Research Article Open Access
Urinary Excretion and Renal Clearance of Allopurinol in Female Gout Patients
Bilal Ahmed*, Imtiaz SohailTahira Iqbal, Abdual Ghaffar and Bushra Munir
Department of Chemistry and Biochemistry, University of Agriculture, Faisalabad, Punjab, Pakistan.
- *Corresponding Author:
- Bilal Ahmed
Department of Chemistry and Biochemistry, University of Agriculture
Faisalabad, Punjab, Pakistan
Tel: +923153133532
E-mail: bilalahmed814@gmail.com
Received date: February 01, 2014; Accepted date: April 30, 2015; Published date: May 07, 2015
Citation: Ahmed B, Iqbal IS, Ghaffar A, Munir B (2015) Urinary Excretion and Renal Clearance of Allopurinol in Female Gout Patients. J Anal Bioanal Tech 6:243. doi: 10.4172/2155-9872.1000243
Copyright: © 2015 Ahmed B. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Drugs are excreted from the human body either unchanged or as metabolite. Gout is treated with allopurinol drug which lowers uric acid level in humans. Xanthine oxidase activity is inhibited with allopurinol which converts hypo-Xanthine and xanthine into uric acid. In case of kidney impairment monitoring of allopurinol drug metabolism was important option for optimization of allopurinol. Allopurinol level was measured in human serum and urine of gout patients after oral intake of allopurinol by using HPLC (high performance liquid chromatography). In female gout patients (n=10) allopurinol was measured in blood and urine samples at 254 nm after taking 300 mg drug. Serum uric acid level, renal function test (RFTs) and urine complete examination was checked before and after oral administration of allopurinol. Statistically results were analyzed and explained.
Introduction
Elimination is a mechanism through which drugs are removed from the human body. Kidneys by which urine is removed are main organs in mammals for elimination of drugs [1]. Renal clearance is concentration of fluid filtered out of the blood plasma which is picked up by the kidneys or amount of blood which is cleared per unit time [2] Gout is treated throughout the world by using allopurinol. It is an isomer of its metabolite oxipurinol and hypoxanthine which stops the Xanthine oxidase activity. Uric acid is produced from Xanthine and hypoxanthine by the enzyme Xanthine oxidase. Allopurinol can be given orally by mouth or by veins (IV). The biologically available allopurinol is (67-90) percent with highest plasma level occurring within 1 hour, The volume of moving back out is approximately 1.6 liter per kg [3] Allopurinol is digested by enzyme aldehyde oxidase to oxipurinol which is a active metabolite. Allopurinol is excreted in urine <10% unchanged and 70% as oxipurinol and 20% in stool [4]. The conservation dose of allopurinol drug must be condensed to avoid side effects due to high oxipurinol serum phases [5]. In case of renal injury, early amount of allopurinol can be check depending upon projected creatinine clearance. Allopurinol dose can be adjusted by pointing quantity of oxipurinol in blood serum [6].
Materials and Methods
This study was conducted to analyze the urinary excretion and renal clearance of Allopurinol and endogenous creatinine in blood and urine samples of female gout patients after the oral administration of 300 mg allopurinol. The experiments were conducted on 10 female gout patients.
Drug administration
The drug Allopurinol 300 mg of (Sami industries Pakistan limited Karachi) was given to each of the female gout patient participate in this study. The drug is given orally with the glass of water. The samples for the analysis were collected in the month of April, 2014 and the samples stored at -20°C temperature until analysis.
HPLC Analysis
Preparation of mobile phase
The mobile phase was prepared by dissolving 2.72 g NaCH3COO·3H2O in 1000 ml distilled water and correcting the pH to 4.5 with acetic acid 30%. The mobile phase was filtered and degassed before use. The mobile phase was filtered in vacuum filtration assembly having cellulose filter which have pore size 0.545 μm (Sartorius ag 37070.) than the filtered mobile phase was sonicated for the removal in any bubbles for 10 minutes. (eyelet sonicator )
Chromatographic conditions
Chromatography was performed with a high performance liquid chromatography. The HPLC system was consisted of Shimadzu SCL-10A system controller, UV visible SPD-10AV detector and LC-10AT pump with FUC-10AL VP flow controller wall. Separation was achieved at ambient temperature with Hypersil C18 BDS 250 × 4.6 column pore size of 5 micron. Chromatographic data was collected and analyzed using CSW32 software. And spectrophotometer (PG Instruments, model T60) was used for creatinine analysis in plasma and urine samples.
Sampling procedure
Collection of blood samples: Before the drug administration blank blood samples were collected from each gout patients. The blood samples of each gout patients were collected after 1 and 3 hours. After the oral intake of Allopurinol 300 mg (Sami industries Pakistan Karachi) these blood samples were stored in eppendorf tubes at -20°C until use for the analysis.
Collection of urine sample: Prior to oral administration of drug the blank urine samples were collected from each gout patients. Urine samples of gout patients were collected after 2, 4, 6, 8, 12 and 24 hours after drug administration. These urine samples were stored in plastic bottles in freezer at -20°C until analysis
Results and Discussion
Amount (mg) excreted
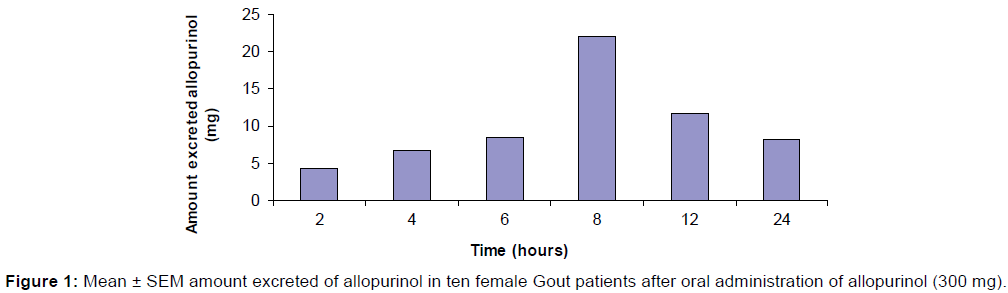
The amount (mg) of allopurinol excreted in urine of ten female Gout patients. At 2 hours after drug administration Mean ± SEM amount (mg) of allopurinol excreted in urine was 4.70 ± 2.28. At 4 hours after drug administration Mean ± SEM amount (mg) of allopurinol excreted in urine was 6.45 ± 3.26. At 6 hours after drug administration Mean ± SEM amount (mg) of allopurinol excreted in urine was 8.88 ± 14.43. At 8 hours after drug administration Mean ± SEM amount (mg) of allopurinol excreted in urine was 21.57 ± 3.03. At 12 hours after drug administration Mean ± SEM amount (mg) of allopurinol excreted in urine was 12.11 ± 2.34. At 24 hours after drug administration Mean ± SEM amount (mg) of allopurinol excreted in urine was 8.60 ± 2.19 (Figure 1).
The principle rout of the drug excretion is the urine. Our kidneys produce urine which contains urea, excess salts, drug metabolites and excess water. Kidneys perform two grand functions. First is to get rid of waste materials and second is to control the composition of the body fluids and the body volume. For water and all electrolytes in the body, balance between output (due to excretion or metabolic consumption) and intake (due to ingestion or metabolic production) is maintained largely by kidneys. The kidneys perform their important function by filtering serum and removing substances from filtrated at variables rates, depending on the needs of the body itself. Ultimately kidneys clear the wasteful materials from the filtrate by excreting them in the urine while returning substances that are needed by the body back to the blood. Kidneys also eliminate most toxic material and other foreign substances that are either produced by the body or ingested, such as pesticides, drugs and food additives. Renal excretion accounts for most drug elimination that are predominately ionized at physiological pH and for polar drugs, drug metabolites with low lipid solubility. Renal drug excretion decreases with aging. Drugs bound to serum proteins remain in the circulation; only unbound drug is contained in the glomerular filtrate. Un-ionized forms of drugs and their metabolites tend to be reabsorbed readily from tubular fluids.
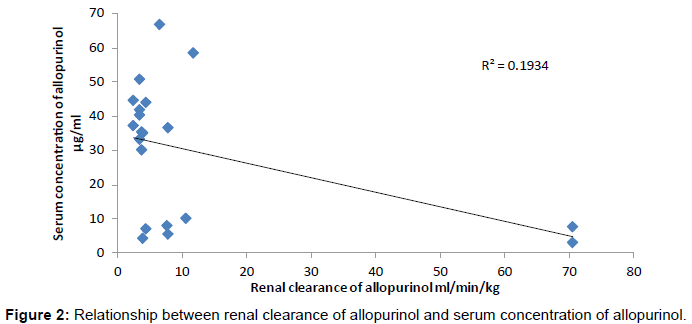
The present study revealed the result non-significant correlation between the pH and urine concentration. It means pH did not affect the urinary excretion of drug. The main functions of kidney are urine formation and water conservation and this is the major channel of water excretion as compared to intestine, skin and lungs. As the glomerular filtrate flow through the tubules, over 99% of its water and varing amount of its solute are normally are absorbed into the vascular system and small amounts of some substances are secreted into the tubules. The remaining tubular water and dissolved substances become urine [7]. In ten female Gout patients the renal clearance of allopurinol was studied and results have been discussed below. The Mean value of allopurinol renal clearance was 359 ± 9.7 ml/min. while another study suggests that the value is 310 ml/min [8]. The Mean value of renal clearance of our study was found to be 11.83 ± 0.6.58 ml/min/ kg. The difference between these values is due to different temperature and environment. Within 24 hours of oral administration, some 50- 70% of the dose on Mean was excreted in the urine as unchanged drug. Over the dose range of 0.3-30 mg/kg allopurinol, there was no dose-dependent effect on total or renal clearance [9]. 70% of an allopurinol dose being excreted unchanged in urine with the major site of elimination which occurs by renal mechanisms. At the glomerulus allopurinol is mainly secreted and filtered by the organic cationic secretory pathway. In this way renal clearance values approximately 4 times greater than GFR [10]. The difference in the urinary excretion of allopurinol under local conditions and reported in literature is due to environmental and genetic influences on glomerular filtration rate which significantly affect the fate of drug in the body. These differences have been elucidated by original term genetics [11] (Figure 2 and Table 1).
| Gout patients | Body Weight | Diuresis | pH | Creatinine concentration(µg/ml) | Allopurinol concentration(µg/ml) | Renal clearance ml/min/kg | Clearance ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| kg | ml/mint/kg | Urine | Serum | Urine | Serum | Urine | Serum | creatinine | allopurinol | ||
| 1 | 56 | 16.106 | 5.6 | 7.36 | 10.4 | 3.32 | 5.737 | 8.702 | 0.015 | 10.618 | 7.79 |
| 2 | 48.5 | 36.880 | 5.15 | 7.45 | 29.5 | 2.88 | 10.649 | 5.564 | 0.059 | 70.581 | 14.9 |
| 3 | 46 | 18.292 | 5.2 | 7.42 | 33.5 | 2.89 | 7.596 | 35.168 | 0.043 | 3.951 | 9.26 |
| 4 | 58 | 12.540 | 5.95 | 7.45 | 35.2 | 2.87 | 7.282 | 20.527 | 0.049 | 4.448 | 9.66 |
| 5 | 64 | 10.104 | 4.85 | 7.44 | 26.7 | 3.17 | 8.442 | 24.967 | 0.037 | 3.416 | 2.71 |
| 6 | 68 | 19.778 | 5.25 | 7.4 | 7.01 | 3.08 | 12.036 | 62.432 | 0.005 | 3.813 | 6.07 |
| 7 | 60 | 19.007 | 5.5 | 7.44 | 3.89 | 2.54 | 7.765 | 42.718 | 0.008 | 3.455 | 4.26 |
| 8 | 50 | 41.655 | 5 | 7.25 | 11.6 | 2.49 | 7.964 | 42.900 | 0.102 | 7.733 | 5.65 |
| 9 | 59 | 12.674 | 5.31 | 7.41 | 19.7 | 2.91 | 11.640 | 18.898 | 0.035 | 7.806 | 3.19 |
| 10 | 48 | 12.711 | 5.23 | 7.46 | 12.3 | 3.04 | 7.219 | 36.878 | 0.025 | 2.488 | 9.71 |
| Mean | 55.75 | 19.975 | 5.304 | 7.408 | 18.98 | 2.919 | 8.633 | 29.876 | 0.038 | 11.831 | 6.86 |
| SEM | 2.3413 | 3.391 | 0.0992 | 0.0199 | 3.6364 | 0.0815 | 0.661 | 5.525 | 0.009 | 6.583 | 11.93 |
Table 1: Mean data of renal clearance of allopurinol and endogenous creatinine in ten female Gout patients after oral administration of allopurinol (300 mg).
Studies on allopurinol suggest that it is extensively secreted from urine even though when given in small amounts. At serum concentrations up to 30-fold the tubular secretion rate of allopurinol gradually increases and higher than those values which are achieved during 300 mg/day typical oral dosing [12]. The retention time for the present study was 6.5 min for serum and 4.2 min for urine while according to a study it is 8.4 min. The difference is probably due to storage of urine and serum samples, environmental conditions and/ or temperature. The Mean value of the percent dose of allopurinol in urine sample was 83% ±16%, in the earlier study was calculated by [13]. The present percent dose is lower calculated as 69.30 ± 2.18. There is difference between present study value and earlier study value due to difference in environment, temperature but major difference in the values is due to non-fasting Gout patients.
References
- Parkison A (1996) Biotransformation of xenobiotic. Casarett and Doull’s Toxicology. In:KlaassenCD (ed.)The basic Science of Poisons (5thedn.) 33: 113-186.
- Seldin DW (2004) The development of the clearance concept. J Nephrol 17: 166-171.
- Reinders MK, Nijdam LC, van Roon EN, Movig KL, Jansen TL, et al. (2007) A simple method for quantification of allopurinol and oxipurinol in human serum by high-performance liquid chromatography with UV-detection. J Pharm Biomed Anal 45: 312-317.
- Hande KR, Noone RM, Stone WJ (1984) Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med 76: 47-56.
- Parekh P, Singh K, Marangoni DG, Bahadur P (2011) Micellization and solubilization of a model hydrophobic drug nimesulide in aqueous salt solutions of Tetronic T904. Colloids Surf B Biointerfaces 83: 69-77.
- Takara K, Hayashi R, Kokufu M, Yamamoto K, Kitada N, et al. (2009) Effects of nonsteroidal anti-inflammatory drugs on the expression and function of P-glycoprotein/MDR1 in Caco-2 cells. Drug ChemToxicol 32: 332-337.
- Ganong WF (2005) Allopurinol excretory mechanism. In: Review of medical physiolog (22ndedn.) 7: 697- 699.
- Lin JE, Auricle S, PasanenM,Juvonen RO(1988) D-Isomer of gly-tyr-pro-cys-pro-his-pro peptide: a novel and sensitive in vitro trapping agent to detect reactive metabolites by electrospray mass spectrometry.J Toxicol 25:411-425
- Laine JE, Auriola S, Pasanen M, Juvonen RO (2011) D-Isomer of gly-tyr-pro-cys-pro-his-pro peptide: a novel and sensitive in vitro trapping agent to detect reactive metabolites by electrospray mass spectrometry. ToxicolIn Vitro 25: 411-425.
- Delanghe J, De Slypere JP, De Buyzere M, Robbrecht J, Wieme R, et al. (1989) Normal reference values for creatine, creatinine, and carnitine are lower in vegetarians. ClinChem 35: 1802-1803.
- Nawaz M, Iqbal T,Nawal R (2001) Geonetical considerations in disposition kinetics evaluation of chemotherapeutic agents. Pakistan Vet J 2: 260.
- Dowling E, PrasnaF, Tongella D (2001) Inhibitory action of allopurinol. E1 Pediatric physiology 7:713-719.
- Sliva J, Dolezal T, Sykora D, Vosmanska M, Krsiak M (2009) Guaifenesin enhances the analgesic potency of ibuprofen, nimesulide and celecoxib in mice. NeuroEndocrinolLett 30: 352-356.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 16475
- [From(publication date):
June-2015 - Nov 21, 2024] - Breakdown by view type
- HTML page views : 11988
- PDF downloads : 4487