Case Report Open Access
Use of a Nutritional Therapy, Serum-Derived Bovine Immunoglobulin/Protein Isolate (SBI), to Achieve Improvement in Two Different Cases of Colitis
Amir Awad1 and Victoria S. Jasion2*1Westchase Gastroenterology, Tampa, FL, United States
2Entera Health, Inc., Medical Affairs, Cary, NC, United States
- *Corresponding Author:
- Victoria S. Jasion, PhD
Entera Health, Department of Medical Affairs
2000 Regency Parkway, Suite 255
Cary NC 27518, USA
Tel: 785-813-2953
E-mail: victoria.jasion@enterahealth.com
Received date: March 10, 2015; Accepted date: April 4, 2015; Published date: April10, 2015
Citation: Awad A, Jasion VS (2015) Use of a Nutritional Therapy, Serum-Derived Bovine Immunoglobulin/Protein Isolate (SBI), to Achieve
Improvement in Two Different Cases of Colitis. J Gastrointest Dig Syst 5:274. doi:10.4172/2161-069X.1000274
Copyright: © 2015 Awad A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Two independent retrospective cases of refractory colitis, one ischemic and one pan ulcerative -colitis, successfully managed over a year by the inclusion of a non-probiotic, non-antibiotic, medical food therapeutic, serum-derived bovine immunoglobulin/protein isolate (SBI), are presented. In addition to patient-reported outcomes and satisfaction regarding their symptomology, both patients had resolved colitis during routine colonoscopies performed at least one year after they had included SBI into their regimens.
Abstract
Two independent retrospective cases of refractory colitis, one ischemic and one pan ulcerative -colitis, successfully managed over a year by the inclusion of a non-probiotic, non-antibiotic, medical food therapeutic, serum-derived bovine immunoglobulin/protein isolate (SBI), are presented. In addition to patient-reported outcomes and satisfaction regarding their symptomology, both patients had resolved colitis during routine colonoscopies performed at least one year after they had included SBI into their regimens.
Keywords
Ulcerative colitis; Ischemic colitis; Serum-derived bovine immunoglobulin/Protein isolate; Medical food; Inflammatory bowel disease
Introduction
Ulcerative colitis (UC) is an autoimmune disorder characterized by mucosal inflammation in the colon and usually the rectum [1]. UC is classified as inflammatory bowel disease (IBD) and patients typically present with the following symptoms: bloody diarrhea, abdominal cramping, tenesmus and urgency. A diagnosis of UC is determined upon multiple factors including colonscopy and biopsies. Treatment options include: 5-ASAs, anti-TNFs, anti-metabolites, corticosteroids, methotrexate and the newer option of adhesion molecule inhibitors [2]. Many of these medicines are associated with complications. In the management of UC patients, it is the goal of the healthcare provider to induce and maintain remission in patients and improve quality of life with the least amount of drug side effects [2].
The exact pathogenesis of UC continues to be delineated, however, the role of diet, gut microbiota and genetic susceptibility have been jointly implicated [3]. Genetics alone cannot determine risk of developing UC since the rate among identical twins is between 16-18.5% [3]. The primary risk factor for UC is a dybiosis in the gut microbiome or disturbances in the intestinal mucosa, leading to a state of altered immune activation including: increased pro-inflammatory cytokines such as IFN-gamma, IL-6, IL-17/IL-23, decreased anti-inflammatory cytokines such as IL-10, IL-22, and decreased barrier function [3]. This state of chronic inflammation is the traditional target for UC therapies, hence the aforementioned medicines which are anti-inflammatory. However, in genetically susceptible murine models it has recently been shown that dybiosis of gut microbiota and a pro-inflammatory immune response can be triggered by the overconsumption of a high-fat, high-caloric diet [4]. More specifically, it was shown that a western-type diet high in saturated fats changed the production of a secondary bile acid, taurocholate, which triggered an increase in a pathobiont, B. wadsworthia, and increased the incidence and severity of spontaneous colitis in an IL-10 knockout mouse population. This work suggests that medicinal algorithms for UC should begin to address both the gut microbiome and diet in combination with the long-standing anti-inflammatory agents [3]. Including specific dietary agents as standard-of-care could help restore homeostasis in patients with enteropathy or colopathy.
We report two cases of chronic colitis, one ischemic and one pan-ulcerative colitis, which have been successfully managed for over a year by the inclusion of serum-derived bovine immunoglobulin/protein isolate (SBI). Immunoglobulin preparations purified from bovine colostrum or human serum have been reported to provide improvement in clinical symptomology in inflammatory bowel disease (IBD) [5,6]. SBI is purified from bovine serum and it is a prescription medical food product intended to provide for the distinctive nutritional requirements unique for the clinical dietary management of intestinal disorders under physician supervision. SBI has been shown to decrease stool frequency while improving consistency in irritable bowel syndrome with diarrhea (IBS-D) and HIV-associated enteropathy [7,8]. Since both patients were refractory to other conventional therapeutic agents over the course of nearly a year prior to beginning SBI therapy, this nutritional intervention was given. Within 4 to 8 weeks of initiating SBI, they reported a decrease in the number of bowel movements per day with improved stool consistency and no longer experienced abdominal cramping, tenesmus and urgency. Follow up colonoscopies of both patients at approximately one year after initiating and continuing SBI therapy revealed resolved colitis. The visual improvements to the mucosa correlated with histological observations from a biopsy in one patient as well as reduction in symptoms strongly suggesting clinical remission in these patients.
Case Presentations
Patient #1
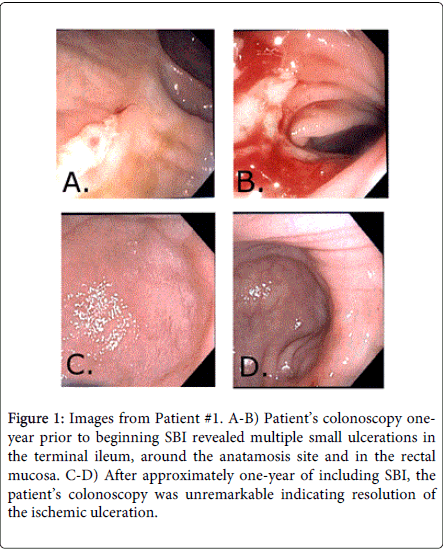
Patient 1 is a 56-year-old Caucasian female patient presented to the office with intractable, chronic, watery diarrhea and fecal incontinence. Three years prior, she underwent subtotal colectomy with ileo-rectal anastomosis secondary to acute mesenteric ischemia, which was due to Factor V deficiency. She was then started on anti-coagulation therapy. She continued to have 5-10 loose stools per day with abdominal cramps, tenesmus, rectal urgency and intermittent fecal incontinence. Laboratory tests were unremarkable, including stool cultures, serum chemistry, blood count, celiac panel, food allergies. An upper esophagogastroduodenoscopy (EGD) with biopsies was also unremarkable. A colonoscopy performed 1.5 years post-surgery revealed multiple small ulcerations in the terminal ileum, around the anatamosis site and in the rectal mucosa (Figure 1). Biopsies showed marked acute inflammation, focal necrosis, ulceration and focal moderate eosinophilia, likely ischemic in etiology. At this point, she was diagnosed with ischemic colitis. Over the next year, her condition remained refractory to all of the medications that were tried before the colonscopy: anti-cholinergics, anti-diarrheal medications, antibiotics (both metronidazole and rifaxamin) and dietary modifications including dairy- and gluten-free. The patient was placed on a dietary therapeutic, serum-derived bovine immunoglobulin/protein isolate (SBI) 5 g QD for 4 weeks, after which time the patient reported a satisfactory management of her cramping, urgency as well as chronic loose and frequent stools, with 1-3 semi-formed stools per day with resolution of fecal incontinence. Her SBI regimen was subsequently decreased to 2.5 g QD, due complaints of abdominal cramps while on 5g QD. The patient had been taking SBI for approximately one year when a repeat colonoscopy was performed that was unremarkable indicating resolution of the ischemic ulceration (Figure 1). The patient remains in remission while maintained on SBI 2.5 g QD, with no further complaints of abdominal cramping.
Figure 1: Images from Patient #1. A-B) Patient’s colonoscopy oneyear prior to beginning SBI revealed multiple small ulcerations in the terminal ileum, around the anatamosis site and in the rectal mucosa. C-D) After approximately one-year of including SBI, the patient’s colonoscopy was unremarkable indicating resolution of the ischemic ulceration.
Patient #2
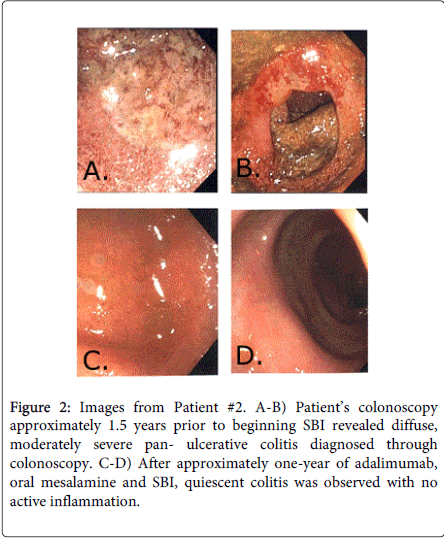
Patient 2 is a 26-year-old male patient of middle-eastern descent who was diagnosed with diffuse, moderately severe pan- ulcerative colitis diagnosed via colonoscopy in January 2012 (Figure 2). Biopsies confirmed UC with marked acute inflammation and ulceration of the mucosa, distortion of the glandular architecture, and crypt abscesses. For the first year after his diagnosis, the patient was placed on oral and rectal mesalamine and repeated courses of oral steroids. However, he was steroid dependent with flares of diarrhea consisting of 5-10 bloody loose stools with tenesmus and cramping, each time his oral steroid dose was decreased. He was started on adalimumab and steroids were tapered since he reported some improvement in his condition. However, he still experienced repeated flares of cramping, diarrhea and rectal bleeding, although less frequent and less severe, while on adalimumab. In order to manage these repeated flares different therapeutic regimens consisting of oral and rectal mesalamines, antibiotics (ciprofloxin and metronidazole), probiotics and oral steroids were attempted for nearly 8 months. Immediately after finishing another course of ciprofloxin and metronidazole and while still on oral steroids and oral mesalamine, he began SBI 5 g BID as part of his therapeutic regimen. After 8 weeks, the patient reported significant satisfaction with management of his chronic loose and frequent stools (1-2 normally formed stools per day) and reported no other symptomatology. He was completely removed from oral steroids at this time and SBI was decreased to 5 g QD. The patient continued to do well and has remained on adalimumab, oral mesalamine and SBI 5g QD for the past year. He remains in a complete clinical and endoscopic remission. Colonoscopy was performed a year after starting SBI during which time quiescent colitis was observed with no active inflammation (Figure 2). Random biopsies confirmed chronic inactive colitis.
Figure 2: Images from Patient #2. A-B) Patient’s colonoscopy approximately 1.5 years prior to beginning SBI revealed diffuse, moderately severe pan- ulcerative colitis diagnosed through colonoscopy. C-D) After approximately one-year of adalimumab, oral mesalamine and SBI, quiescent colitis was observed with no active inflammation.
Discussion
Reported are two distinct cases of refractory colitis, one ischemic and one pan-ulcerative colitis, where patients were able to manage their disease through the inclusion of a medical food that contains a specially-formulated bovine immunoglobulin. Both patients reported improved symptomology between 4 to 8 weeks after beginning SBI therapy. Furthermore, both had resolved colitis during routine colonscopies after they had been on SBI for approximately one year. Patient #1 had the co-morbidity of Factor V deficiency and had tried multiple other therapies for over two years for her chronic loose and frequent stools without any satisfactory response. Within 4 weeks of initiating SBI 5 g QD, she denied experiencing further tenesmus, rectal urgency and reported no further episodes of fecal incontinence. She did complain of abdominal cramps and was thus advised to decrease and continue SBI at 2.5 g QD. After a year of continuous, daily SBI, resolution of ulcerations and normalization of the mucosa at ileo-colonic anastomosis were noted. She continues her regimen of SBI 2.5 QD without further complaints of abdominal cramps. Patient #2 had steroid-refractory colitis that had first been managed by the inclusion of an anti-TNF agent, adalimumab, along with rectal and oral mesalamine. However, this patient continued to have intermittent flares which required repeat rounds of antibiotics and oral steroids. He finally reported symptom management after 8 weeks of SBI 5 g BID in combination with adalimumab, oral steroids and oral mesalamine, with no further complaints of 5-10 bloody loose stools, tenesmus or cramping. At this point, the patient was able to stop the steroids for the first time since his diagnosis while remaining on adalimumab, oral mesalamine and SBI 5 g BID reporting 1-2 normally formed stools per day. He was also advised to decrease his SBI to 5 g QD. A routine colonoscopy after approximately one year after incorporating SBI into his therapy revealed the patient’s colitis was quiescent. In addition, he had no flares throughout the year, requiring no further steroids. He continues his regimen of adalimumab, oral mesalamine and SBI 5 g QD.
These results highlight the utility of including a specially formulated medical food like SBI for the management and maintenance of patients with chronic loose and frequent stools associated with large bowel disorders. For patient #2, however, it should be discussed that the clinical course of UC can include improved symptomology with spontaneous remission [9]. Therefore, it could be argued that the improvement in mucosa observed in patient #2 could have been spontaneous. Patients who have spontaneous remission are likely to relapse within 2 months [9] and due to the length of time patient #2 has remained in remission (over a year), it was not interpreted to be a spontaneous event. In addition, prior to including SBI, patient #2 had been clinically refractory to common therapeutics for over a year. It was only after the addition of SBI to his therapeutic regimen that he achieved improved clinical symptomology. This clinical improvement has been maintained for over a year; he has experienced no flares and has not required steroids while on the combination of SBI, oral mesalamine and adalimumab. All of these considerations are highly suggestive that the improved mucosa in patient #2 was not due to a spontaneous event of remission. In addition, the evidence of mucosal healing after long-term ingestion of SBI presented in these two cases is consistent with a previous case of colopathy; a UC patient with a flare precipitated from Clostridium difficile infection who had been steroid-refractory had evidence of mucosal healing after two months of ingesting SBI along with steroids [10]. This patient was able to avoid surgery, taper her steroids, and remains on 5 mg prednisone and SBI 5 g QD [10]. The outcomes described for these cases are consistent with results from pre-clinical studies in mice, where SBI has been shown to improve clinical signs of mucosal inflammation in dextran-sodium sulfate (DSS)-induced colitis mouse model of IBD [7].
As discussed earlier, there is growing evidence showing that the etiology of UC is a complex interplay between diet, gut microbiota and genetic predisposition. Therefore, it is reasonable that both diet and gut microbiota should be addressed in therapeutic algorithms for UC patients. In fact, medicinal agents that affect gut microbiota, such as antibiotic combinations or the probiotic medical food VSL#3, have been shown to be effective in inducing remission in UC patients [11-13]. On the other hand, tetracyclines have been associated with a higher risk of developing UC, underscoring the delicate balance of microbial populations in the gut and the need for agents which are not broad-spectrum antibiotics [14].
SBI is a dietary therapeutic, a medical food, and these patients were counseled to include it due to the high safety profile, the unique mechanism of action and other clinical publications regarding the use of enteral administration of immunoglobulins which will be discussed below [5,6,15]. As far as mechanism, it is known that diets containing SBI have various downstream effects, including both maintenance of GI immune balance and management of gut barrier function [16]. It is also known that SBI improves nutrient utilization in both HIV-associated enteropathy and malnourished pediatric populations [16]. Improved nutrient utilization is most likely a direct downstream effect of SBI in the management of gut barrier function. It has been illustrated that SBI reduces both ileal and colonic permeability, most likely from improved tight junction regulation, in pig and rodent models [17]. How SBI was efficacious in these patients needs to be further studied, however, it is possible that SBI might have altered the gut microbiota in these two patients since SBI has been shown to alter fecal microbiota in patients with HIV-associated enteropathy [18].
How SBI alters the microbiota in patients with HIV-associated enteropathy is unknown and if it alters the microbial population in colitis remains to be shown. If it does, one explanation may be that SBI, as a specially-formulated protein isolate, may alter the bacterial metabolome (bacterial metabolites) and subsequently affect the microbiota. Devkota et al. have illustrated that diet can alter the metabolome, which alters the microbiota population and induces more severe colitis in a genetically susceptible model of colitis4. On the other hand, SBI could modulate microbiota in a more direct manner. For example, the simple action of the immunoglobulins (Igs) in SBI binding directly to various microbial components in the lumen of the GI tract could be enough to impact the microbiota population. Alternately, ingestion of SBI influences immune activation within the GI tract and manages gut barrier function, which might affect the microbiota indirectly [19].
Immunoglobulins preparations from either human or bovine sources have been studied in a variety of intestinal disorders. A review of bovine colostrum summarizes that most applications are those with infectious etiologies [15]. Yet, bovine colostrum administered via enema and used combination with mesalazine showed potential for left-sided colitis over mesalazine monotheraphy in a pilot study6. In another form of inflammatory bowel disease (IBD), orally-administered human immunoglobulins from serum has illustrated efficacy in clinical improvement in two cases of refractory pediatric Crohn’s patients [5]. Hence, SBI was chosen due to clinical reports of other immunoglobulin preparations with improvement of symptomology. The two cases presented in this manuscript serve as preliminary evidence for the potential of including SBI as a dietary therapeutic in colonic disorders. As clinical retrospective observations in a private practice, these cases underscore the need for formal randomized, controlled clinical trials of SBI in colonic disorders.
Conclusion
Reported are two patients with refractory chronic loose and frequent stools with other symptomology. Both of the patients incorporated a dietary therapeutic, SBI, into their regimens to assist in managing their conditions. Patient #1 reported improved symptomology after 4 weeks on SBI 5 g QD at which time she reduced her SBI to 2.5 g QD. After one year on SBI 2.5 g QD, a colonoscopy illustrated improved mucosa with resolution of ulcerations. Patient #2 reported improved symptomology after 8 weeks of therapy with adalimumab, oral steroids, oral mesalamine and SBI 5 g BID. At this time he was able to completely stop steroids. He remained on adalimumab, oral mesalamine and SBI 5 g QD for about one year when a colonoscopy revealed mucosal healing and histological evidence of quiescent colitis. Both patients continue on SBI therapy. These retrospective cases, which are summarized in Table 1, suggest that colitis patients with refractory symptomology may have a distinct nutritional requirement that is satisfied by SBI. This has been suggested for enteropathies where SBI may help restore intestinal homeostasis [17]. The utility of SBI in colopathies is clearly suggested by these two cases and both cases support a need for prospective, well-designed future studies.
| Symptoms prior to SBI | Therapeutics prior to SBI | Other therapeutics while on SBI | Initial response to SBI | Long-term use of SBI and results | |
| Patient #1 Ischemic Colitis 56-year-old Caucasian female | 5-10 loose stools daily with abdominal cramps, tenesmus, rectal urgency and intermittent fecal incontinence | Anti-cholinergics, anti-diarrheals, antibiotics (metronidazole and rifaximin), dairy-free, gluten-free | None | After 4 weeks of SBI 5 g QD, patient reported satisfactory management of symptoms with 1-3 semi-formed stools per day with resolution of fecal incontinence. | Advised to continue on SBI 2.5 g QD indefinitely. Decreased SBI to 2.5 g QD due to complaints of abdominal cramps. After a year on SBI 2.5 g QD, colonoscopy was unremarkable. |
| Patient #2 Pan-Ulcerative Colitis 26-year-old male | Repeated flares of 5-10 bloody loose stools with tenesmus and cramping | Oral and rectal mesalamine, antibiotics (ciprofloxin and metronidazole), probiotics, oral steroids, adalimumab | Adalimumab, oral mesalamine, oral steroids (for the first 8 weeks) | After 8 weeks, patient reported significant satisfaction with 1-2 normally formed stools per day and reported no other symptomology. He was completely removed from oral steroids. | Advised to continue on SBI 5 g QD indefinitely while still on adalimumab and oral mesalamine. After a year with no flares while on all three therapies and no need for steroids, colonoscopy illustrated quiescent colitis with no active inflammation. |
Table 1: Summary of patient’s refractory symptoms, other therapeutics, and initial and long-term responses to the inclusion of SBI.
Conflict of Interest Disclosure and Declaration of Funding Sources
A. Awad is an Advisor for Entera Health. A. Awad is on the speakers bureau for Entera Health and QOL Medical. V.S. Jasion is a salaried employee of Entera Health, which markets a product containing SBI. Each author contributed to the writing, proofreading and approval of this manuscript. A. Awad is the article guarantor and the senior author. This is the original work of both authors. A Awad wrote each case presentation; V Jasion reviewed and edited. V Jasion wrote the introduction and discussion section; A Awad reviewed and edited. Financial Support: The submission of this manuscript will be supported by Entera Health.
Acknowledgements
Writing Assistance - Editing of this manuscript was provided by Bruce Burnett, PhD, Raymond Panas, PhD and Hayley Young, PhD. All are salaried employees of Entera Health, Inc., Cary, NC which markets a product containing SBI.
References
- Kornbluth A, Sachar DB (2010) Practice Parameters Committee of the American College of G. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. The American journal of gastroenterology 105: 501-523.
- Feuerstein JD, Cheifetz AS (2014) Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc 89: 1553-1563.
- Leone VA, Cham CM, Chang EB2 (2014) Diet, gut microbes, and genetics in immune function: can we leverage our current knowledge to achieve better outcomes in inflammatory bowel diseases? Curr Opin Immunol 31: 16-23.
- Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, et al. (2012) Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 487: 104-108.
- Tjellström B, Stenhammar L, Magnusson KE, Sandqvist T (1997) Oral immunoglobulin treatment in Crohn's disease. Acta Paediatr 86: 221-223.
- Khan Z, Macdonald C, Wicks AC, Holt MP, Floyd D, et al. (2002) Use of the 'nutriceutical', bovine colostrum, for the treatment of distal colitis: results from an initial study. Alimentary pharmacology & therapeutics 16:1917-1922.
- Maas KJ, Henderson AL, Brand MW (2014) Serum-Derived Bovine Immunoglobulin/Protein Isolate Attenuated DSS-Induced Colitis in a Defined Floral Model. Poster Presented at DDW Chicago, IL. May 3-6.
- Asmuth DM, Ma ZM, Albanese A, Sandler NG, Devaraj S, et al. (2013) Oral serum-derived bovine immunoglobulin improves duodenal immune reconstitution and absorption function in patients with HIV enteropathy. AIDS 27: 2207-2217.
- Meyers S, Janowitz HD (1989) The "natural history" of ulcerative colitis: an analysis of the placebo response. J Clin Gastroenterol 11: 33-37.
- Dryden GW (2014) Use of Serum-Derived Bovine Immunoglobulin/Protein Isolate (SBI) to Manage Refractory Ulcerative Colitis Symptoms and Avoid Surgery. Poster Presented at ACG Philadelphia, PA. October 17-22.
- Sood A, Midha V, Makharia GK, Ahuja V, Singal D, et al. (2009) The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 7: 1202-1209.
- Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, et al. (2010) Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. The American journal of gastroenterology 105:2218-2227.
- Kato K, Ohkusa T, Terao S, Chiba T, Murakami K, et al. (2014) Adjunct antibiotic combination therapy for steroid-refractory or -dependent ulcerative colitis: an open-label multicentre study. Alimentary pharmacology & therapeutics 39:949-956.
- Shaw SY, Blanchard JF, Bernstein CN (2011) Association between the use of antibiotics and new diagnoses of Crohn's disease and ulcerative colitis. Am J Gastroenterol 106: 2133-2142.
- Struff WG, Sprotte G (2008) Bovine colostrum as a biologic in clinical medicine: a review--Part II: clinical studies. International journal of clinical pharmacology and therapeutics 46:211-225.
- Petschow BW, Burnett B, Shaw AL, Weaver EM, Klein GL (2014) Serum-derived bovine immunoglobulin/protein isolate: postulated mechanism of action for management of enteropathy. Clin Exp Gastroenterol 7: 181-190.
- Petschow BW, Burnett BP, Shaw AL, Weaver EM, Klein GL (2015) Dietary requirement for serum-derived bovine immunoglobulins in the clinical management of patients with enteropathy. Dig Dis Sci 60: 13-23.
- Asmuth DM, Strombaugh J, Ursell L (2013) Changes in Stool Microbiota, Bacterial Translocation and Mucosal Immunity after Oral Serum-Derived Bovine Immunoglobulin (SBI) Administration. Poster Presented at Conference on Retroviruses and Opportunistic Infections (CROI) Atlanta, GA. March 3-6.
- Detzel CJ, Horgan A, Henderson AL, Petschow BW, Warner CD, et al. Development of a co-culture model of the intestinal epithelium to study barrier function and immune exclusion by bovine IgG. J Parenter Enteral Nutr 38:127.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 35579
- [From(publication date):
April-2015 - Jul 02, 2025] - Breakdown by view type
- HTML page views : 30756
- PDF downloads : 4823