Unraveling the Roles and Dysregulation of Adult Hippocampal Neurogenesis: Implications for Regenerative Processes and Pathological Conditions
Received: 01-Mar-2024 / Editor assigned: 04-Mar-2024 / Reviewed: 18-Mar-2024 / Revised: 25-Mar-2024 / Published Date: 29-Mar-2024 DOI: 10.4172/2314-7326.1000495
Abstract
The hippocampus is the only known brain region where physiological neurogenesis continues into adulthood across mammalian species and in humans. However, disease and injury can change the level of adult hippocampal neurogenesis, which plays an important role in regulating cognitive and emotional abilities. Alterations in hippocampal neurogenesis can mediate treatment of mental illness or affect the brain’s capacity for repair and regeneration. In the present review, we evaluate how adult neurogenesis contributes to the repair and regeneration of hippocampal circuitry in the face of diseases and injuries. We also discuss possible future directions for harnessing adult neurogenesis for therapeutic use.
Keywords
Introduction
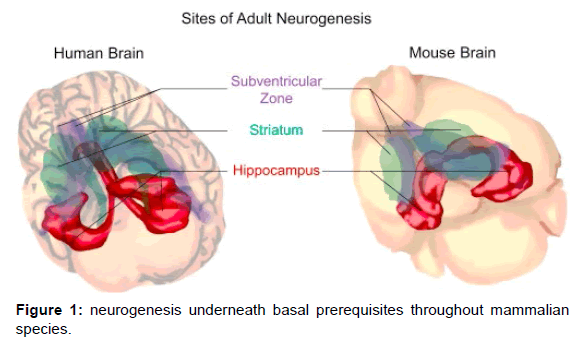
The grownup Genius has lengthy been regarded restricted in its regenerative ability in contrast with different organs or tissues in the body. For most of the twentieth century, it was once believed that neurogenesis ceased after development [1]. However, in 1962, Altman1 injected person rats with radioactive thymidine to label replicating cells and then confirmed that mitotically lively progenitors stay in the person rat talent and provide start to new neurons. A collection of subsequent research over a long time confirmed that person neurogenesis exists in almost all mammals,2 along with humans.3, four Under ordinary conditions, excessive ranges of neurogenesis manifest in two person rodent regions: the subventricular sector of the lateral ventricles and the dentate gyrus of the hippocampus. Of the two regions, the dentate gyrus of the hippocampus is the solely location succesful of neurogenesis underneath basal prerequisites throughout mammalian species, along with human beings (Figure 1). This region’s living neural stem cells can generate useful new neurons and glia in response to pathologic and pharmacologic stimuli, retaining each community plasticity and tissue homeostasis, with viable for restore and regeneration on sickness and injury [2-6].
Figure 1. Adult neurogenesis regions. Adult neurogenesis is constrained in unique intelligence areas in human beings and mice. Left panel: Human person neurogenesis takes place below basal prerequisites in the hippocampus (red) and the striatum (green). Right panel: Murine person neurogenesis happens in the hippocampus (red) and the subventricular quarter (purple). The hippocampus serves as the solely intelligence location the place grownup neurogenesis is conserved throughout mammalian species. Adult hippocampal neurogenesis has been functionally linked to learning and memory and emotional processing, such as stress and depression. In addition, hippocampal neurogenesis is highly regulated by the local and extrinsic environment. Stress suppresses neurogenesis through the corticosteroid and hypothalamic-adrenal axis. In turn, the level of neurogenesis also affects animals' sensitivity to stress. We will discuss both the positive and negative relationships among neurogenesis, postinjury repair, and neural disease development [7,8].
Regenerative processes: Adult hippocampal neurogenesis holds promise for regenerative medicine and neural repair. The continuous generation of new neurons provides a substrate for neural plasticity and adaptability, enabling the brain to integrate new experiences and information. Studies have shown that environmental enrichment, physical exercise, and cognitive stimulation can enhance neurogenesis, highlighting the potential for lifestyle interventions to promote brain health and resilience against age-related cognitive decline [9].
Furthermore, recent findings suggest that manipulating neurogenic processes may facilitate recovery following brain injury or neurodegenerative diseases. Preclinical studies utilizing animal models have demonstrated that enhancing neurogenesis can improve cognitive function and promote functional recovery in conditions such as stroke, traumatic brain injury, and Alzheimer's disease. These findings underscore the therapeutic potential of targeting adult hippocampal neurogenesis in regenerative approaches for neurological disorders [10].
Pathological conditions: Conversely, dysregulation of adult hippocampal neurogenesis has been implicated in the pathogenesis of various psychiatric and neurological disorders. Chronic stress, a known suppressor of neurogenesis, has been linked to the development of mood disorders such as depression and anxiety. Moreover, alterations in neurogenic processes have been observed in psychiatric conditions like schizophrenia and bipolar disorder, highlighting the complex interplay between neurogenesis and mental health [11].
Conclusion
The roles and dysregulation of adult hippocampal neurogenesis are multifaceted, encompassing both regenerative processes and pathological conditions. Understanding the intricate molecular and cellular mechanisms underlying neurogenic regulation is crucial for developing targeted interventions to harness the regenerative potential of the adult brain and mitigate the impact of neurological and psychiatric disorders. Future research aimed at unraveling the complexities of neurogenesis holds promise for advancing therapeutic approaches to promote brain health and resilience across the lifespan.
Acknowledgment
None
Conflict of Interest
None
References
- Ingadottir AR, Bjorgvinsdottir EB, Beck A, Baldwin C, Weekes C, et al. (2020) Effect of two different nutritional supplements on postprandial glucose response and energy- and protein intake in hospitalised patients with COPD: A randomised cross-over study. Clin Nutr 39: 1085-1091.
- Ogan N, Yildirim F, Süzen B, Baha A, Akpinar EE (2020) Does Nutritional Risk Screening 2002 Correlate with the Dyspnea Status of Patients with GOLD Stage C-D Chronic Obstructive Pulmonary Disease?. Turkish Thorac J 21: 49–53.
- Maia I, Peleteiro B, Xará S, Amaral TF (2017) Undernutrition Risk and Undernutrition in Pulmonology Department Inpatients: A Systematic Review and Meta-Analysis. J Am Coll Nutr 36: 137–147.
- Law S, Kumar P, Woods S, Sriram KB (2016) Malnutrition screening in patients admitted to hospital with an exacerbation of chronic obstructive pulmonary disease and its association with patient outcomes. Hosp Pract (1995) 44: 207–212.
- Vermeeren MA, Schols AM, Wouters EF (1997) Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J 10: 2264-2269.
- van Bokhorst-de van der Schueren MAE, Guaitoli PR, Jansma EP, De-Vet HCW (2014) Nutrition screening tools: does one size fit all? A systematic review of screening tools for the hospital setting. Clin Nutr 33: 39–58.
- Kovelis D, Segretti N, Probst V, Lareau S, Brunetto A, et al. (2008) Validation of the Modified Pulmonary Functional Status and Dyspnea Questionnaire and the Medical Research Council scale for use in Brazilian patients with chronic obstructive pulmonary disease. J Bras Pneumol 34: 1008–1018.
- Fischer M, JeVenn A, Hipskind P (2015) Evaluation of muscle and fat loss as diagnostic criteria for malnutrition. Nutr Clin Pract 30: 239–248.
- Landis J, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33: 159–174.
- Neelemaat F, Meijers J, Kruizenga H, Ballegooijen HV, van Bokhorst-de van der Schueren M (2011) Comparison of five malnutrition screening tools in one hospital inpatient sample. J Clin Nurs 20: 2144–2152.
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Google Scholar, Cross Ref, Indexed at
Citation: Peng M (2024) Unraveling the Roles and Dysregulation of AdultHippocampal Neurogenesis: Implications for Regenerative Processes andPathological Conditions. J Neuroinfect Dis 15: 495. DOI: 10.4172/2314-7326.1000495
Copyright: © 2024 Peng M. This is an open-access article distributed under theterms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 399
- [From(publication date): 0-2024 - Mar 13, 2025]
- Breakdown by view type
- HTML page views: 293
- PDF downloads: 106