Research Article Open Access
Use of Ecopiling to Remediate PAH-Contaminated Storm-water Lagoon Sediment
Xuemei Liu1, Samuel Kiwanuka2, Kevin Cleary3, David Ryan2, David N Dowling2 and Kieran J Germaine2*
1MicroGen Biotech Ltd., Enterprise, Research and Innovation Centre, Carlow, Ireland
2EnviroCore, Dargan Research Centre, Institute of Technology Carlow, Carlow, Ireland
3Verde Environmental Consultants Ltd., Kilcoole, Co. Wicklow, Ireland
- *Corresponding Author:
- Kieran J Germaine
EnviroCore, Dargan Research Centre
Institute of Technology Carlow
Kilkenny Road
Carlow, Ireland
Tel: +353(0)599175580
E-mail: kieran.germaine@itcarlow.ie
Received date: April 24, 2016; Accepted date: May 18, 2016; Published date: May 25, 2016
Citation: Liu X, Kiwanuka S, Cleary K, Ryan D, Dowling DN, et al. (2016) Use of Ecopiling to Remediate PAH-Contaminated Storm-water Lagoon Sediment. J Bioremed Biodeg 7: 355. doi:10.4172/2155-6199.1000355
Copyright: © 2016 Liu X, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Polycyclic Aromatic Hydrocarbons (PAHs) are widely distributed in the environment originating from both natural and anthropogenic sources. Consequently, they are found ubiquitously in the lithosphere, hydrosphere, atmosphere and biosphere. They are of major concern due to their recalcitrant nature, ability to bio-accumulate and their toxic, carcinogenic and mutagenic effects on biological systems. One major anthropogenic source of PAHs is creosote which is used as a wood preservative and contains 85% PAHs (w/w). Industrial facilities carrying out creosote treatment activities can result in major contamination of soil and groundwater at these sites. The current study investigates the potential of Ecopiling as a bioremediation strategy for creosote contaminated soil/sediment at a former wood preservation facility. The study involved greenhouse experiments to assess the phytoremediation potential of perennial ryegrass (Lolium perenne L.) and onsite construction of two small-scale, trial Ecopiles. The greenhouse study showed that the creosote contaminated sediment had an inhibitory effect on plant growth and reduced the effectiveness of rhizoremediation. However, the use of a clean soil overlay led to a significant decrease in the phyto-toxicity experienced by the ryegrass plants and subsequently resulted in greater PAH degradation in these pots. The use of Ecopiling was found to reduce the sum of EPA 16 PAH concentrations in the sediment by 94-96% after 730 days. The incorporation of spent-mushroom compost into the Ecopile, did initially increase the rate of PAH degradation, however, after 730 days there was no significant difference between the PAH concentrations remaining in the two Ecopiles. Although Ecopiling is a slow process compared to thermal and chemical treatments it has proven to be an effective and low cost process for the remediation of PAHs from industrial sediments.
Keywords
Ecopiling; Bioremediation; Phytoremediation; PAHs; Field trials
Introduction
Polycyclic Aromatic Hydrocarbons (PAHs) are a group of compounds composed of fused aromatic rings. There are 1896 possible PAH structures containing two to eight fused rings. Many PAHs occur in the environment as a result of natural combustion processes such as forest fires and volcanic activity [1]. Anthropogenic inputs of PAHs into the environment stem from burning of fossils fuels, waste incineration, smelting operations and the use of oil and coal products such as asphalt and creosote. With the exception of leachate and spills, the vast majority of anthropogenic PAHs enter the environment in the form of air pollutants and over time are transported into water, soils, sediments and biota [2]. Low molecular weight PAHs (2-3 ring compounds) tend to be more water soluble than the higher molecular weight PAHs (5-8 rings) and these tend to partition between the vapour and solid phases. Heavier PAHs tend to be exclusively found in the solid phase and so are generally adsorbed onto dissolved organic matter (DOM) or soil particulates [3]. The tendency of PAHs to accumulate in soils, sediments and biota increases with increasing molecular weight of the PAH and the corresponding increase in the soil/sediment partition coefficient (Koc). PAHs themselves are generally unreactive and have low acute toxicity. However, their metabolites can be potent mutagens and carcinogens [4].
Creosote is a mixture containing 85% PAHs (w/w), 10% phenolics and 5% heterocyclic compounds and is commonly used as a wood preservative to treat wooden structures such as rail-road sleepers, electricity line poles and wooden marine structures. It is a dense, nonaqueous phase liquid (DNAPL) that contaminates soil and water at many wood-preservation sites [5,6]. In the past, inefficiencies in the wood impregnation process led to losses of up to 2% of the creosote charge in the form of fugitive air emissions and drip loss to the underlying ground (with typical charges being 120 L of creosote per m3 of wood). Remediation of these sites is difficult as aged PAHs tend to be tightly bound to soil particles reducing their bioavailability for microbial degradation. Soils and sediments with high clay content are particularly problematic due to the strong absorption and adsorption of PAHs to clay particles [7]. Currently, there are no European wide soil quality standards for PAHs in soil. However, the Dutch target and invention levels are widely used as benchmarks in remediation efforts. These values are based on the sum of 10 individual PAHs (anthracene, benzo[a]anthracene, benzo[k]fluoranthracene, benzo[a]pyrene, chrysene, phenanthrene, fluoranthene, indeno[1,2,3 c-d]pyrene, naphthalene and benzo[ghi]perylene) in the soil/sediment. The Dutch guidelines set a target value of 1 mg kg-1 and an intervention value (i.e., the value that remedial action should be taken) of 40 mg kg-1 [8].
A wide variety of remediation techniques are available for PAH removal from soils and sediments, with three of the most effective being thermal treatment, use of oxidants and bioremediation. Of these, bioremediation techniques are the most environmentally-friendly and cost effective. Bioremediation utilises the inherent ability of the soil and plant-associated microbiome to partially or completely degrade organic pollutants. Bacteria are the dominant micro-organisms in PAHcontaminated soil and play a crucial role in degradation of a wide range of PAH species [9]. However, the main drawback of bioremediation is that considerably more time is needed to complete the remediation process [10]. The rate of PAH biodegradation from contaminated soils varies depending on the molecular weight, water solubility and bioavailability of the constituent PAHs as well as other abiotc factors (soil pH, temperature, salinity, concentration of other toxic substances (e.g., metals), water content, availability of macro and micronutrients), and the population sizes of active PAH degrading micro-organisms [11]. Common bioremediation strategies including biostimulation, composting, biopiling and phytoremediation have been used with mixed success on PAH contaminated soil. All of these bioremediation strategies are aerobic processes and consequently, for them to be effective, the oxygen content of the soil needs to be relatively high. This oxygen content can be increased through processes of periodic tillage, continuous sparging with air, or by using oxygen release compounds (ORCs) such as hydrogen or magnesium peroxides. Oxygen diffusion in the soil is also effected by soil texture and structures. Soils and sediments with high clay content tend to have poor structure and consequently, low oxygen, water and nutrient permeability. These soils and sediments can be improved by incorporating materials like gypsum or organic matter such as compost into the soil [12].
The use of plants in conjunction with hydrocarbon degrading and/or plant growth-promoting bacteria (PGPB) has great potential for the remediation of hydrocarbon contaminated soils [13]. Germaine et al. [14] reported the successful remediation of 4800 m3 of soil containing low levels (<2000 ppm) of petrochemicals through Ecopiling over a 2 year period. The Ecopile process involves biostimulation of indigenous hydrocarbon degraders, bio-augmentation through inoculation with known PAH degrading consortia and phytoremediation, through the effect of root growth and penetration throughout the soil and the resulting stimulation of microbial activity in the rhizosphere.
The current study describes a small scale Ecopiling trial on a former wood preserving facility in Ireland. The contaminated soil had originated from sediment that had collected in a storm-water lagoon. During operation, the pond was regularly dredged and the sediment placed in an onsite landfill. This sediment was found to be grossly contaminated with PAHs (200-10,000 mg PAHs kg-1 soil). In addition, the high clay content of this material made it particularly challenging to work with and to remediate.
Materials and Methods
Soil conditions
Ecopiling was utilised as a remediation technology on a former industrial site in the Republic of Ireland. The sediment from the on-site landfill had high clay content (23.3% sand, 28.8% silt and 47.9% clay, 1.5-2% organic matter) and contained 575 ± 63.00 mg PAHs kg-1 soil.
Greenhouse phytoremediation trials
A greenhouse trial was initiated to establish the toxicity of the soil to perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens). 15 kg of PAH-contaminated sediment was air dried for 24 hrs and passed through a 2 mm sieve, to remove pebbles and debris. The sieved sediment was homogenised by mixing. Triplicate glass beakers containing 1 kg PAH contaminated soil were set up. An additional three beakers containing 1 kg of PAH contaminated sediment were set up and the sediment surface in each of these beakers was covered with uncontaminated soil (from the grounds of IT Carlow) to a depth of 2 cm. A set of control beakers were set up containing 1 kg of the same uncontaminated soil.
Into each glass beaker, 15 seeds of either ryegrass or clover were sown. The plants were cultivated under greenhouse conditions (16 hrs at 24°C, 8 hr at 16°C) for 12 weeks. Another set of control beakers were set up containing PAH contaminated sediment with no plants. Each beaker was watered with 100 ml of deionised water once per week. At the end of the experiment plant height and biomass were measured in each of the beakers.
All of the sediment was removed from each of the glass beakers, air dried for 24 hrs, crushed, sieved and homogenised as described above. 10 g air dried soil samples were subjected to Soxhlet extraction (hexane:acetone solvent 1:1) for 24 hours. The EPA 16 PAHs in these extractions were quantified by Gas Chromatography coupled with Flame Ionisation Detection (GC-FID) using a DB-5 30 m × 0.25 mm capillary column (oven profile 40°C for 4 minutes, ramp to 270°C (10°C min-1) held for 10 minutes, nitrogen gas flow rate 1.5 ml min-1, inlet temperature 220°C, FID temperature 270°C, injection volume 5 μl).
Field trial Ecopile constructions
Two tons of sediment was excavated from the landfill for use in a small scale field trial. This sediment was treated with a rotovator to break-up the large soil clods and was divided up into two 1 ton piles which were used to construct two Ecopiles.
The sediment in the two Ecopile systems were treated as follows: Nutrients were mixed into the sediment in the form of nitrogen: phosphorus (25:4) fertiliser at a rate of 5 kg m3. The sediment was also augmented with consortia of PAH degrading bacteria that had been isolated from the contaminated soil from the same site. This consortia was isolated by incubating 10 g of PAH contaminated soil in 500 ml minimal media [15] at 30°C, 100 rpm for 2 weeks and sub-culturing in the same media, supplemented with either naphthalene, fluorene, anthracene or phenanthrene, every 2 weeks for 3 months. This consortia was immobilised in an alginate bead carrier as described in Ref. [16]. Typical bacterial numbers in these beads range from 108-109 CFU per bead. The alginate beads were applied at a rate of 37 g m-3.
Ecopile 1 had the additional amendment of 0.1 tons of spent mushroom compost in order to improve texture and air diffusion. The Ecopiles were constructed so that they were perpendicular to the prevailing wind. The base layer of sediment (50 mm) was placed over a heavy-duty polythene liner and 1 m lengths of 50 mm drainage pipe were placed at approximately 0.5 m centers, laid laterally across the pile to allow for passive ventilation. The Ecopile was then raised in consecutive 200 cm layers, comprising PAH contaminated sediment and drainage piping to a height of 0.5 m. The Ecopiles were constructed to be trapezoidal in shape and were approximately 5 × 1 × 0.5 m (L × B × H) in dimension. Finally each Ecopile was capped with uncontaminated topsoil (~5 cm deep) and seeded with a 50/50 Clover: Ryegrass seed mix. A control pile consisting of untreated sediment was set up for the purposes of comparison.
Sampling
Three individual sediment samples were taken from each Ecopile, every 2-4 weeks over a three month period. Each of these samples were analysed for pH, water content, total aerobic bacterial counts, PAH degrader counts and PAH levels. TVC was estimated using standard plate count methods [17] while PAH degraders were estimated using a modified most probable number method [18]. Triplicate sediment samples were collected at day 0, 56, 161 and 730 and were sent to an independent testing laboratory (TE laboratories, Carlow, Ireland) for PAH quantification and speciation.
Results
Analysis of the PAH contaminated sediment revealed that fluorene, phenanthracene, fluoranthene, pyrene were the predominant PAHs in the soil and accounted for over 70% of the total EPA 16 PAHs present. A feasibility study investigating the use of phytoremediation as a treatment strategy for remediating this creosote contaminated soil/sediment was carried out in the greenhouse.
Toxicity of PAH contaminated sediment on growth of rye grass and white clover
This study involved growing either perennial ryegrass or white clover on creosote contaminated sediment with and without a clean soil overlay. The results showed that the creosote contaminated sediment exhibited significant (P=0.007) toxicity to perennial ryegrass and even greater toxicity on white clover. Biomass production of perennial ryegrass in the creosote contaminated sediment was inhibited by 94% (Figure 1; Table 1). The addition of a 2 cm clean soil overlay on top of the contaminated sediment significantly (P=0.002) reduced this growth inhibition, with perennial ryegrass plants showing a 53% reduction in their biomass compared to plants cultivated in pristine soils. White clover proved to be much more sensitive to the sediment contaminants than perennial ryegrass. The biomass of the clover plants cultivated in creosote contaminated sediment was reduced by 97% compared to plants cultivated in pristine soils. The clean soil overlay did improve the growth of the clover (82% inhibition) but not as dramatically as that seen in ryegrass.
| Total Biomass (g) | Stem Biomass (g) | Root Biomass(g) | |
|---|---|---|---|
| Ryegrassin Uncontaminated soil | 1.96± 0.53 | 1.07± 0.14 | 1.14± 0.22 |
| Rye grassin PAH sediment+overlay | 1.59± 0.10 | 0.29± 0.01 | 0.75± 0.11 |
| Rye grass+PAH sediment | 0.53± 0.30 | 0.04± 0.02 | 0.09± 0.13 |
Table 1: Biomass (dry weight) of perennial ryegrass in sediment treatments after 12 weeks (Values represent the average of three independent replicates (n=3) and the standard deviation).
Phyto-remediation of PAHs in greenhouse trial
PAH analysis of the soils at the end of these greenhouse studies showed that the growth of perennial ryegrass on creosote contaminated soil resulted in a 27% reduction in EPA 16 PAH concentrations compared to those present in the control sediment (Table 2). However, the perennial ryegrass cultivated on creosote contaminated soil having the clean soil overlay resulted in a 55% reduction in PAH concentrations compared to those present in the control soil. In glass beakers planted with clover there was no significant difference in the PAH concentrations in either the creosote contaminated or clean soil overlay treatments compared with the controls (Data not shown).
| PAH Concentration (mg kg-1) | |||
|---|---|---|---|
| Unplanted Control soil | Rye Grass Contaminated sediment | Ryegrass soil overlay | |
| Naphthalene | 0.000 | 0.000 | 0.000 |
| Acenaphthene | 1.739 | 0.725 | 0.000 |
| Acenaphthylene | 0.000 | 0.000 | 0.000 |
| Fluorene | 4.500 | 4.416 | 0.000 |
| Phenanthrene | 18.927 | 17.596 | 5.564 |
| Anthracene | 12.071 | 7.896 | 4.182 |
| Fluoranthene | 116.928 | 70.841 | 48.442 |
| Pyrene | 55.100 | 41.364 | 31.626 |
| Benzo[a]anthracene | 16.303 | 15.311 | 11.090 |
| Chrysene | 23.395 | 16.307 | 13.130 |
| Benzo[b,k]fluoranthene | 10.020 | 9.171 | 5.948 |
| Benzo[a]pyrene | 11.004 | 8.837 | 6.246 |
| Indeno[1,2,3,cd]pyrene | 13.528 | 13.569 | 0.000 |
| Dibenzo[a,h]anthracene | 1.039 | 0.943 | 0.000 |
| Benzo[g,h,i]perylene | 2.433 | 3.191 | 2.560 |
| Total | 286.985 | 210.167 | 128.788 |
| Stddev | 16.823 | 26.444 | 47.120 |
Table 2: PAH concentrations in sediments after 12 week of cultivation in the greenhouse (Values represent the average of three independent replicates, n=3).
Effectiveness of Ecopiling as a remediation strategy for PAH contaminated sediment
Two small-scale field trials were constructed to investigate the feasibility of remediating creosote contaminated soil/sediment using Ecopiles (Figure 2). The Ecopiles were monitored over an 86 day period for water content, pH, TVC and PAH degrader count. PAHs concentrations were also determined after 0, 56, 161 and 730 days. Water content and pH values did significantly fluctuate over the course of the 86 day monitoring period in Ecopile 1 but not in Ecopile 2. The addition of the spent mushroom compost was intended to increase aeration and improve soil texture in Ecopile 1. The increased aeration may have led to the increased water content loss observed. There was an unusually warm and dry period between day 14 and day 28, which may account for the 8% drop in water content levels in Ecopile 1 within this period. There was also a steady reduction in pH in Ecopile 1 over the monitoring period dropping from pH 7.6 to 6.8. Once again, the addition of the spent compost is thought to be responsible for this as the compost is high in acidic compounds such as fulvic and humic acids. The pH of Ecopile 2 remained relatively constant ranging from pH 7.2- 7.5.
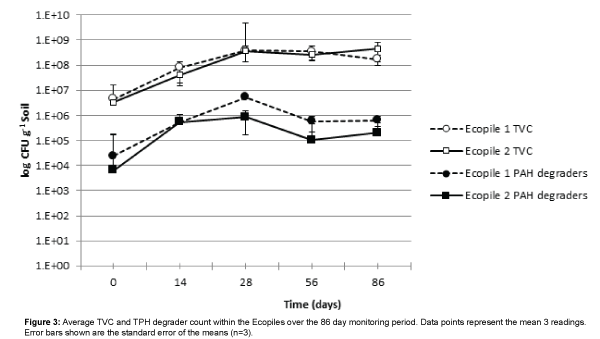
Total viable aerobic bacterial counts (TVC) increased in both Ecopiles over the course of the first 28 days of the trial, rising by nearly two orders of magnitude and remained constant thereafter (Figure 3). This rise is likely to be due to a combination of the effect of inoculation and biostimulation of the indigenous bacterial communities as a result of the macronutrient addition. There was no significant difference between the TVC counts of the two Ecopiles at any of the five sampling times. The addition of the spent mushroom compost did not significantly effect TVC counts in Ecopile 1 compared to Ecopile 2 (p>0.05). PAH degrader counts also increased in both Ecopiles over the course of the first 28 days of the trial, rising by nearly two orders of magnitude and decreased by one order of magnitude thereafter.
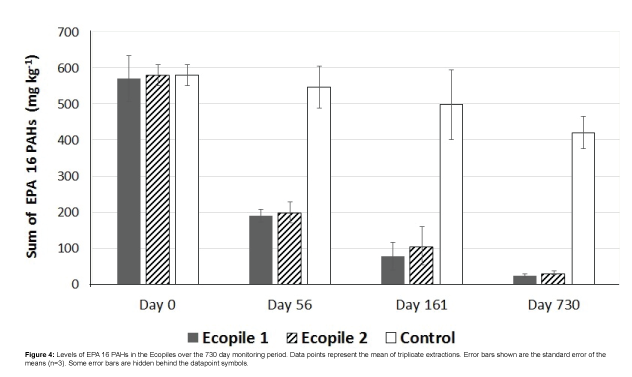
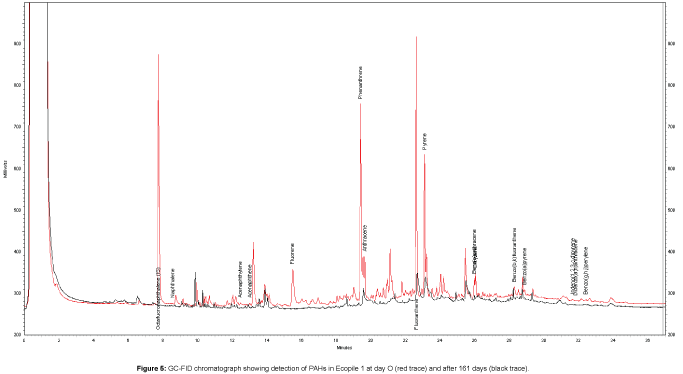
PAH quantification showed initial PAH levels of 570 ± 63 mg kg-1 and 580 ± 29 mg kg-1 for Ecopiles 1 and 2, respectively. Monitoring of the bioremediation progress showed that there was a rapid drop in PAHs concentrations in the Ecopiles (Figure 4). After 56 days, PAH concentrations remaining in Ecopiles 1 and 2 were 200 and 190 mg kg-1 respectively, corresponding to 66% and 65% reduction in PAH concentrations. After 161 days, the levels of EPA 16 PAHs detected within the Ecopiles soils showed a further reduction in the PAH concentrations with 86% degraded in Ecopile 1 and 81% degraded in Ecopile 2. After 730 days, EPA 16 PAH concentrations were reduced by 96% and 94% in Ecopile 1 and Ecopile 2, respectively (Table 3). The most recalcitrant PAHs proved to be Chrysene and Benzo[g,h,i] perylene, which showed very low degradation rates over the 730 day period. These were followed by Benzo[b,k]fluoranthene and Benzo[a] pyrene which had 44-62% and 46-65% degradation rates, respectively, over the 730 day period. The other remaining PAHs were degraded by 80-99%. The PAH analysis of the Ecopile soils suggested that there was considerably more degradation of phenanthrene, fluoranthene, benzo[a]anthracene, benzo[a]pyrene and indeno[1,2,3,cd]pyrene in Ecopile 1 than in Ecopile 2. Many of the unidentified compounds present in the initial contaminated sediment (unidentified peaks) were not observed in the GC-FID profiles after 161 days, suggesting that these compounds were also biodegraded (Figure 5). A control pile containing just untreated sediment showed a 27.5% reduction in EPA 16 PAH concentrations over the 730 day period.
| Ecopile 1 | Ecopile 2 | |||||
|---|---|---|---|---|---|---|
| Initial concentration (mg Kg-1) | Final concentration (mg Kg-1) | % Degraded | Initial concentration (mg Kg-1) | Final concentration (mg Kg-1) | % Degraded | |
| Naphthalene | 35.50± 0.11 | 0.15± 0.07 | 99.58 | 35.43± 0.04 | 0.15+0.07 | 99.57 |
| Acenaphthylene | 12.91± 0.38 | 0.45± 0.49 | 96.51 | 12.78± 0.15 | 0.7+0.85 | 94.52 |
| Acenaphthene | 4.02± 1.19 | 0.15± 0.07 | 96.27 | 4.35± 0.04 | 0.15+0.07 | 96.55 |
| Fluorene | 54.8± 4.98 | 0.30± 0.28 | 99.45 | 51.07± 0.04 | 0.5+0.02 | 99.02 |
| Phenanthrene | 160.85± 0.20 | 0.55± 0.21 | 99.66 | 152.75± 3.78 | 0.55+0.07 | 99.64 |
| Anthracene | 17.69± 3.56 | 1.90± 1.13 | 89.26 | 21.47± 0.31 | 1.75+0.21 | 91.85 |
| Fluoranthene | 141.13± 26.43 | 2.65± 1.34 | 98.12 | 153.78± 10.8 | 4.45+1.06 | 97.11 |
| Pyrene | 63.31± 12.06 | 5.90± 2.82 | 90.68 | 71.63± 4.16 | 6.6+0.71 | 90.79 |
| Benzo[a]anthracene | 35.92± 7.32 | 1.15± 0.35 | 96.80 | 36.39± 1.68 | 2.2+0.99 | 93.95 |
| Chrysene | 2.11± 0.00 | 2.10± 0.28 | 0.31 | 3.39± 0.08 | 3.55+1.2 | 0.00 |
| Benzo[b,k]fluoranthene | 7.24± 1.30 | 2.70± 0.56 | 62.70 | 7.74± 2.10 | 4.3+8.4 | 44.46 |
| Benzo[a]pyrene | 5.74± 0.73 | 2.00± 0.35 | 65.19 | 5.23± 2.33 | 2.8+4.2 | 46.41 |
| Indeno [1,2,3 cd] pyrene | 26.54± 3.71 | 1.60± 0.28 | 93.97 | 20.40± 3.09 | 2.05+0.78 | 89.95 |
| Dibenzo [a,h] anthracene | 0.77± 0.00 | 0.15± 0.07 | 80.42 | 1.53± 0.38 | 0.45+0.99 | 70.50 |
| Benzo [g,h,i] perylene | 1.38± 0.50 | 1.35± 0.35 | 2.09 | 2.07± 0.27 | 1.9+0.71 | 8.25 |
| Total | 570.00± 63.91 | 23.00± 8.65 | 95.95 | 580.00± 29.21 | 32.10± 8.97 | 94.46 |
Table 3: Percentage degradation of individual PAHs within the Ecopiles after 730 days (n=3).
Discussion
This study examined the feasibility of using Ecopiling as a possible remediation system for the removal of PAHs from creosote contaminated sediment. Ecopiling is a modification of traditional passive composting (biopiling) by incorporating phytoremediation into the remediation process. The Ecopile process also involves biostimulation of indigenous hydrocarbon degraders, bio-augmentation through inoculation with known hydrocarbon degrading consortia and phytoremediation, through the effect of root growth and penetration throughout the soil and the resulting stimulation of microbial activity in the rhizosphere. Greenhouse trials were set up to examine the feasibility of using perennial ryegrass and clover for the purpose of phytoremediating creosote contaminated soil. When grown in creosote contaminated sediment both ryegrass and clover plants had significant reductions in biomass production. Liste and Felgentreu [19] found that ryegrass was the most PAH-tolerant in their study of plant species growing in soil from a coal gasification site (PAH content: 71.4 mg kg-1). However, they reported up to a 50% decrease in root and shoot yield in ryegrass. The reduction in growth was thought to be due to toxicity of low molecular weight constituents. Yu et al. [20] showed that both phenanthrene and pyrene significantly decreased the biomass of ryegrass plants. While in an earlier report, Yu et al. [21] found no effects on plant growth or any sign of stress in white clover and perennial ryegrass grown in soil spiked with phenanthrene and pyrene at rates up to 375 mg kg-1. Saison et al. [22] found that the growth of Italian rye-grass (Lolium multiflorum var. Fastyl) and red clover (Trifolium pratense) on coking plant soils with 2077 mg PAH kg-1 soil was similar to plant growth in an uncontaminated control. In the current study we found that sowing the plant seeds in a thin layer of clean soil led to significant improvements in plant biomass. Seed germination is a very sensitive phase of plant growth, and many classical toxicity assays are based upon germination or seedling emergence in the presence of contaminants. Henner et al. [23] found that seed germination was strongly inhibited by low molecular weight, water-soluble compounds, for example toluene, styrene and naphthalene. Kuiper et al. [24] also found that naphthalene reduced seedling emergence. Seedlings grown in naphthalene contaminated (200 mg kg-1) soil produced no above ground growth. Liu et al. [25] found the high levels of phenanthrene (200 mg kg-1) exhibited toxic effects on ryegrass but this toxicity was only observed after 75 days. The analysis of the soils in the current study showed that there were low molecular weights PAHs present which may have been responsible for the reduced biomass observed.
In the greenhouse trials the growth of ryegrass in the creosote contaminated soil did result in greater PAH degradation than in the unplanted controls. Glass beakers with greater ryegrass biomass had greater levels of PAH biodegradation. Yu et al. [21] showed that ryegrass enhanced the dissipation of phenanthrene and pyrene through the significant (p<0.05) increase in peroxidase activities in soil. Olson et al. [26] found that planting soil with perennial ryegrass led to 85% dissipation of PAHs in contaminated soil. There are also many other reports of successfully using ryegrass in PAH phytoremediation studies (e.g., Ref. [27] and Ref. [28]).
The effectiveness of phytoremediation of PAHs in contaminated soil can be enhanced through the introduction of PAH degrading microbes. Afzal et al. [29] studied the effect of Italian ryegrass when it was grown in non-sterilised soil polluted with hydrocarbons and inoculated with different alkane-degrading strains of Pantoea sp. and Pseudomonas sp. This inoculation had a beneficial effect on plant biomass production and hydrocarbon degradation. Teng et al. [30] examined the effects of inoculation with Rhizobium meliloti on phytoremediation of aged PAHs by alfalfa. Planting with uninoculated Alfalfa and Alfalfa inoculated with R. meliloti significantly lowered the initial soil PAH concentrations by 37.2 and 51.4% respectively compared with unplanted control soil. However, the inoculation of soils with hydrocarbon degraders is not always guaranteed to be successful in increasing biodegradation rates. Pritchard et al. [31] bioaugmented PAH contaminated soil with a PAH degrading Sphingomonas sp., along with macronutrients. However, PAH degradation rates were not enhanced compared to untreated controls. On further analysis they found that undetermined compounds in the alkaline fraction of creosote extracts caused the highest level of inhibition in PAH degradation activity. It is generally accepted that microbes introduced into soil through liquid applications show poor survival rates [32]. For successful bioaugmentation, the added microorganisms must fill a unique metabolic niche in the soil microbial community or be protected by encapsulation [33,34]. In the current study, a constructed functional microbiome of PAH degrading bacteria (previously isolated from the same site) were encapsulated in calcium alginate beads. It has been previously shown that this is a useful way of inoculating polychlorinated biphenyl and diesel degrading microbes into soil [14,16]. These beads degrade slowly over time, giving the inoculum time to adapt to the conditions of the soil and protecting them from predation and competition.
In the current study, we also report a 95% reduction in PAH concentration after 730 days in Ecopile 1 and a 94% reduction in Ecopile 2. There have been a number of studies demonstrating the successful use of biopiling to remediate PAH contaminated soil. Lors et al. [35] used large field scale windrows (5000 tons each) to remediate soils heavily contaminated with PAHs (3000 mg kg-1). After 181 days they reported 85% degradation of EPA 16 PAHs. This reduction of PAH concentration was linked to the bacterial community, which was characterised by having a high diversity and the persistence of an introduced bacterial consortium during the entire biotreatment process. Llado et al. [36] used a dynamic biopile as a remediation strategy for a creosote-contaminated soil. This led to a 98% depletion of the low molecular weight PAH fraction while some high molecular weight PAHs (4-5 rings) were either partially or not degraded at all after a 180-day treatment. They found that additional biostimulation was necessary after the biopiling process to remove these residual PAHs. Gandolfi et al. [37] constructed small scale biopiles and used compost amendments on PAH contaminated soil. Their results showed that the addition of compost was effective in enhancing the degradation of PAHs (particularly the four-ringed PAHs) and in reducing the over-all toxicity of the soil. In the current study the addition of spent mushroom compost initially significantly enhanced the degradation of EPA 16 PAHs (by 30%) in the compost amended Ecopile compared to the Ecopile without compost. However, after 730 days there was no significant difference in the concentrations of the 16 EPA PAHs.
One of the possible disadvantages of using a phytoremediation system to remediate toxic compounds from the soil is the potential for transfer of the toxic components or their metabolites into plant tissues and so enter into the ecosystems food chain via herbivorous primary consumers. This is unlikely to be an issue with PAHs as their low water solubility and high log Kow values result in very little plant uptake and translocation. For example, Reilley et al. [38] found the total accumulation of pyrene and anthracene in roots and shoots of different plants accounted for <0.03% of total added PAHs. Thus, uptake of PAH by vegetation is insignificant. The potential for dermal contact and dust generation is reduced in the Ecopile system as the contaminated soil is covered with a layer of clean soil and the roots of the plants prevent soil erosion by the wind and thus reduce the generation of contaminated dust particles leaving the site during the remediation process.
Conclusion
The creosote contaminated sediment used in this study was found to significantly inhibit the growth of ryegrass and clover, and consequently reduced the effectiveness of the phytoremediation of the PAHs. The use of a thin layer of clean soil over the PAH contaminated sediment significantly increased the growth of ryegrass with a corresponding increase in PAH degradation. The incorporation of spent mushroom compost, as a soil conditioning agent, had no significant effect on the PAH levels within the Ecopiles after 730 days. Ecopiling proved to be an effective bioremediation technique for the removal of PAHs from industrial clayey sediments, with a reduction of 94-96% in the sum of EPA 16 PAH concentrations in the sediment after 730 days. One of the key advantages to this system is the reduced costs of the remediation process, as once constructed, there is little additional cost in terms of labour and maintenance (although the longer process time may incur additional monitoring costs). The other major advantage is that the aesthetics and many ecological functions are rapidly restored to the site. Ecopiling is a relatively slow process but it is a useful technique for remediating abandoned or very large sites where the cost of thermal/ chemical treatment would be prohibitively high.
Acknowledgements
This work was funded in part by the Irish Higher Education Authority (HEA) under the TSR III project ‘Agribiotics’ and the Technology Innovation Development Award 2011 funded by the Science Foundation Ireland (SFI).
References
- Abdel-Shafya HI, Mansourb MSM (2016) A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum 25:107-123.
- Gan S, Lau EV, Ng HK (2009) Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J Hazard Mater 172: 532-549.
- Haritash AK,Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169: 1-15.
- Dubrovskaya EV, Pozdnyakova NN, Muratova AY, Turkovskaya OV (2016) Changes in phyto-toxicity of polycyclic aromatic hydrocarbons in the course of microbial degradation. Russian Journal of Plant Physiology 63:172-179.
- Rogers SW, Ong SK, Moorman TB (2007) Mineralization of PAHs in coal-tar impacted aquifer sediments and associated microbial community structure investigated with FISH. Chemosphere 69:1563-1573.
- ATSDR (1995) Toxicological profile for polycyclic aromatic hydrocarbons (PAHs). Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services, Public Health Service, Atlanta, GA.
- Lau EV,Gan S, Ng HK (2010) Extraction techniques for polycyclic aromatic hydrocarbons in soils. Int J Anal Chem 2010: 398381.
- Esdat (2000) Dutch Target and Intervention Values.
- Deka H, Lahkar J (2016) Soil bacteria for Polycyclic Aromatic Hydrocarbon (PAH) remediation: Application Potentialities and Limitations. In: Hakeem KR, Akhtar MS, Abdullah SNA (Eds) Plant, Soil and Microbes. Springer pp. 291-316.
- De Boer J, Wagelmans (2016) Polycyclic Aromatic Hydrocarbons in soils-practical options for remediation. J Clean Soil, Air, Water 44:1-6.
- Harmsen J, Rulkens WH, Sims RC, Rijtema PE, Zweers AJ (2007) Theory and application of land farming to remediate polycyclic aromatic hydrocarbons and mineral oil-contaminated sediments: Beneficial reuse. J EnvironQual 36:1112-1122.
- UqabB, Mudasir S, Qayoom A, Nazir R (2016) Bioremediation: A Management Tool. J BioremedBiodeg 7: 331.
- Khan S,Afzal M, Iqbal S, Khan QM (2013) Plant-bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 90: 1317-1332.
- Germaine KJ, Byrne J, Liu X, Culhane J, Keohane J, et al. (2014) Ecopiling: A combined phytoremediation and passive biopiling System for remediating hydrocarbon impacted soils at field scale. Front Plant Sci 5: 756.
- Abraham WR, Nogales B, Golyshin PN, Pieper DH, Timmis KN (2002) Polychlorinated biphenyl-degrading microbial communities in soils and sediments. CurrOpinMicrobiol 5:246-253.
- Power B, Liu X, Germaine KJ, Ryan D, Brazil D, et al. (2011) Alginate beads as a storage, delivery and containment system for genetically modified PCB degrader and PCB biosensor derivatives of Pseudomonas fluorescens F113. J ApplMicrobiol 110:1351-1358.
- Skinner FA, Jones PCT, Mollison JE (1952) A Comparison of a direct- and a plate-counting technique for the quantitative estimation of soil micro-organisms. J Gen Micro 6: 261-271.
- Johnsen AR, Bendixen K, Karlson U (2002) Detection of microbial growth on Polycyclic Aromatic Hydrocarbons in microtiter plates by using the respiration indicator WST-1. Appl EnvironMicrobiol 68: 2683-2689.
- Liste HH, Felgentreu D (2006) Crop growth, culturable bacteria, and degradation of petrol hydrocarbons (PHCs) in a long-term contaminated field soil. Appl Soil Ecol 31: 43-52.
- Yu XZ, Wu SC, Wu FY, Wong MH (2011) Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J Hazard Mater 186: 1206-1217.
- Xu SY, Chen YX, Wu WX, Wang KX, Lin Q, et al. (2006) Enhanced dissipation of phenanthrene and pyrene in spiked soils by combined plants cultivation. Sci Total Environ 363: 206-215.
- Saison C, Perrin-Ganier C, Schiavon M, Morel JL (2004) Effect of cropping and tillage on the dissipation of PAH contamination in soil. Environ Pollut 130: 275-285.
- Henner P, Schiavon M, Druelle V, Lichtfouse E (1999) Phytotoxicity of ancient gaswork soils. Effect of polycyclic aromatic hydrocarbons (PAHs) on plant germination. Organic Geochemistry 30: 963-969.
- Kuiper I,Bloemberg GV, Lugtenberg BJ (2001) Selection of a plant-bacterium pair as a novel tool for rhizostimulation of polycyclic aromatic hydrocarbon-degrading bacteria. Mol Plant Microbe Interact 14: 1197-1205.
- Liu SL, Cao ZH, Liu HE (2013) Effect of ryegrass (Loliummultiflorum L.) growth on degradation of phenanthrene and enzyme activity in soil. Plant Soil Environ 59: 247-253.
- Olson PE, Castro A, Joern M, Duteau NM, Pilon-Smits E, et al. (2008) Effects of agronomic practices on phytoremediation of an aged PAH-contaminated soil. J Environ Qual 37: 1439-1446.
- Günther T,Dornberger U, Fritsche W (1996) Effects of ryegrass on biodegradation of hydrocarbons in soil. Chemosphere 33: 203-215.
- Binet P, Portal J, Layval C (2000) Fate of polycyclic aromatic hydrocarbons in the rhizosphere and mycorrhizosphere of Ryegrass. Plant and Soil 227: 207-213.
- Afzal M,Yousaf S, Reichenauer TG, Sessitsch A (2012) The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int J Phytoremediation 14: 35-47.
- Teng Y,Shen Y, Luo Y, Sun X, Sun M, et al. (2011) Influence of Rhizobium meliloti on phytoremediation of polycyclic aromatic hydrocarbons by alfalfa in an aged contaminated soil. J Hazard Mater 186: 1271-1276.
- Pritchard PH, Jones-Meehan J, Straube W, Nestler C, Jones W, et al. (2002) Polycyclic aromatic hydrocarbons (PAHs): Improved land treatment with bioaugmentation.p. B1-B101. In: Bajpal R, Felt DR, Nestler CC, Wani A, Spain JC, et al. (Eds) Federal Integrated Biotreatment Research Consortium (FIBRC): Flask to Field Initiative (ERDC/EL TR-02-37). US Army Engineer Research and Development Center, Vicksburg, MS.
- O’ReillyKT, Crawford RL (1989) Degradation of pentachlorophenol by polyurethane-immobilized Flavobacterium cells. Appl Environ Microbiol 55: 2113-2118.
- Watanabe K,Teramoto M, Harayama S (2002) Stable bioaugmentation of activated sludge with foreign catabolic genes harboured by an indigenous dominant bacterium. EnvironMicrobiol 4:577-583.
- El Fantroussi S,Agathos SN (2005) Isbioaugmentation a feasible strategy for pollutant removal and site remediation? CurrOpinMicrobiol 8: 268-275.
- Lors C,Damidot D, Ponge JF, Périé F (2012) Comparison of a bioremediation process of PAHs in a PAH-contaminated soil at field and laboratory scales. Environ Pollut 165: 11-17.
- Lladó S, Covino S, Solanas AM, Viñas M, Petruccioli A (2013) Comparative assessment of bioremediation approaches to highly recalcitrant PAH degradation in a real industrial polluted soil. Journal of Hazardous Materials 249: 407-414.
- Gandolfi I,Sicolo M, Franzetti A, Fontanarosa E, Santagostino A, et al. (2010) Influence of compost amendment on microbial community and ecotoxicity of hydrocarbon-contaminated soils. BioresourTechnol 101: 568-575.
- Reilley KA, Banks MK, Schwab AP (1994) Dissipation of polycyclic aromatic hydrocarbons in the rhizosphere. Journal of Environmental Quality 25: 212-219.
--
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 12185
- [From(publication date):
July-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 11086
- PDF downloads : 1099