprosate actually have a higher risk of hospitalization for an alcohol use disorder [7]. Only naltrexone seems to be effective in reducing heavy drinking, but less efficacious in promoting abstinence [8].
In recent years evidence from animal studies have shown that intestinal hormones such as ghrelin and glucagon-like-peptide 1 (GLP- 1), which are well-known as regulators of appetite and food intake, might also affect alcohol-related behaviours [9]. GLP-1 is a hormone secreted by the intestine in response to food intake and together with other gut hormones, it signals to the pancreas increasing insulin secretion and reducing glucagon secretion [10]. GLP-1 receptor agonists have therefore been developed for the treatment of type 2 diabetes. Apart from the peripheral effects, GLP-1 has also been found to function in the central nervous system [11]. Here, GLP-1 has been found to decrease consummatory behaviour by inhibition and modulation of dopaminergic neurons in the ventral tegmental area and the nucleus accumbens which are central areas involved in the mesolimbic reward system [12-14]. To date, the GLP-1 receptor agonist’s liraglutide and semaglutide are approved for treating obesity [15].
Studies on the effect of GLP-1 receptor agonists on alcohol addiction have reported reduction in alcohol drinking behaviour in rodents and monkeys [16,17], and polymorphisms in the GLP-1 receptor gene have been associated with alcohol use disorders [18]. However, to date no published studies on humans have examined the potential association between GLP-1 receptor agonists and alcohol use disorders. Thus, the aim of this study was to examine the association between use of GLP-1 receptor agonists and the risk of subsequent alcohol-related events in Danish adults.
Methods
Study population
We conducted a cohort study using information on the entire Danish population from nationwide registers. The study population was identified in the Danish Civil Registration System using the unique personal identification number (CPR) which enables accurate linkage of recorded individual-level information in all registers [19]. The cohort consisted of all persons from 18 years of age and living in Denmark at the start of the follow-up period (January 1, 2010) (n=4,299,646).
Main exposure: GLP-1 receptor agonists
All refills of prescriptions for medication were identified in the Danish National Prescription Registry, which contains information on all prescribed drugs dispensed at pharmacies since 1995 including date of prescription redemption and anatomical therapeutic chemical (ATC) codes [20].
The main exposure was treatment with GLP-1 receptor agonists which were defined as a redeemed prescription of drugs with ATC code A10BJ. In Denmark, the first GLP-1 receptor agonist, Exernatide, was approved for antidiabetic treatment in 2006, but the number of GLP-1 receptor agonist users was not stable until 2010 due to the initiation of liraglutide to the Danish marked in 2009. Consequently, we excluded 3,376 who had initiated GLP-1 receptor agonist treatment before 2010 as they might represent a selected group of patients [21], leaving a study population of 4,296,270 individuals.
Negative control exposures: insulin and metformin
To compare the potential effect of GLP-1 receptor agonists on alcohol-related events to the potential effect of other antidiabetic agents, all redeemed prescriptions of insulin and metformin was included using ATC codes A10A and A10BA02.
Outcomes: Alcohol-related events
The outcome of interest was alcohol-related events measured by: 1) Hospital contacts with a main diagnosis of alcohol use disorders (international classification of diseases (ICD)-10 code DF10) in the Danish National Patient Registry; 2) Registered treatments for alcoholism in the National Registry of Alcohol Treatment; or 3) Purchase of the benzodiazepine chlordiazepoxide (ATC code N05BA02), which is used for alcohol withdrawal syndrome or purchase of a medication against alcohol dependence (ATC code N07BB), registered in the Danish National Prescription Registry.
The Danish National Patient Registry includes information on physicians’ diagnoses for all inpatient, outpatient and emergency contacts at Danish hospitals since 1995, whereas the National Registry of Alcohol Treatment includes data on public treatment for alcoholism treatment since 2006 [22,23]. For individuals with more than one outpatient hospital contact, events recorded within 180 days of each other were categorized as the same, whereas emergency and inpatient events recorded within 15 days of each other were categorized as the same (e.g. due to discharge from psychiatric hospital to go to somatic hospital). If an individual had more than one purchase of an alcoholrelated medication, the following purchases within 180 days of each were assumed to belong to the same treatment event. For details see Supplementary Table 2 in the Supplement for included data and Supplementary Table 3 for the distribution of alcohol-related events on data source for the different populations.
Covariables
We selected several factors assumed to be associated with selection of patients for GLP-1 receptor agonists and risk of alcohol-related events as covariates. From the Danish National Patient Registry and Danish National Prescription Registry we obtained information on history of heart disease, stroke, diabetes, obesity, schizophrenia, major depression, alcohol, and substance abuse. From the Danish Civil Registration System, we obtained information on sex, age, marital status, country of origin and the region of place of living. Data on highest achieved educational level was obtained from the Population’s Education register. Educational level was categorized into four groups (low: primary school; middle: high school, vocational education (educations aimed towards manual work), and high: higher education, and higher advanced education and missing (patients born before 1920, older immigrants, or still receiving education)). Codes used for definitions of covariates are given in supplementary Table 4.
Ethical aspects
The study was approved by the Regional Data Protection Agency. As the data only included register-data, informed consent from participants was not required. The data used in the study are anonymized and located at Statistics Denmark and at the Danish Clinical Registries and cannot directly be accessed by others due to privacy restrictions.
Statistical analysis
The associations between GLP-1 receptor agonist’s prescriptions and alcohol-related events were analysed using the following approaches to address potential confounding by indication: Multiple adjustments, propensity score matching and self-controlled case-series design. Finally, we included two potential negative control exposures, insulin, and metformin, assuming they would have similar confounding but no plausible biological connection with the outcome. All analyses were performed in STATA-16.
First, we used Cox proportional hazard regression to calculate hazard ratio (HR) and 95% Confidence Intervals (CI) and included GLP-1 receptor agonist treatment as a time-dependent variable. Thus, if a patient started GLP-1 receptor agonist treatment during follow-up,this patient would change exposure status from unexposed to expose.Cohort members were followed from January 1st, 2010 or initiation of GLP-1 receptor agonist treatment until first registration of an alcoholrelated event, emigration, death, or end of follow-up (December 31st, 2018), whichever came first. To explore if the risk varied during followup, we examined the incidence rates for 0-90 and 90-365 days and later after first prescription of GLP-1 receptor agonists by splitting the data on follow-up time. We accounted for potential confounders by multiple adjustments for age, sex, marital status, education, country of origin, somatic and psychiatric comorbidity, diabetes, and time since diabetes diagnosis/treatment at baseline. Since the risk of alcoholrelated events vary with sex, age and comorbid obesity, we explored potential interactions with these variables in stratified analyses and, if applicable, tested by including interaction terms using likelihood ratio tests. This showed no significant interactions when analyses were stratified by these covariates.
Next, we did a propensity score (PS) calibration [24], where the PS was estimated by use of Cox proportional hazard regression as the relative hazard of exposure (to GLP-1 receptor agonists) conditional on the baseline covariates. With this score, we performed PS matching with a one-to-one match on the nearest neighbour. In Cox proportion hazard regression models, we subsequently estimated the hazard of alcohol-related event in the PS-matched sample.
To further reduce some potential residual confounder, we secondly included a self-controlled case-series design [25]. In this design, only included participants who purchased GLP-1 receptor agonists during follow-up were included, and the potential association between GLP-1 receptor agonist treatment and an alcohol-related event was investigated by comparing different time periods for each individual. In this analysis, the available follow-up time for each individual was classified into five different categories:
1. Time when unexposed to GLP-1 receptor agonists (nontreatment period);
2. A three months preexposure period up to and including the date of first prescription of GLP-1 receptor agonists;
3. The first three months following GLP-1 agonist initiation (initial exposed time);
4. 4-12 months following GLP-1 agonist initiating (late exposed time); and
5. The following 13-24 months (consolidated exposed time) after initiation.
The pre-exposure period was defined because of concerns about confounding by indication. We used Poisson regression conditional on the individual person to calculate the incidence of alcohol-related events in the different study periods and generated incidence rate ratios, with 95% confidence intervals, using the non-treatment period as reference. Individuals who discontinued or died were censored one month after the last prescription of GLP-1 receptor agonists or at time of death. Self-controlled time series remove time constant confounders but are still sensitive towards time varying factors such as age which we adjusted for [26].
Finally, all the above analyses were repeated using insulin and metformin as negative control exposures.
Results
Of the 4,296,270 participants with a mean age 49.2 years, 50.9% were women, 85,456 (2.0%) had a previous diabetes diagnosis, while 251,435 (5.1%) had a history of alcohol or substance abuse. GLP-1 receptor agonist treatment was initiated in 44,649 individuals (1.0 %), while 55,157 (1.1%) individuals started insulin, and 174,467 (4.1%) started metformin. At baseline, GLP-1 agonist users generally had higher mean age, were more often men and had more comorbidities compared with non-users, but in the PS matched sample, there were only small differences in the distribution of covariates between GLP-1 agonist treated and non-treated subjects (Table 1). During follow-up of a mean of 8.2 years (SD 2.0), a total of 97,868 (2.3%) participants experienced an alcohol-related event corresponding to an incidence rate (IR) of 27.3 (95% CI 27.1-27.5) cases per 10,000 person-years.
Association of GLP-1 receptor agonists with alcohol-related events
| Item No | Recommendation | Page No | ||
|---|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | P1-2 | |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | P2 | |||
| Introduction | ||||
| Background/ rationale | 2 | Explain the scientific background and rationale for the investigation being reported | P4 | |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | P4-5 | |
| Methods | ||||
| Study design | 4 | Present key elements of study design early in the paper | P5 | |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | P5-6 | |
| Participants | 6 | (a) Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up | P5 | |
| (b) For matched studies, give matching criteria and number of exposed and unexposed | N/A | |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | P5-6 | |
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | P5-6 | |
| Bias | 9 | Describe any efforts to address potential sources of bias | P5-8 | |
| Study size | 10 | Explain how the study size was arrived at | P5 | |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | P6 | |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | P6-8 | |
| (b) Describe any methods used to examine subgroups and interactions | P7 | |||
| (c) Explain how missing data were addressed | P6 | |||
| (d) If applicable, explain how loss to follow-up was addressed | N/A | |||
| (e) Describe any sensitivity analyses | N/A | |||
| Results | ||||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | P5 and P6 | |
| (b) Give reasons for non-participation at each stage | P5 | |||
| (c) Consider use of a flow diagram | N/A | |||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | P8 | |
| (b) Indicate number of participants with missing data for each variable of interest | Table 1 and suppl table 4 | |||
| (c) Summarise follow-up time (eg, average and total amount) | P8 | |||
| Outcome data | 15* | Report numbers of outcome events or summary measures over time | P8 | |
Main results |
16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | P8-9 Fig 1-3 Suppl Fig 1-2 |
|
| (b) Report category boundaries when continuous variables were categorized | N/A | |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | N/A | |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | Validations- study in supplementary |
|
| Discussion | ||||
| Key results | 18 | Summarise key results with reference to study objectives | P9-10 | |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | P11-12 | |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | P10-11 | |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | P11 | |
| Other information | ||||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | P12 | |
| *Give information separately for exposed and unexposed groups. Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at http://www.strobe-statement.org. |
||||
Table 1: STROBE Statement: Checklist of items that should be included in reports of cohort studies.
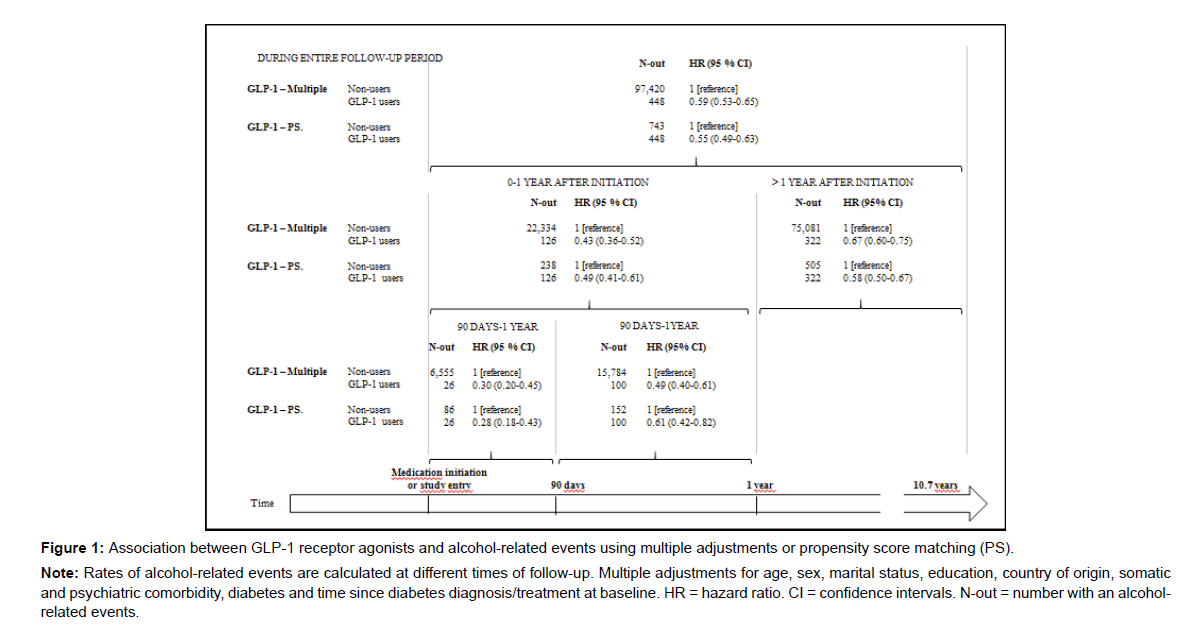
In the multiple adjusted Cox proportional hazard regression model, GLP-1 receptor agonist use was associated with a lower risk of a subsequent alcohol-related event (HR 0.59 (95% CI 0.53-0.65)) compared to non-use (Figure 1). When splitting into time periods, the lower risk was more pronounced during time periods closest to initiation (HR1 year after 0.43 (95% CI 0.36-0.52), HR90 days after 0.30 (95% CI 0.20-0.45)). In the PS matched sample, the results from the Cox proportional hazard regression models were similar to those obtained by multiple adjustment (HR 0.55 (95% CI 0.49-0.63)) and with the lowest risk in time-period closest to GLP-1 agonist initiation (Figure 1).
Note: Rates of alcohol-related events are calculated at different times of follow-up. Multiple adjustments for age, sex, marital status, education, country of origin, somatic and psychiatric comorbidity, diabetes and time since diabetes diagnosis/treatment at baseline. HR = hazard ratio. CI = confidence intervals. N-out = number with an alcoholrelated events.
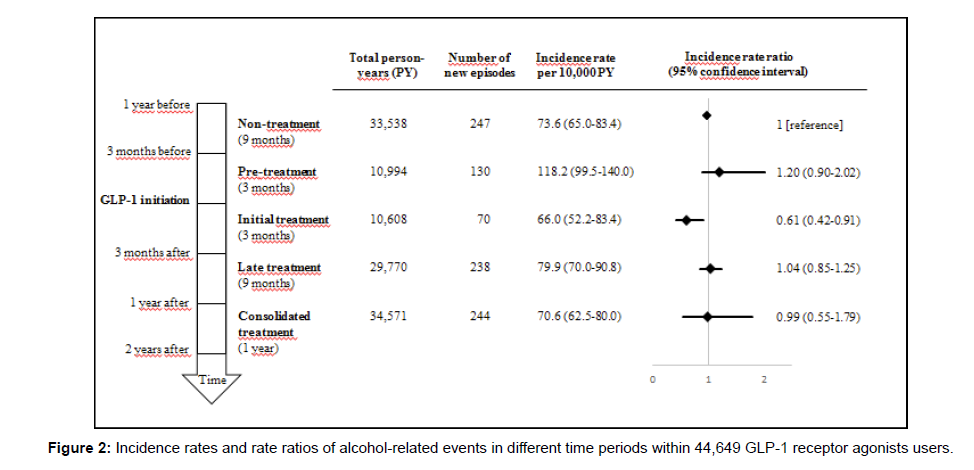
In the self-controlled analysis, we compared time periods before and after initiation of GLP-1 receptor agonist treatment. This showed that the IR of an alcohol-related event was highest during the last three months before GLP-1 receptor agonist initiation (pre-treatment period IR 118.2 (95% CI 99.5-140.0) per 10,000 person-years), while the lowest rate was found in the three months following GLP-1 receptor agonist treatment initiation (initial treatment period, IR 66.0 (95%CI 52.2- 83.4) per 10,000 person-years) (Figure 2). Thus, when compared to the non-treatment period, the incidence rate ratio (IRR) was slightly higher (IRR 1.20 (95% CI 0.90-2.02)) in the pre-treatment period and significantly lower (0.61 (95% CI 0.42-0.91)) in the initial treatment period. During the following years of continued treatment, the risk did not vary from the non-treatment period.
Associations of the negative control exposures with alcoholrelated events
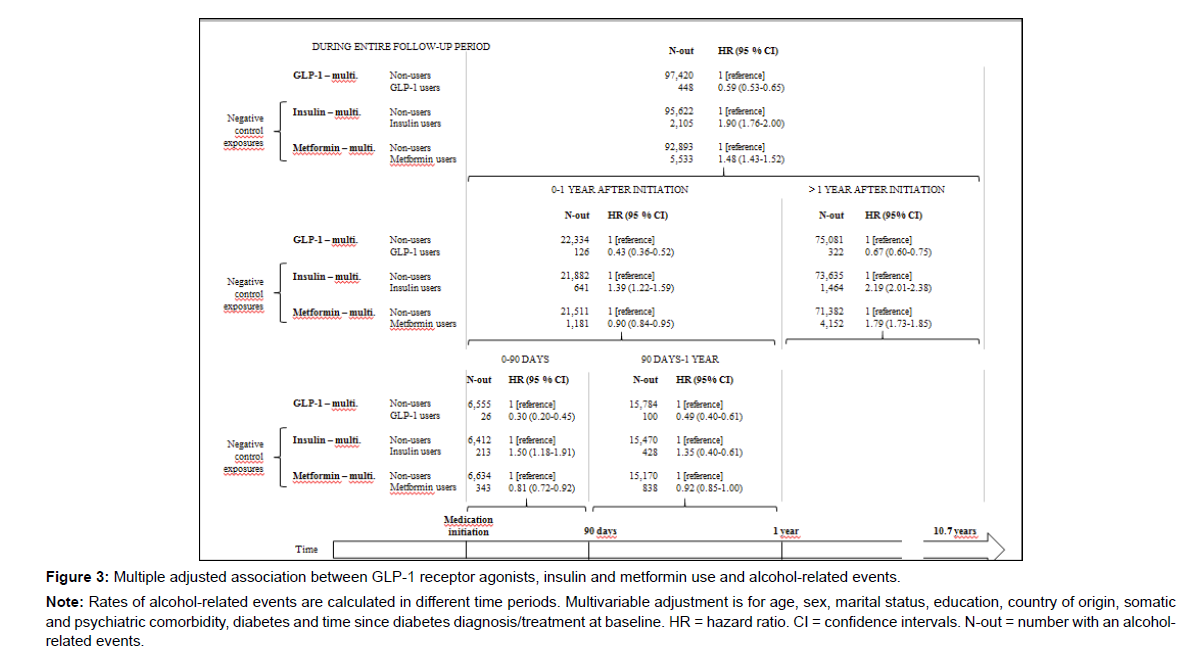
Characteristics of individuals treated with metformin or insulin at the time of treatment initiation is shown in supplementary Table 5. The multiple adjusted Cox proportional hazard regression models with insulin or metformin as negative control exposures showed that both insulin and metformin users had a higher risk of an alcoholrelated event during the entire follow-up period (HRInsulin 1.90 (95% CI 1.79-2.00), HRMetformin 1.48 (95% CI 1.43-1.52) vs. HRGLP-1 0.59 (95% CI 0.53-0.65)) (Figure 3). For insulin users, this risk was higher during all time periods, while for metformin the risk was slightly lower than in non-users during the first year and especially, during the first 90 days (HRMetformin 0.81 (95% CI 0.72-0.92). In the PS-matched samples, results were similar to results from the multiple adjusted models (Figure 1).
Note: Rates of alcohol-related events are calculated in different time periods. Multivariable adjustment is for age, sex, marital status, education, country of origin, somatic and psychiatric comorbidity, diabetes and time since diabetes diagnosis/treatment at baseline. HR = hazard ratio. CI = confidence intervals. N-out = number with an alcoholrelated events.
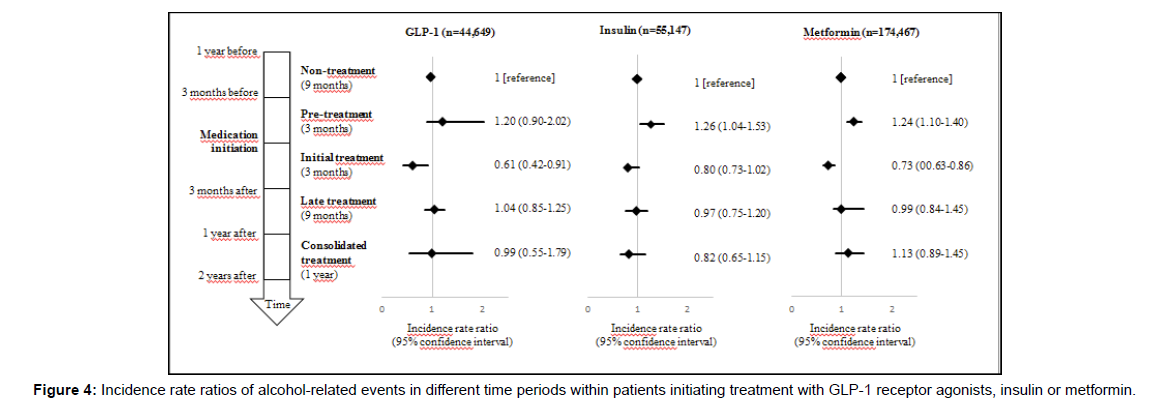
The self-controlled analysis showed that compared to in the nontreatment period, the IRR was highest in the three months preceding treatment initiation and lowest in the three months following initiation of insulin or metformin treatment (Figure 2 and Figure 4).
Discussion
Principal findings
In this nationwide register-based cohort study, we found that use of GLP-1 receptor agonists was associated with a lower risk of a subsequent alcohol-related event after adjustment for covariates. In self-controlled analysis, initiation of GLP-1 receptor agonist treatment was also associated with a lower risk of an alcohol-related event compared with the non-treatment period but only during the first 3 month after start of treatment. The negative control exposures insulin and metformin were both associated with higher risk of a subsequent alcohol-related event in cohort analyses, whereas in the self-controlled design, the risk of alcohol-related events were highest in the three months preceding treatment initiation and lowest in the three months after initiation.
Comparison with related studies
Available data on the effects of GLP-1 receptor agonists on alcohol intake in patients with alcohol use disorder are extremely limited [14]. Data in abstract format reported that liraglutide treatment reduce alcohol intake in patients with diabetes [27]. Thus, most evidence of an effect of GLP-1 receptor agonists on alcohol consumption is based on rodent studies [17] and studies in velvet monkeys [28], all showing that GLP-1 receptor agonists reduce intake of alcohol. The present study was based on the hypothesis that GLP-1 receptor agonists attenuate the ability of alcohol to activate the mesolimbic dopamine system, and through this decreases motivation for alcohol consumption [29,30]. At first, our findings seemed to support that initiation of GLP-1 receptor agonist treatment is associated with fewer alcohol-related events, which we used as proxy for a lowered alcohol intake. However, data from different approaches to address confounding indicated that a part of this relation might be explained by unmeasured confounding: While the multiple adjusted and propensity score matched analyses both showed lower rates of alcohol-related events in individuals treated with GLP-1 receptor agonists, the self-controlled design - which efficiently accounts for unmeasured between person confounding - showed that GLP-1 agonist users had low rates of alcoholic events during the first three months after treatment start but similar rates the following months. In the latter analysis, individuals who discontinued treatment were censored and as the potential effect of GLP-1 receptor agonists on alcohol behaviour seemed to attenuate with time. Thus, it is possible that the effect is either only observed in the initial treatment phase, i.e. 3 months, or is caused by GLP-1 receptor agonist-related adverse events, is due to unspecified factors e.g. more frequent contact to doctors, healthy life style advices or other lifestyle choices during this specific time-period. Most previous experimental studies have examined potential acute effects of GLP-1 receptor agonists, while evidence on chronic or sub-chronic use of GLP-1 receptor agonists and alcohol use is sparse [17]. It is thus possible, that only acute but not chronic use of GLP-1 receptor agonists affects alcohol consumption. In human studies testing the effect of the GLP-1 receptor agonist liraglutide on CNS activation in response to viewing food pictures or to chocolate milk in obese subject, liraglutide significantly reduced CNS activation compared to insulin during the first 12 weeks, however, the effect ceased to be significant after 12 weeks of treatment [31, 32]. Common side effects of GLP-1 receptor agonists after initiation include malaise and nausea [33], and it is also a possibility that these side effects could cause lower alcohol consumption during the first three months. In the self-controlled analyses, results were similar for users of GLP-1 receptor agonists, metformin and insulin, suggesting that the effect of GLP-1 may also be due to non-drug related factors during the specific time-period after initiating a new drug treatment.
Strengths and weaknesses
Our study benefits from a longitudinal design and the use of nationwide population-based registers in Denmark, a country with free access to health care. Register linkage enabled us to minimize probable selection or recall bias as all exposures were recorded independently of the outcome. Information on drug exposures was relative complete and entered as time-varying covariate in cohort analysis. Finally, we used different strategies to account for confounding by indication as suggested by others [34].
This observational study has two main limitations. First, we used patients registers to identify alcohol-related events as proxies for alcohol consumption. To test this approach, we did a validation study comparing the register-based definition with information on self-reported data on alcohol consumption in a longitudinal study of 2725 middle-aged men (Online-only Validation study). This study showed that low self-reported alcohol consumption was associated with fewer alcohol-related events, which indicates that our outcome measure may reflect changes in heavy alcohol consumption. The other main limitation is confounding by indication. Although we used several approaches to address potential confounding, each method has limitations. In the cohort and PS matched sample, all data used for adjustment were register-based and the information on education, comorbidities and at baseline might not fully reflect difference e.g. in relation to lifestyle and social relations between patients selected for GLP-1 receptor agonist treatment or not. The self-controlled case-series design, on the other hand, account for time invariant confounders, e.g. personality traits and family or genetic disposition but time-varying confounders may still influence the association. For example, the higher risk of outcomes during the pre-treatment period might be due to excessive drinking which might contribute to metabolic dysregulation leading to antidiabetic treatment. Similar, the lower risk during the initial treatment period may partly have been because of the support from health care facilities. Finally, we brought insulin and metformin into the analysis as negative control exposures. We assumed that these individuals in many ways would be similar to those treated with GLP-1 receptor agonists. However, this assumption was not fulfilled as users of these antidiabetics had other sociodemographic profiles and health status than GLP-1 receptor agonist users.
Conclusion
In conclusion, the incidence of alcohol-related events was low in individuals who started treatment with GLP-1 receptor agonists, but self-controlled analyses indicated that this might be explained by confounding by indication. However, there might be a transient preventive effect on alcohol-related events the first months after treatment initiation.
Overall, this study does not support that treatment with the presently available GLP-1 receptor agonists is an effective alternative to the existing treatment of alcohol use disorders, but the present study considers changes in heavy alcohol consumption only and our results may not be applicable to moderate alcohol use.
Funding
The work was supported by the Lundbeck Foundation (Grant number R249-2017-1074 and R231-2016-2550), Jaschafonden, The Danish Medical Association (Grant number 22017-1064/26 HBN) and M.D. Gerhard Linds Grant.
The sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Contributor Statement
IWA and MWA have made the first draft of the paper. MO was responsible for the data collection and analyzing the data. All authors took part in design, interpretation of the results, and have read and approved the final version of the article.
The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of Competing Interests
All other authors declare no competing interests.
References
- Carvalho AF, Heilig M, Perez A, Probst C, Rehm J (2019) Alcohol use disorders. Lancet 394(10200): 781-792.
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, et al. (2015) Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72(8): 757-766.
- Mohler-Kuo M, Foster S, Gmel G, Dey M, Dermota P (2015) DSM-IV and DSM-5 alcohol use disorder among young Swiss men. Addict Abingdon Engl 110(3): 429-440.
- Plana-Ripoll O, Pedersen CB, Agerbo E, Holtz Y, Erlangsen A, et al. (2019) A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet Lond Engl 394(10211):1827-1835.
- Rehm J (2011) The risks associated with alcohol use and alcoholism. Alcohol Res Health 34(2):135-143.
- Schuckit MA (2009) Alcohol-use disorders. Lancet 373(9662):492-501.
- Heikkinen M, Taipale H, Tanskanen A, Mittendorfer‐Rutz E, Lähteenvuo M, et al (2021) Real‐world effectiveness of pharmacological treatments of alcohol use disorders in a Swedish nation‐wide cohort of 125 556 patients. Addiction 116(8):1990-1998.
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW (2013) Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful?. Addiction 108(2): 275-293.
- Jerlhag E (2020) Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1. Brain Res 1727: 146562.
- Holst JJ, Seino Y (2009) GLP-1 receptor agonists: Targeting both hyperglycaemia and disease processes in diabetes. Diabetes Res Clin Pract 85(1):1-3.
- Nilsson M, Gjedde A, Brock B, Gejl M, Rungby J (2018) The effects of incretin hormones on cerebral glucose metabolism in health and disease. Neuropharmacology 136:243-250.
- Volkow ND, Wise RA, Baler R (2017) The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18(12):741-752.
- Eren-Yazicioglu CY, Yigit A, Dogruoz RE, Yapici-Eser H (2021) Can GLP-1 Be a Target for Reward System Related Disorders? A Qualitative Synthesis and Systematic Review Analysis of Studies on Palatable Food, Drugs of Abuse, and Alcohol. Front Behav Neurosci 14:614884.
- Klausen KM, Thomsen M, Wortwein G, Fink‐Jensen A (2022) The role of glucagon‐like peptide 1 (GLP‐1) in addictive disorders. Br J Pharmacol 179(4):625-641.
- Singh G, Krauthamer M, Bjalme-Evans M (2022) Wegovy (semaglutide): a new weight loss drug for chronic weight management. J Investig Med 70(1): 5-13.
- Jerlhag E (2020) Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1. Brain Res 1727: 146562.
- Brunchmann A, Thomsen M, Fink-Jensen A (2019) The effect of glucagon-like peptide-1 (GLP-1) receptor agonists on substance use disorder (SUD)-related behavioural effects of drugs and alcohol: A systematic review. Physiol Behav 206: 232-242.
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, et al. (2015) The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Transl Psychiatry 5: e583.
- Pedersen CB, Gotzsche H, Moller JO, Mortensen PB (2006) The Danish Civil Registration System: A cohort of eight million persons. Dan Med Bull 53(4): 441-449.
- Pottegard A, Schmidt SA, Wallach-Kildemoes H, Sorensen HT, Hallas J, et al. (2016) Data Resource Profile: The Danish National Prescription Registry. Int J Epidemiol 46(3):798-798f.
- Ankarfeldt MZ, Thorsted BL, Groenwold RH, Adalsteinsson E, Ali MS, et al. (2017) Assessment of channeling bias among initiators of glucose-lowering drugs: A UK cohort study. Clin Epidemiol 9: 19-30.
- Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, et al. (2015) The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 7: 449-490.
- Schwarz AS, Nielsen B, Nielsen AS (2018) Changes in profile of patients seeking alcohol treatment and treatment outcomes following policy changes. J Public Health 26(1): 59-67.
- Austin PC (2014) The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 33(7): 1242-1258.
- Gibson JE, Hubbard RB, Smith CJP, Tata LJ, Britton JR, et al. (2009) Use of Self-controlled Analytical Techniques to Assess the Association Between Use of Prescription Medications and the Risk of Motor Vehicle Crashes. Am J Epidemiol 169(6): 761-768.
- Petersen I, Douglas I, Whitaker H (2016) Self controlled case series methods: an alternative to standard epidemiological study designs. Bio Med J 354: i4515.
- Kalra S, Kalra B, Sharma A (2011) Change in alcohol consumption following liraglutide initiation: A real-life experience. Am Diabetes Assoc Conf 1029: 93.
- Thomsen M, Holst JJ, Molander A, Linnet K, Ptito M, et al. (2019) Effects of glucagon-like peptide 1 analogs on alcohol intake in alcohol-preferring vervet monkeys. Psychopharmacology (Berl) 236(2): 603-611.
- Jerlhag E (2018) GLP-1 signaling and alcohol-mediated behaviors; preclinical and clinical evidence. Neuropharmacology 136(Pt B): 343-349.
- Urban NBL, Kegeles LS, Slifstein M, Xu X, Martinez D, et al. (2010) Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [11C]raclopride. Biol Psychiatry 68(8):689-696.
- ten Kulve JS, Veltman DJ, van Bloemendaal L, Groot PFC, Ruhé HG, et al. (2016) Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J Endocrinol 229(1):1-12.
- ten Kulve JS, Veltman DJ, van Bloemendaal L, Barkhof F, Drent ML, et al. (2015) Liraglutide Reduces CNS Activation in Response to Visual Food Cues Only After Short-term Treatment in Patients With Type 2 Diabetes. Diabetes Care 39(2): 214-221.
- Filippatos TD, Panagiotopoulou TV, Elisaf MS (2014) Adverse Effects of GLP-1 Receptor Agonists. Rev Diabet Stud 11(3-4): 202-230.
- Gage SH, Munafo MR, Smith GD (2016) Causal Inference in Developmental Origins of Health and Disease (DOHaD) Research. Annu Rev Psychol 67:567-585.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Wium-Andersen IK, Wium-Andersen MK, Fink-Jensen A, Rungby J, Jorgensen MB, et al. (2022) Use of GLP-1 Receptor Agonists and Subsequent Risk of Alcohol-Related Events: A Nationwide Register-Based Cohort and Self- Controlled Case Series Study. J Addict Res Ther 13: 466. DOI: 10.4172/2155-6105.100466
Copyright: © 2022 Wium-Andersen IK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1144
- [From(publication date): 0-2022 - Mar 14, 2025]
- Breakdown by view type
- HTML page views: 813
- PDF downloads: 331