Research Article Open Access
Urban Pollution Increases Sensitivity to Temperature Changes in Elliptio complanata Mussels Exposed to Municipal Wastewaters
Chantale André, Joëlle Auclair and J François Gagné*
Aquatic Contaminants Research Division, Environment and Climate Change Canada, Montreal, QC, Canada
- *Corresponding Author:
- Francois Gagné
Aquatic Contaminants Research Division
Environment and Climate Change Canada
105 McGill, Montreal, QC, Canada, H2Y 2E7
Tel: 514 496 7105
E-mail: francois.gagne@canada.ca
Received date: March 28, 2017; Accepted date: April 10, 2017; Published date: April 17, 2017
Citation: André C, Auclair J, Gagné JF (2017) Urban Pollution Increases Sensitivity to Temperature Changes in Elliptio complanata Mussels Exposed to Municipal Wastewaters. Environ Pollut Climate Change 1:122
Copyright: © 2017 André C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Environment Pollution and Climate Change
Abstract
The cumulative effects of pollution in the context of global warming are not well understood at the present time. The purpose of this study was to determine whether organisms exposed to urban pollution are more susceptible to temperature changes. Feral Elliptio complanata freshwater mussels were caged and immersed for 1, 12 and 24 days in the final aeration pond treating domestic wastewaters from a township with a population of about 25,000. Mussels were also placed in the river upstream from the township to serve as reference. Following each exposure times, mussels were collected for temperature-dependent mitochondrial electron transport (METT) activity assessments. Other biomarkers were used, including xenobiotic metabolism (cytochrome P4503A4, glutathione S-tranferase and metallothioneins), energy budget (MET at 20ºC and total lipids) and gonad status endpoints (gonadosomatic index [GSI], lipid peroxidation [LPO] and vitellogenin-like proteins). The data revealed that mussels exposed to the aeration pond had elevated METT and MET activities, suggesting that they were more sensitive to temperature changes and spent more energy than the controls. METT activity was negatively correlated with lipid stores in gonad, pointing to a negative energy budget. METT activity was also influenced by all xenobiotic metabolism and gonad status markers, with the exception of GSI. This suggests that temperature sensitivity is increased in mussels exposed to urban pollution and involves the expression of xenobiotic biotransformation, oxidative stress and endocrine disruption.
Keywords
Municipal wastewaters; Mussel; Mitochondria; Energy expenses toxicity; Climate changes
Introduction
Global warming will bring about pronounced temperature variations in the warmer range at the expense of cold climates. Warmer air temperatures at the poles will drive sea level rise and increase the amount of water vapor that is released to the atmosphere. This will contribute to increased precipitation (rain) in temperate zones owing to evaporation and atmosphere humidity loadings [1]. These extremes in weather and precipitation could have serious consequences for both human populations and ecosystems. The recent increases in the frequency of heavy precipitation events in the Northern Hemisphere are linked to the effect of warming on atmospheric water-holding capacity [1]. One potential consequence of increased precipitation frequency is that current wastewater treatment plants may not be able to handle overflows of rainfall and wastewater. Existing wastewater treatment plants were designed to handle a limited volume of rainfall and when their capacity is reached, the excess rainfall and untreated wastewater is released directly into the environment. This results in an increase in urban pollution (untreated wastewater diluted by rain), which can lead to increased contamination of watercourses and deleterious effects on aquatic biota. Municipal wastewaters are recognized as a source of pollutants such as polyaromatic hydrocarbons, heavy metals, cyclic aliphatic hydrocarbons (steroids), antibiotics and many other pharmaceutical products [2,3]. Municipal effluents are well known to contain endocrine disrupters which can cause reproductive, developmental and behavioral changes in fish and other organisms. In addition to inducing oxidative damage, municipal effluents have been shown to feminize fish and mussels, owing, in part at least, to their estrogenic and serotonergic effects [4-6]. In a context where the frequency of heavy rainfall events is expected to increase due to global warming, minimizing pollution of water bodies by sewage, along with the associated toxic effects will be a challenge. Moreover, the influence of pollution on tolerance to temperature changes is not well understood at the present time.
Cold-blooded (ectothermic) organisms are dependent on the ambient temperature of their habitats and have developed means to balance their energy expenditure and reserves for survival, growth and reproduction. Changes in ambient temperature could increase susceptibility to various contaminants and reduce their ability to tolerate the contaminants typically present in urban/industrialized areas. For example, the increased metabolic energy required for xenobiotic biotransformation and elimination could result in decreased growth rates and gametogenesis in mussels [7]. This could also limit the capacity of mussels to adapt to further stresses such as anoxia and temperature changes [8]. Physiological biomarkers that integrate the effects of both temperature and pollution are urgently needed at present, and the lack of such markers is hindering risk assessment related to chemical pollutants in the context of global warming.
Increased temperature has been shown to increase cellular respiration and resulting in the production of reactive oxygen species [9]. During oxidative phosphorylation, the flow of electrons to support the proton gradient for ATP production represents the major source of reactive oxygen species in cells. When uncoupling occurs, leakage in the proton gradient in the inner membrane occurs and reactive oxygen species are formed from the flow of electrons and the transmembrane gradient of protons. The increased presence of radical scavengers (pollutants) could facilitate electron displacements and increase the production of H2O2, which would lead to oxidative stress such as lipid peroxidation (LPO). Mitochondrial electron transport (MET) is closely correlated with oxygen consumption in the mitochrondria from various aquatic organisms [10]. MET activity was readily increased in the mitochondria of mussels exposed to pharmaceutical products known to be found in municipal effluents [11]. Furthermore, temperaturedependent MET (METT), determined by measuring MET activity in the same species at cold and warm temperatures, was also increased by the pharmaceuticals and was significantly correlated with LPO in the gonad tissues. Mussels exposed to effluents for 30 days in an aerated pond used to treat domestic wastewaters were also found to have elevated levels of MET and METT activity, suggesting increased sensitivity to temperature changes [11]. Changes in MET and METT activities could represent an interesting biomarker of the cumulative effects of pollution and temperature, provided that the results are associated with toxic stress endpoints such as xenobiotic metabolism, oxidative stress and endocrine disruption usually observed in urban effluent contaminated environments.
The purpose of this study was to examine the influence of urban pollution on sensitivity to temperature changes in freshwater mussels exposed in situ (experimental pens) to the terminal aerated lagoon used to treat municipal wastewaters. Xenobiotic metabolism was determined by changes in the activity of cytochrome P450 3A4 (CYP3A4), the major enzyme involved in the metabolism of most drugs used in therapeutics [12]; and glutathione S-transferase activity (GST), the major enzyme involved in conjugation of polar compounds in mussels. The levels of metallothioneins (MT) were also determined to determine metal and reactive oxygen species sequestration. Oxidative stress was determined by measuring LPO, and gonad status was assessed by following changes in total lipid contents and the levels of vitellogenin-like proteins based on alkali-labile phosphate (ALP). An attempt was made to relate MET and METT activities to changes in energy reserves, gonad activity and xenobiotic metabolism in order to evaluate the adverse impact of pollution in relation to temperature changes.
Materials and Methods
Mussel handling and exposure experiments
Elliptio complanata mussels were collected by hand in the Richelieu River, Quebec, Canada, in June 2011. They were maintained in 300 L aerated tanks for 30 days at 15ºC and fed with commercial algal preparations. Mussels (N=12) were then placed in 6 cylinder nets (24 cm diameter × 48 cm height). The immersion site consisted of the final aerated pond in a system used to treat domestic and industrial wastewaters from a municipality of circa 25,000 inhabitants. The reference site consisted of a site in the Richelieu River located 3 km upstream from the City of Chambly (Québec, Canada). The mussel cages were immersed at depths between 2 and 3 m using buoys and exposed for 1, 12 and 24 days from August to September. At each exposure times (1, 12, and 24 days), one cage (N=12 mussels) were removed and were placed in dechlorinated and charcoal-treated water at 15°C for 12 h. The mussels were measured (shell length), weighed and their tissues (gonad, digestive gland) were weighed and dissected on ice. The tissues were homogenized in a Teflon pestle tissue grinder in 10 mM Hepes-NaOH (pH 7.4) containing 100 mM NaCl, 1 mM EDTA, 1 mM β-mercaptoethanol and 0.1 μg/mL apoprotinin at a 20% weight/volume ratio. The homogenate was centrifuged first at 1500x g for 10 min at 2ºC, the supernatant removed and centrifuged at 10 000x g for 30 min at 2ºC. The pellet contained the crude mitochondria fraction for MET determination, was resuspended in 0.5 mL of homogenization buffer, and the supernatant (S10 fraction) was stored at -85ºC. The amount of total protein in the homogenate, S10 and mitochondria fractions were determined using the protein-dye binding principle of Bradford [13]. Serum bovine albumin was used for calibration.
Xenobiotic biotransformation activity
Biotransformation activity was determined in the S10 fraction of the digestive gland homogenates using the following measures: metallothioneins (MT) for heavy metal metabolism, CYP3A4 activity for polar cyclic aliphatic compounds (some drugs and steroids) (phase I biotranformation) and glutathione S-transferase (GST) activity for the conjugation of xenobiotics (phase II biotranformation). MT levels were determined using a modified version of the spectrophotometric assay methodology [14]. The S10 fraction was pre-treated first with 50 mM of tris(2-carboxyethyl)phosphine, a strong reducer, for 15 min on ice before the fractionation step. Freshly prepared standard solutions of reduced glutathione (GSH) were used as standards. The data were expressed as μmole GSH (equivalents)/mg proteins. The CYP3A activity was determined using the dibenzyloxyfluorescein substrate methodology [15]. Briefly, 50 μL of the S10 fraction was incubated with 0.2 mM NAPDH and 20 μM of dibenzyloxyfluorescein in 50 mM NaCl, 25 mM MOPS (pH 7.2) and 1 mM EDTA for 0, 15, 30 and 60 min. At the end of each incubation time, fluorescence was measured at 485 nm excitation and 520 nm emission using standard solutions of fluorescein (0.1, 0.5 and 1 μM) for calibration. The data were expressed in relative fluorescence units formed per min per mg of proteins. Glutathione S-transferase (GST) was determined by the spectrophotometric method of Boryslawskyj et al. [16] using 2,4-dichloronitrobenzene and reduced glutathione as substrates. Data were expressed as increase in absorbance at 412 nm/min/mg of proteins.
Gonad status
The gonadosomatic index (GSI) was determined by gonad weight per soft tissue weight. Measurement of alkali-labile phosphate (ALP), an indirect method for vitellogenin like properties (egg yolk precursor), was determined as described elsewhere [6]. Briefly, the gonad homogenate S10 fraction was treated with 35% acetone on ice and centrifuged at 10 000x g for 5 min at 4ºC. The pellet was washed in 50% acetone, resuspended in 1 M NaOH and incubated at 60ºC for 30 min. The levels of inorganic phosphates were then determined using the phosphomolybdate methodology. The samples were centrifuged 10 000 x g for 5 min if turbidity was observed. The data were expressed as μg phosphates per mg proteins in the S10 fraction. Lipid peroxidation was determined in gonad tissues according to the thiobarbituric acid reactants (TBARS) method as described elsewhere. The levels of TBARS were determined by fluorescence at 520 nm for excitation and 590 nm for emission using a fluorescence microplate reader (Bioscan, USA). The data were expressed in μg of TBARS/mg of homogenate protein.
Cellular energy status and temperature sensitivity
Total lipids in gonad tissues (visceral mass without the digestive gland) were determined using the sulfo-phospho-vanillin methodology [17]. Absorbance was measured at 540 nm and Triton X-100 (0, 0.1, 0.5, 1.0, 2.0 mg/mL) was used as a surrogate lipid for calibration. The data were expressed as milligrams of lipids per gonad wet weight. Cellular energy expense was also determined in gonad tissues using the mitochondria electron transport (MET) activity methodology [10]. The mitochondria fraction (50–100 μg/mL) were mixed with two volumes of 0.1 M Tris-HCl (pH 8.5) containing 5% polyvinylpyrrolidone, 0.1 mM MgSO4 and 0.1% Triton X-100. Freshly prepared NADH and NADPH were added at a final concentration of 1 mM and 0.2 mM, respectively. The reaction was started after adding 1 mM p-iodonitrotetrazolium. The reaction mix was placed in two clear microplates and incubated at 4ºC and 20ºC for 30 min. Absorbance readings were taken at t0-1 min and t30 min at 520 nm. The data were expressed as change in absorbance at 520 nm per min per mg of protein in mitochondria fraction. Temperature-dependent MET (METT) was derived from the difference in MET activity at 20ºC and MET activity at 4ºC: (A520/min at 20°CA520/ min at 4ºC)/(20-4=16ºC).
Data analysis
Biomarkers were determined from N=12 mussels at each exposure times (day 1, 12 and 24) in the aerated pond and upstream river water reference. The data were checked for homogeneity of variance and normality using the Bartlett’s test and the Shapiro-Wilk test, respectively. The data were then analyzed using analysis of variance, and critical differences between controls and day 1 mussels were determined using the Fisher’s Least Square Difference (LSD) test. Correlation analysis was performed to identify trends for biomarkers. Significance was set at p < 0.05 (Statistica version 8 software package, France).
Results
Mussels were exposed to wastewaters in the final aeration lagoon for increasing amounts of time: 1, 12 and 24 days. The wastewaters in that lagoon, which came from a city of 25,000 inhabitants, had a pH level between 6.1 to 7.1, conductivity of 410 to 760 μS/cm and total coliform counts of 500 to 1,000/100 mL. The river water to which mussels were exposed had a pH level of 7.8 to 8.2, conductivity of 200 μS/cm and total coliform counts less than 100.
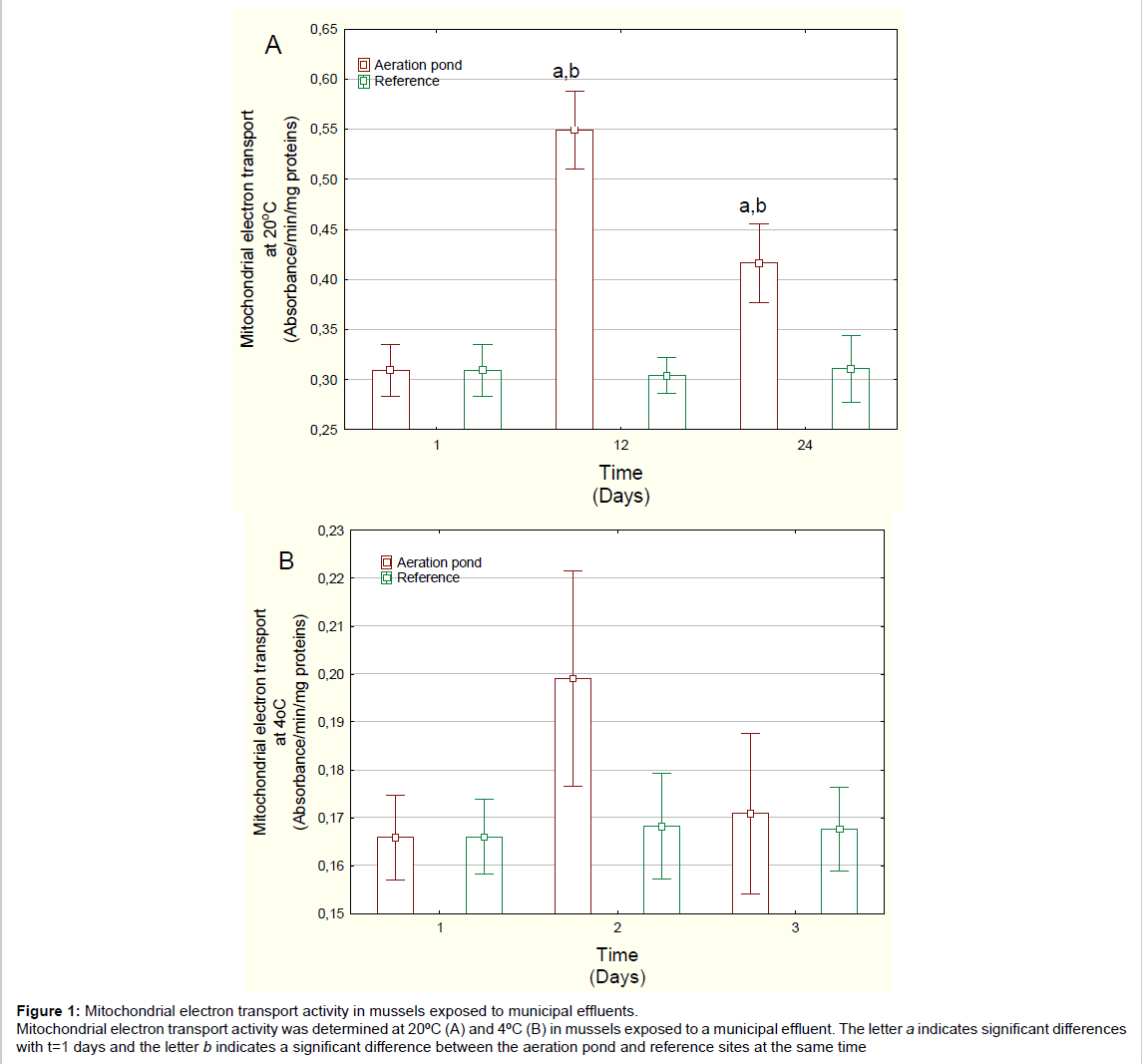
Figure 1: Mitochondrial electron transport activity in mussels exposed to municipal effluents.
Mitochondrial electron transport activity was determined at 20├?┬║C (A) and 4├?┬║C (B) in mussels exposed to a municipal effluent. The letter a indicates significant differences
with t=1 days and the letter b indicates a significant difference between the aeration pond and reference sites at the same time.
| Sites | Time (days) |
Condition factor (g/mm) |
Soft tissues (g tissues/g total) |
GSI | Gonad total lipids (mg/g tissues) |
|---|---|---|---|---|---|
| Reference | 1 | 0.470 ├?┬▒ 0.02 | 0.363 ├?┬▒ 0.02 | 0.086 ├?┬▒ 0.008 | 2.49 ├?┬▒ 0.20 |
| 12 | 0.457 ├?┬▒ 0.03 | 0.329 ├?┬▒ 0.06 | 0.085 ├?┬▒ 0.007 | 1.79 ├?┬▒ 0.18 | |
| 24 | 0.487 ├?┬▒ 0.02 | 0.276 ├?┬▒ 0.03 | 0.120 ├?┬▒ 0.006 | 2.44 ├?┬▒ 0.24 | |
| Aeration Pond | 1 | 0.394 ├?┬▒ 0.02 | 0.336 ├?┬▒ 0.02 | 0.098 ├?┬▒ 0.01 | 2.51 ├?┬▒ 0.22 |
| 12 | 0.454 ├?┬▒ 0.03 | 0.328 ├?┬▒ 0.04 | 0.101 ├?┬▒ 0.007 | 1.78 ├?┬▒ 0.1 | |
| 24 | 0.400 ├?┬▒ 0.007 | 0.246 ├?┬▒ 0.03a | 0.102 ├?┬▒ 0.005b | 1.92 ├?┬▒ 0.18ab |
Table 1: Morphometric analysis of mussels.
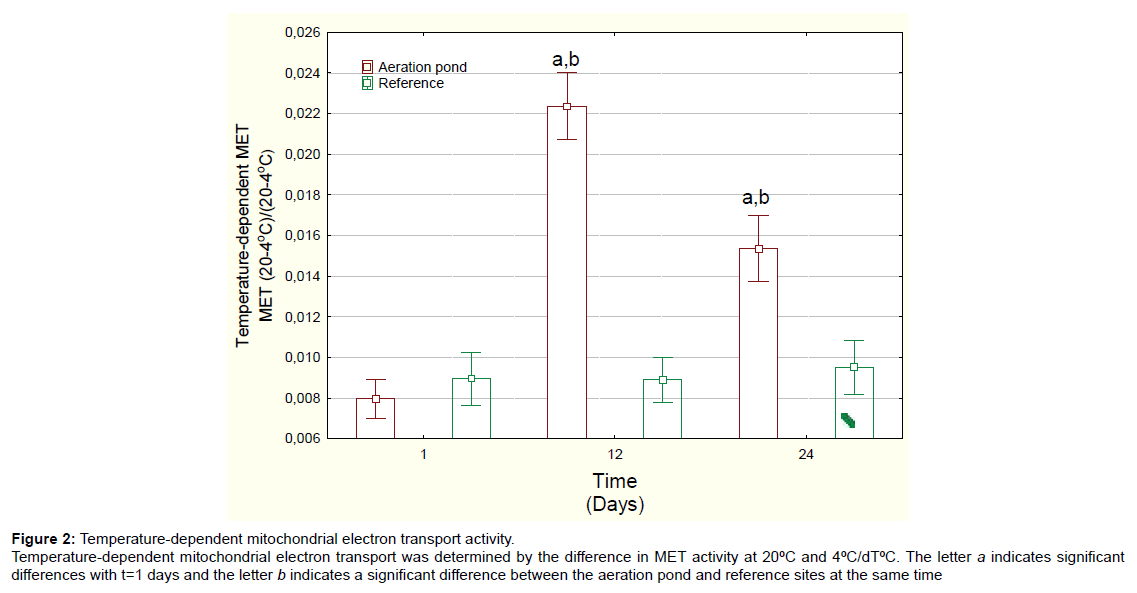
Figure 2: Temperature-dependent mitochondrial electron transport activity. Temperature-dependent mitochondrial electron transport was determined by the difference in MET activity at 20├?┬║C and 4├?┬║C/dT├?┬║C. The letter a indicates significant differences with t=1 days and the letter b indicates a significant difference between the aeration pond and reference sites at the same time
| GSI | ALP | LPO | MET 20├?┬║C | MET 6├?┬║C | Temp MET | Lipids | GST | MT | CYP3A4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| GSI | 1 | -0.49 p=0.001 | -0.27 p=0.1 | -0.33 p<0.05 | -0.19 | -0.29 p<0.1 | 0.04 | 0.32 p=0.1 | -0.18 | -0.51 p=0.01 |
| ALP | 1 | 0.27 p<0.1 | 0.28 p<0.1 | 0.05 | 0.32 p<0.05 | 0,04 | 0.05 | 0.37 p=0.01 | 0.60 p=0.001 | |
| LPO | 1 | 0.26 p<0.1 | -0.03 | 0.35 p<0.05 | -0.42 p<0.01 | -0.14 | 0.14 | 0.23 | ||
| MET 20├?┬░C | 1 | 0.57 p<0.001 | 0.93 p<0.001 | -0.32 p<0.05 | 0.34 p<0.05 | 0.26 p<0.1 | 0.38 p=0.05 | |||
| MET 4├?┬░C | 1 | 0.22 p=0.1 | -0.12 | 0.03 | -0.10 | 0.20 | ||||
| Temp MET | 1 | -0.33 p<0.05 | 0.36 p<0.05 | 0.31 p<0.05 | 0.39 p=0.05 | |||||
| Lipids | 1 | -0.05 | 0.05 | -0.09 | ||||||
| GST | 1 | 0.03 | 0.76 p=0.001 | |||||||
| MT | 1 | 0.32 p=0.1 |
Table 2: Correlation analysis of biomarker data.
Mussels exposed to domestic wastewaters showed no significant changes in condition factor (mussel weight/shell length) (Table 1). The soft tissue content was significantly decreased at day 24 for both the reference site and the aeration pond but there was no difference between aeration pond and reference site at day 24. MET activity at 20°C was determined in mussels exposed to the final aerated pond (Figure 1A). MET activity was significantly increased at 12 and 24 days compared to either day 1 or to reference mussels at each corresponding time. Mussels exposed to domestic wastewaters spend more energy than those exposed to river water. MET activity changes were not significant at 4ºC. Temperature-dependent MET (METT) activity was also determined in mussels exposed to domestic wastewaters (Figure 2). METT was significantly higher at days 12 and 24 in mussels caged in the aerated pond. Correlation analysis revealed that METT was significantly correlated with gonad lipids (r=-0.33) and MET activity at 20ºC (r=0.93) (Table 2).
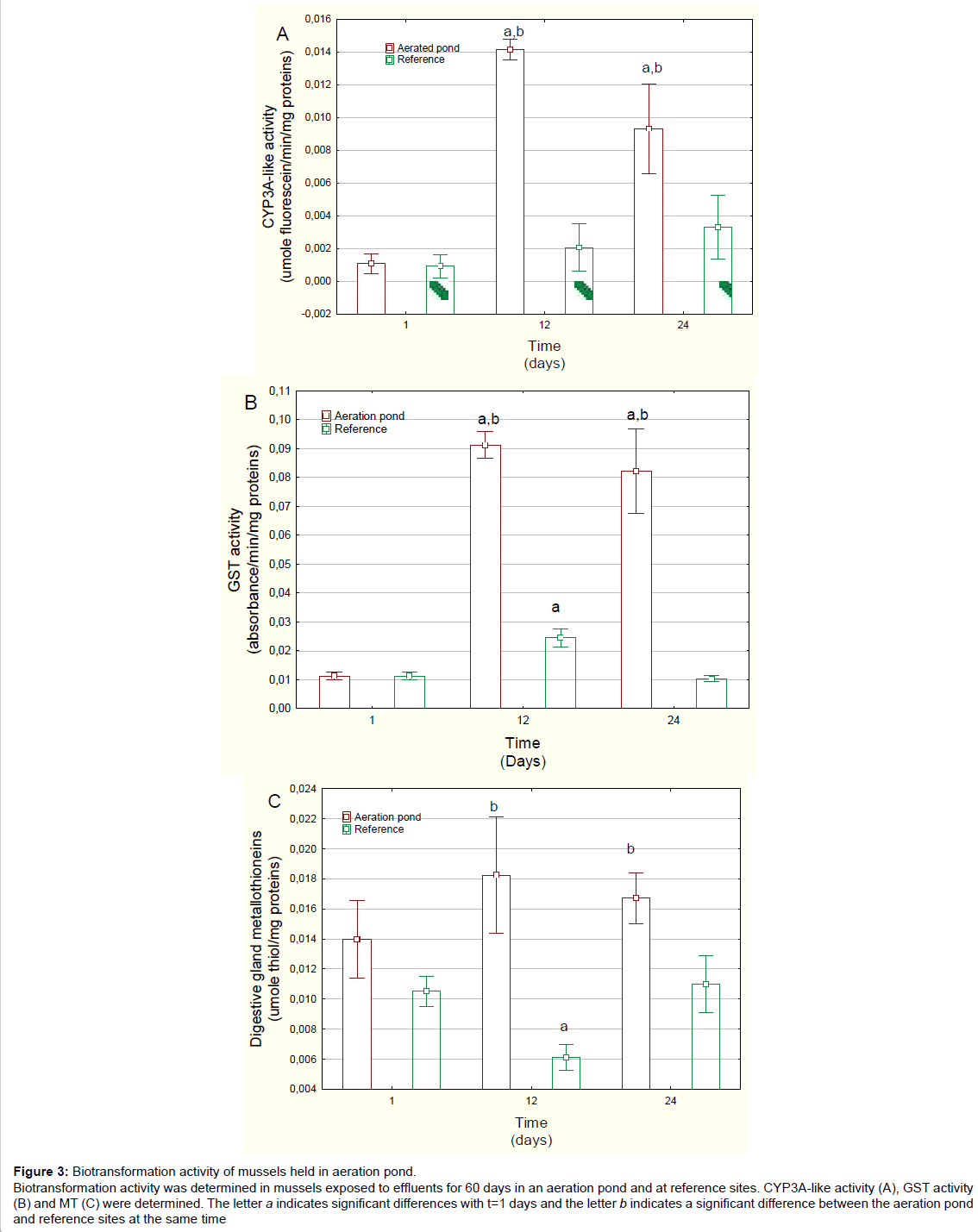
Figure 3: Biotransformation activity of mussels held in aeration pond. Biotransformation activity was determined in mussels exposed to effluents for 60 days in an aeration pond and at reference sites. CYP3A-like activity (A), GST activity (B) and MT (C) were determined. The letter a indicates significant differences with t=1 days and the letter b indicates a significant difference between the aeration pond and reference sites at the same time
Xenobiotic biotransformation was examined by following changes in CYP3A-like activity, GST activity and MT levels in the digestive gland (Figure 3). Exposure to the treated domestic wastewater strongly increased Cyp3A activity around 14- and 8-fold at days 12 and 24, respectively, in relation to day 1 mussels caged in the aeration pond. Cyp3A activity was also significantly increased in relation to reference mussels at each corresponding time. Correlation analysis revealed that CYP3A activity was significantly correlated with the GSI (r=-0.51), MET at 20ºC (r=0.38) and METT (r=0.39). The activity of xenobiotic conjugation enzyme GST was also highly induced in mussels exposed to aerated pond wastewaters (Figure 3B). GST activity was 9 and 8 times higher than day 1 values, at days 12 and 24, respectively. GST activity was also significantly increased in relation to reference mussels for each corresponding time. Correlation analysis revealed that GST activity was significantly correlated with MET activity at 20ºC (r=0.34), METT activity (r=0.36) and CYP3A4 activity (r=0.76). MT levels were also determined in the digestive gland of mussels exposed to aeration pond effluents (Figure 3C). MT levels were significantly increased at 12 and 24 days compared with the levels in reference mussels. However, MT levels were unchanged when compared to day 1. Correlation analysis revealed that MT levels were correlated with METT (r=0.31).
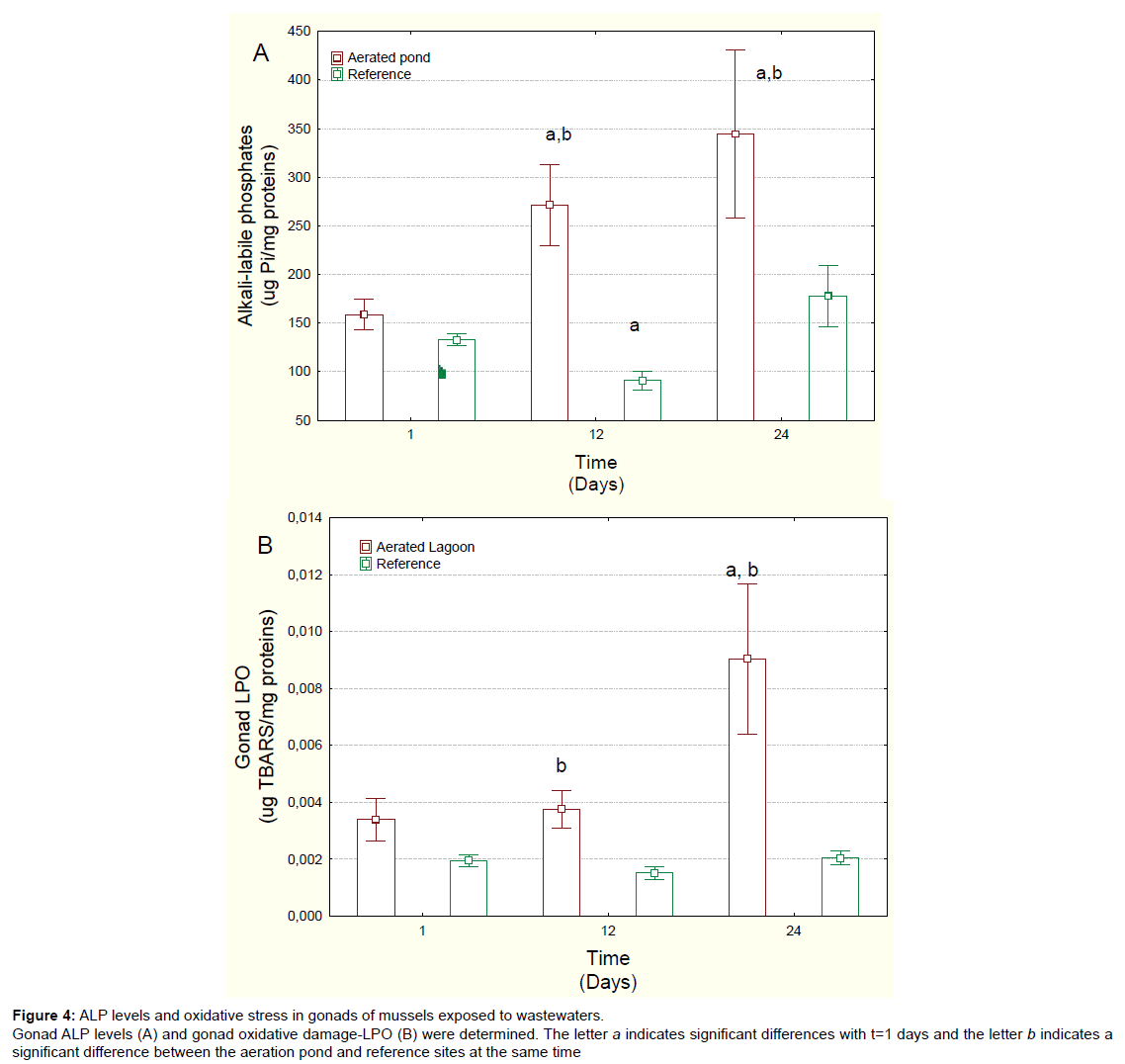
Gonad status was examined by determining LPO and ALP levels (Figure 4) and the GSI and lipid contents (Table 1). The GSI did not significantly change between males and females; however, it was significantly lower in mussels exposed to aerated pond effluents compared to those at the reference site at 24 days. Correlation analysis revealed that the GSI values were significantly correlated with ALP (r=-0.49), MET at 20ºC (r=-0.33) and CYP3A4 activity (r=-0.51). Total lipids in gonad were significantly lower at day 24 compared to either reference site at day 24 or day 1 in the aerated pond. Correlation analysis revealed that lipid levels were correlated with METT (r=-0.33) and LPO (r=-0.42), suggesting that temperature susceptibility was negatively correlated with energy budget in mussels. ALP levels, an indirect measure of vitellogenin, were considerably higher in exposed mussels at days 12 and 24 in relation to day 1 mussels or reference mussels, with a 2.2-fold increase recorded in relation to day 1 mussels (Figure 4A). Correlation analysis revealed that ALP levels were significantly correlated with METT (r=0.32), MT (r=0.37) and CYP3A4 activity (r=0.60). Finally, the integrity of gonad was examined using LPO as an index of oxidative damage (Figure 4B). Gonad LPO was significantly increased at day 12 relative to the reference site and at day 24 relative to both day 1 and the reference site (at same time) mussels. Correlation analysis revealed that LPO was significantly correlated withMETT (r=0.35) and total lipids (r=-0.42).
Figure 4: ALP levels and oxidative stress in gonads of mussels exposed to wastew aters.
Gonad ALP levels (A) and gonad oxidative damage-LPO (B) were determined. The letter a indicates significant differences with t=1 days and the letter b indicates a
significant difference between the aeration pond and reference sites at the same time.
Discussion
Warming water temperatures were shown to induce oxidative stress in unionids [18]. In Anodonta anatina, exposure to a temperature of 30ºC increased LPO in the digestive gland and gills; however, in polluted habitats, LPO was apparent at 25ºC, indicating a cumulative effect of temperature and pollution on LPO levels. In another study, lysosomal membrane destabilization (swelling) was greater in mussels exposed to cadmium in combination with heat stress (from 12 to 22ºC or from 18 to 28ºC for the winter and summer, respectively) compared to mussels subjected to heat only. In another study, which focused on soft-shell clams (Mya arenaria), interaction between pollution and habitat quality was observed [20,21]. MET activity, a source of reactive oxygen species, was more sensitive to temperature changes in clam populations from polluted sites than those from pristine sites. Gradual warming is therefore likely to worsen the negative effects of pollution on clam habitats. This observation appears to be supported by the present study, in which mussels exposed to treated domestic wastewaters exhibited elevated levels of MET activity, METT activity and oxidative stress (LPO).
Reproductive activity such as gametogenesis (vitellogenesis in females) could also be affected by to the cumulative effects of temperature and pollution. Seasonal variation in oxidative stress was shown to follow temperature changes with gametogenesis usually occurring during the warmer months of the year [21]. The increase of ALP levels was associated with METT activity, which suggests that the production of ALP in the gonad could influence temperature sensitivity to energy expenses. The production of egg yolk proteins during gametogenesis is an energy demanding process where temperature increase could facilitate gamete growth and development. Increased METT was also associated with oxidative damage in the gonad and with CYP3A activity, suggesting that steroid (testosterone) or other polar polycyclic aliphatic hydrocarbons contributed to METT. The strong association between ALP levels and CYP3A4 activity is consistent with the involvement of CYP3A4 in sexual steroid metabolism and regulation (6β-testosterone hydroxylase activity) [22] in addition to its major role in drug metabolism [12] which abound in treated municipal wastewaters. An increase in CYP3A4 activity could therefore result from increased exposures to steroids or other pharmaceuticals and could be an adaptive mechanism to lower (steroid) estrogen signaling in mussels exposed to municipal effluent. This suggests that the process of energy metabolism to support gametogenesis contributes to oxidative stress, in conjunction with temperature sensitivity. However, pollution could disrupt reproduction at the neuroendocrine level in mussels [23]. Altered production of vitellogenin-like proteins was associated with increased inflammation as determined by LPO, cyclooxygenase activity at the high dopamine/serotonin ratio. Cellular energy allocation could be qualitatively determined by the ratio between MET and lipid and carbohydrate contents [24]. For example, mussels from an estuary (combined pollution and salinity stress) were shown to have a decreased ratio of MET to energy reserves (lipids and carbohydrate) compared to mussels at a coastal site. In the present study, MET activity was negatively correlated with total lipids, indicating that increased energy expenditure (MET) was associated with lower lipids (energy reserves) in mussels exposed to aerated pond wastewater. The ratio of MET to lipids in mussels exposed to aerated pond effluents was 0.31 and 0.2 for day 12 and day 24, respectively, compared to 0.1 for reference mussels and day 1 mussels. This suggests a negative energy budget for effluent-exposed mussels during the first 24 days compared to mussels in river water (reference). This lower energy budget could be the result of increased defense responses to pollutants in the wastewaters such as CYP3A-like activity, GST and MT and sensitivity to temperature changes (METT). It was found that in Mytilus galloprovincialis clams, co-exposure to nickel along a temperature gradient from 18ºC to 26ºC led to decreased antioxidant activity (catalase, glutathione S-transferase) and detoxification (MT) [25]. However, we found that METT was positively correlated with MT levels, suggesting that temperature sensitivity was correlated with detoxification within a temperatures range of 4ºC to 20ºC for Elliptio complanata. Exposure of oysters to 50 μg/L cadmium and heat stress (acclimation temperature of 20ºC increased to 30ºC) led to increased proton leakage in mitochondria [26]. The increase in electron transport activity through enhanced phosphorylation and proton flux capacity requires a constant O2 supply to support respiration. This suggests that Cd exposure and acute temperature stress may lead to the loss of mitochondrial resilience to change in O2 levels (hypoxia) in the surrounding environment, which can compromise oysters’ ability to adapt to periodic oxygen deprivation. In freshwater mussel habitats, the changes in oxygenation that occur during water level changes in the summer (higher temperature and drying of river banks) or due to eutrophication processes could also be harmful. Mussels in warmer climates had lower mitochondria density and increased proton leakage in gills and digestive gland compared to their colder climate counterparts [27]. These mussels were also more sensitive to Cd inhibition of mitochondria respiration or electron transport activity when the temperature reached 28ºC to 30ºC, suggesting that warmer temperatures increase sensitivity to Cd contamination.
Conclusion
In conclusion, exposure of freshwater mussels to treated wastewater in an aerated pond led to increase METT. This increase was associated with negative lipid contents and increased xenobiotic biotransformation such as GST, MT and CYP3A4 activity and oxidative stress, which suggests that the increased temperature dependence of energy expenditure associated with pollution could cause a decrease in energy reserves. Hence, organisms exposed to urban pollution are more sensitive to temperature changes, which are expected to occur more often in the present context of climate change.
References
- Min SK, Zhang X, Zwiers FW, Hegerl GC (2011) Human contribution to more-intense precipitation extremes. Nature 470: 378-381.
- Holeton C, Chambers PA, Grace L (2011) Wastewater release and its impacts on Canadian waters. Canadian J Fish Aquat Sci 68: 1836-1859.
- Marcogliese DJ, Blaise C, Cyr D, de Lafontaine Y, Fournier M, et al. (2015) Effects of a major municipal effluent on the St. Lawrence River: A case study. Ambio 44: 257-274
- McArdle M, Elskus A, McElroy A, Larsen
- B, Benson W
- et al. (2000) Estrogenic and CYP1A response of mummichogs and sunshine bass to sewage effluent. Mar
- Environ Res 50: 175-179
- Gagn├?┬ę F, Bouchard B, Andr├?┬ę C, Farcy E, Fournier M (2011) Evidence of feminization in wild Elliptio complanata mussels in the receiving waters downstream of a municipal effluent outfall. Comp Biochem Physiol 153C: 99-106
- Gagn├?┬ę F, Fournier M, Blaise C (2004) Serotonergic effects of municipal effluents: Induced spawning activity in freshwater mussels. Fresenius Environ Bull 13: 1099-1103.
- Smolders R, Bervoet L, De Coen W, Blust R (2004) Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ Poll 129: 99-112.
- Gagn├?┬ę F, Blaise C, Andr├?┬ę C, Salazar M (2006) Effects of municipal effluents and pharmaceutical products on temperature-dependent mitochondrial electron transport activity in Elliptio complanata mussels. Comp Biochem Physiol 143C: 388-393.
- Viarengo A, Banesi L, Pertica M, Mancinelli G, Accomando R, et al. (1995) Stress on stress response: A simple monitoring tool in the assessment of general stress syndrome in mussels. Mar Environ Res 39: 245-248.
- Heise K, Puntarulo S, Portner HO, Abele D (2003) Production of reactive oxygen species by isolated mitochondria of the Antarctic bivalve Laternula elliptica under heat stress. Comp Biochem Physiol 134A: 79-90.
- King F, Packard TT (1975) Respiration and the activity of the respiratory electron transport system in marine zooplankton. Limnol Oceanogr 20: 849-854.
- Crespi CL, Miller,VP, Penman, BW (1997) Microtiter plate assays for inhibition of human, drug-metabolizing cytochromes P450. Anal Biochem 248: 188-190.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem72: 248-254.
- Viarengo A, Ponzanon E, Dondero F, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to Mediterranean and Antarctic molluscs. Mar Environ Res44: 69-84.
- Quinn B, Gagn├?┬ę F, Blaise C (2004) Oxidative metabolism activity in Hydra attenuata exposed to carbamazepine. Fresenius Environ Bull 13: 783-788.
- Boryslawskyj M, Garrood AC, Pearson JT (1988) Elevation of glutathione-S-transferase activity as a stress response to organochlorine compounds, in the freshwater mussel, Sphaerium corneum. Mar Environ Res 24: 101-104.
- Frings CS, Fendley TW, Dunn RT, Queen CA (1972) Improved determination of total serum lipids by the sulfo-phospho-vanillin reaction. Clin Chem 18: 673-674.
- Falfushynska H, Gnatyshyna L, Yurchak I, Ivanina A, Stoliar O, et al. (2014) Habitat pollution and thermal regime modify molecular stress responses to elevated temperature in freshwater mussels (Anodonta anatina: Unionidae).
- Sci Total Environ 500-501: 339-350
- Gagn├?┬ę F, Blaise C, Andr├?┬ę C, Pellerin J (2007a) Implication of site quality on mitochondrial electron transport activity and its interaction with temperature in feral Mya arenaria clams from the Saguenay Fjord. Environ Res 103: 238-246.
- Gagn├?┬ę F, Blaise C, Pellerin J (2007b) Neuroendocrine disruption in Mya arenaria clams during gametogenesis at sites under pollution stress. Mar Environ Res 64: 87-107.
- Jarque S, Prats E, Olivares A, Casado M, Ram├?┬│n M, et al. (2014) Seasonal variations of gene expression biomarkers in Mytilus galloprovincialis cultured populations: Temperature, oxidative stress and reproductive cycle as major modulators. Sci Total Environ 499: 363-372.
- Krauser JA, Guengerich FP (2005) Cytochrome P450 3A4-catalyzed Testosterone 6-Hydroxylation Stereochemistry, Kinetic Deuterium Isotope Effects, and Rate-limiting Steps. J Biol Chem 280: 19496-19506.
- Gagn├?┬ę F, Blaise C, Andr├?┬ę C, Gagnon C, Salazar M (2007) Neuroendocrine disruption and health effects in Elliptio complanata mussels exposed to aeration lagoons for wastewater treatment. Chemosphere 68: 731-743.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 2551
- [From(publication date):
April-2017 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 1742
- PDF downloads : 809