Understanding the Polyethylene Glycol-Induced Mouse Model of Retinal Degeneration and Choroidal Neovascularization
Received: 29-Nov-2017 / Accepted Date: 30-Dec-2017 / Published Date: 07-Jan-2018
Abstract
This review discusses a novel and inexpensive model for studying wet and dry AMD using polyethylene glycol (PEG). In this model, various doses of PEG are injected into the subretinal space of mice. The morphology of the subretinal space and the biochemical and immunological parameters have been evaluated over a period of time. In vitro, human adult retinal pigment epithelial-19 (ARPE-19) cells were treated with various doses of PEG, and cell death, CD59, C3, and CD46 were measured. PEG injections into the eyes of wild type mice induce RPE cell atrophy and proliferation, resulting in consequent loss of photoreceptors. Changes in morphology and gene expression, involved in complement autophagy and/or phagocytosis, also provide evidence that the PEG-induced pathological changes observed in mouse eyes are similar to the changes seen in human dry AMD. The formation of choroidal neovascularization (CNV) in PEG-treated mice indicates that the PEG can be used to induce CNV. This review article focuses exclusively on PEG-induced AMD models. The applications of the PEG model are to study the changes that occur during AMD development in response to complement activation in vivo and in vitro. This model also makes it possible to test potential drug candidates for AMD treatment and prevention.
Keywords: Age-Related Macular Degeneration; Pathology; Drugs; Choroidal Neovascularization; Polyethylene Glycol
Introduction
Age-related macular degeneration (AMD) is the leading cause of severe visual impairment and blindness in the elderly [1-3]. AMD is defined as the presence of choroidal neovascularization (CNV) and/or geographic atrophy in the eye [4]. As the average life expectancy in developed countries nears 80 years, cases of AMD in the United States are expected to reach 4 million by 2020 [5,6]. Not only is the number of cases predicted to rise, but the cost of treatment is expected to skyrocket unless a successful treatment is developed that decreases cases by 10% [5]. The available, somewhat effective, preventative strategies for AMD include antioxidant and zinc supplementation and laser photocoagulation [4]. It is estimated that over $8 billion will be spent on AMD treatment and management in the next few years [5,6]. However, this amount will increase as the elderly population grows.
Currently, only three drugs are available to treat AMD, Lucentis® by Genentech/Novartis, Eyelea® by Regeneron/Bayer and Macugen, however Macugen is not used to treat patients anymore because of its minimal benefits to patients [7,8]. Both drugs are antibodies against vascular endothelial growth factor (VEGF) [9]. Unfortunately, these drugs have some serious side effects (e.g., hemorrhage and tissue damage) [10,11]. The drug delivery is accomplished through frequent (once or twice a month) and expensive (about $2000 per injection) ocular injections [12-16]. The ability to deliver an AMD drug with an eye drop as opposed to an injection would make the product very economical and preferred. With the rates of AMD rising, there is an unmet need not only for health and social services for the affected individuals but also for better ways to prevent the early stages of AMD.
The availability of appropriate and effective animal models for AMD is a pivotal point in accomplishing the stated goals regarding AMD treatment. This review is the first to discuss an innovative method of inducing CNV and geographic atrophy in mice with polyethylene glycol (PEG) and its applications in studying AMD. Although several research and review articles have been written on AMD models, here we focus on PEG-induced models of CNV and retinal degeneration mimicking dry AMD. Therefore, we only discuss PEG-induced AMD models, not previously described AMD models.
Later stages of AMD are characterized by central vision loss and the development of CNV [3,17]. In addition, complement activation is well-known as being critical to the development of AMD [3,18-22]. Studies in mice have shown that laser-induced CNV and angiogenic growth-factor generation and release depend upon the formation of the membrane attack complex (MAC) and its deposition on retinal pigment epithelial (RPE) and choroidal cells [19-21,23-25]. The complement system is composed of a number of proteins that circulate throughout the body via plasma and those that can be membrane bound [26-28]. The interactions among these proteins are highly regulated due to the role of the complement system in innate immunity [19,29-32]. Patients with AMD display elevated levels of the complement proteins C3, C5, C5b-9 (MAC), and CFH [33,34]. Importantly, PEG is an activator of the classical and alternative pathways of the complement system [35,36]. Overall, the benefits of using PEG to induce CNV include its low molecular weight and quick absorption and secretion.
PEG Models and Their Uses
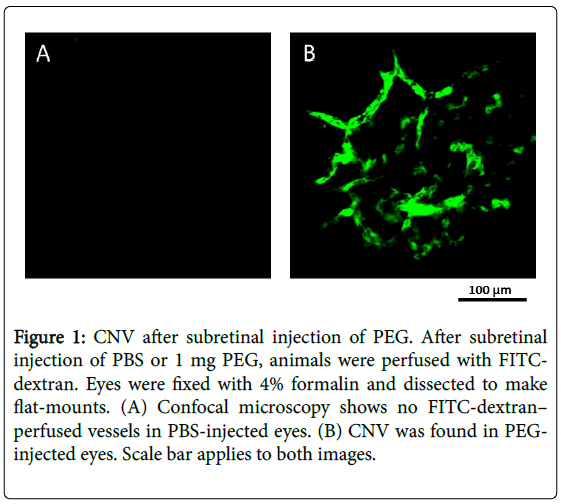
We published a report in the Journal of Biological Chemistry titled “Polyethylene glycol (PEG)-induced mouse model of choroidal neovascularization” as a baseline study describing an animal model for CNV. We performed a series of studies to evaluate the angiogenic effects of different doses of subretinal PEG. The methodology described includes the injection protocol (PEG doses 2.0, 1.0, 0.5, 0.25, and 0.125 mg), evaluation of CNV size, histological and immunohistochemical studies of MAC components, complement hemolytic assays, inhibition of the systemic complement system by cobra venom factor, and ELISA. The results supported the hypothesis that subretinal PEG effectively induces CNV, and the effects of subretinal PEG on the complement system are representative of CNV development during AMD (Figure 1).
Figure 1: CNV after subretinal injection of PEG. After subretinal injection of PBS or 1 mg PEG, animals were perfused with FITCdextran. Eyes were fixed with 4% formalin and dissected to make flat-mounts. (A) Confocal microscopy shows no FITC-dextran– perfused vessels in PBS-injected eyes. (B) CNV was found in PEGinjected eyes. Scale bar applies to both images.
This study revealed a dose-dependent effect of PEG on CNV severity in mice. It also showed that a single subretinal injection of PEG was sufficient to produce a prolonged effect on the mouse retina (for at least 42 days after injection), and there was a significant impact on tissue integrity near the injection site. The paper also discusses how PEG can activate the complement system. There was a significant increase of C3 split products and C9 in the eye; however, we did not find any changes in complement activity in response to PEG injections in systemic circulation. Given the results of this study, it is undoubtedly important to recognize the potential application of PEG in studying CNV in AMD. The remainder of this review will focus on the use of PEG to induce AMD.
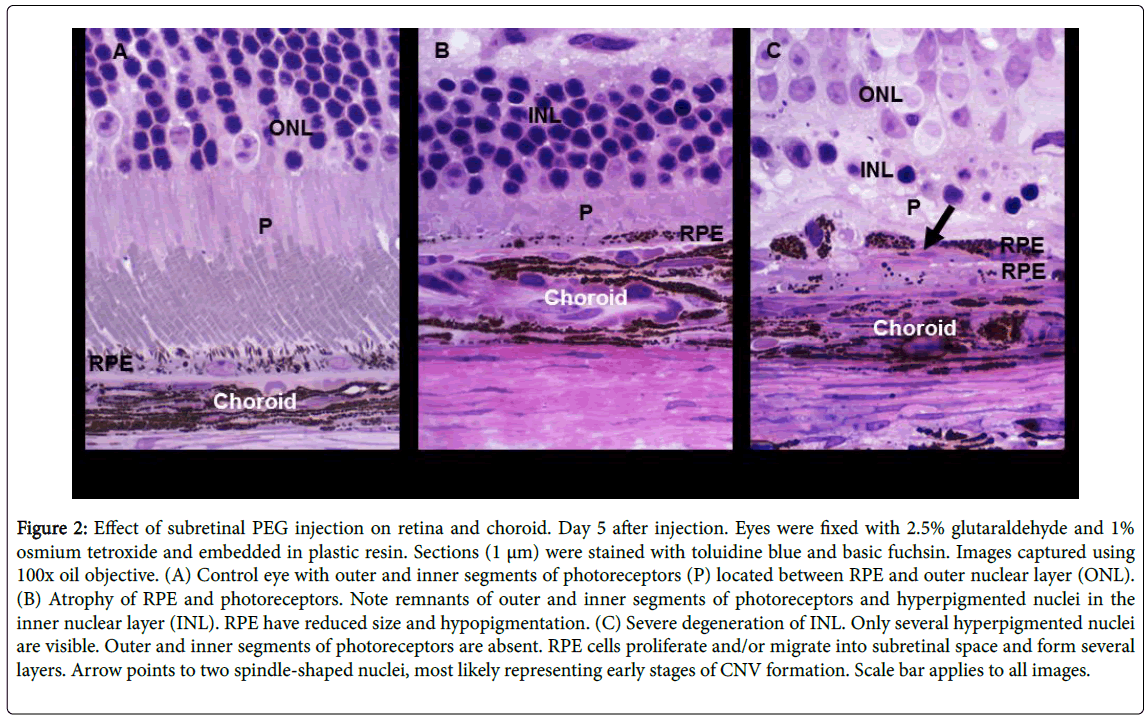
In a second paper published in 2014 in Experimental Eye Research titled “Polyethylene glycol induced mouse model of retinal degradation,” we evaluated the potential role of PEG to induce dry AMD-like changes. Dry AMD is defined as the degeneration of RPE cells and photoreceptors, RPE proliferation, and drusen accumulation [17,37]. Here, the focus was on the impact of PEG injection into the subretinal space on the morphology of the retina and choroid and corresponding changes in gene expression in these tissues. The approach used in this study included PEG injection into mice followed by tissue collection and processing for light and electron microscopy. The morphological parameters that were evaluated included outer nuclear layer (ONL) thickness; lengths of photoreceptor inner segment (PIS) and photoreceptor outer segment (POS); density of nuclei in the ONL; and number of RPE cells. The number of apoptotic cells was measured with the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method. Lastly, total RNA was analyzed for changes in gene expression. The results of this study revealed the potential for using a low dose of PEG (0.5 mg) to create a model of dry AMD (Figure 2).
Figure 2: Effect of subretinal PEG injection on retina and choroid. Day 5 after injection. Eyes were fixed with 2.5% glutaraldehyde and 1% osmium tetroxide and embedded in plastic resin. Sections (1 μm) were stained with toluidine blue and basic fuchsin. Images captured using 100x oil objective. (A) Control eye with outer and inner segments of photoreceptors (P) located between RPE and outer nuclear layer (ONL). (B) Atrophy of RPE and photoreceptors. Note remnants of outer and inner segments of photoreceptors and hyperpigmented nuclei in the inner nuclear layer (INL). RPE have reduced size and hypopigmentation. (C) Severe degeneration of INL. Only several hyperpigmented nuclei are visible. Outer and inner segments of photoreceptors are absent. RPE cells proliferate and/or migrate into subretinal space and form several layers. Arrow points to two spindle-shaped nuclei, most likely representing early stages of CNV formation. Scale bar applies to all images.
Some of the observations made for this model were a reduction in ONL thickness due to photoreceptor death; RPE degeneration, depigmentation, and proliferation; autophagosome formation in RPEs; and upregulation of the genes involved in a response to injury, immune system regulation, cell death, and proliferation, all of which resemble the course of action of human AMD pathology.
The papers summarized above provide strong support for the use of PEG in simulating wet and dry AMD. PEG is not only readily available, but it is also non-toxic to animals and is excreted in a short period of time. It is now important to discuss how the two models introduced above - the PEG-induced mouse model of choroidal neovascularization and PEG-induced mouse model of retinal degradation can be used to evaluate potential treatments for AMD. One example is the use of aspirin to treat AMD.
These models were used in an article published by Goyal et al. in 2016 in Clinical & Experimental Ophthalmology titled “Effect of aspirin on models of RPE pathology”; this report covers the impact of aspirin on two major models of AMD: laser photocoagulation and PEG injection [38]. Aspirin was chosen as the topic of the study in this paper due to its usage/prescription associated with wet and dry AMD in humans and its anti-inflammatory activity [39,40]. The researchers first used an argon laser to induce CNV in mice and then administered aspirin at 300 mg/kg of food, which is equivalent to 350 mg in humans. Next, one group of mice was sacrificed, and eye tissues were used to measure CNV size and VEGF levels. A second group of mice was injected with PEG and then used for histological analysis. The RPE layer and choroid thicknesses were used as morphological measurements for the dry AMD model. In addition to in vivo aspirin administration, an in vitro experiment was performed using human adult RPE-19 (ARPE-19) cells in which the effect of aspirin on VEGF and other complement-associated molecules was tested; the restorative effect of aspirin on damaged cells was also examined.
In both cases, ELISA was used to measure protein levels. The outcomes of this study indicated no significant effects of aspirin on CNV size and VEGF expression; however, aspirin did have a positive effect on the ability of RPE cells to regenerate in the in vitro damage experiment.
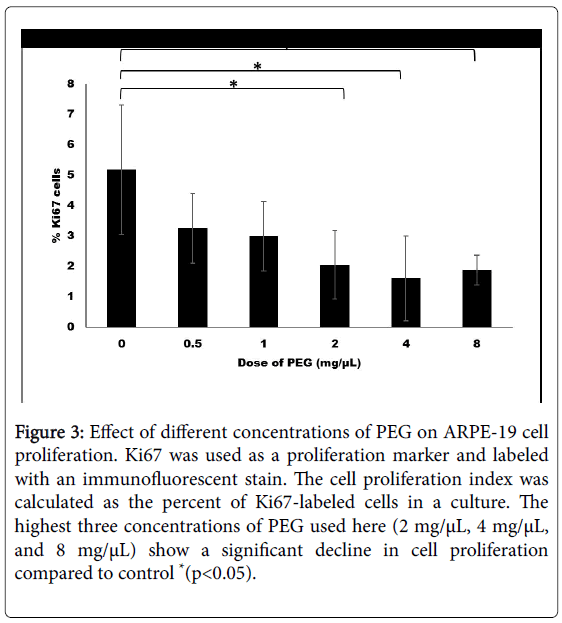
The most recent in vitro study of PEG applications defined the effective complement system-activating dose of PEG for RPE cells. Before any in vivo experiments are conducted, it is critical to determine a dose or range of doses that cause an appropriate cell response (i.e., a dose that does not disturb cellular processes and does not increase cell mortality). Recent data from our lab show a significant decline in human ARPE-19 cell proliferation when treated with 2, 4, or 8 mg/μL PEG (Figure 3).
Figure 3: Effect of different concentrations of PEG on ARPE-19 cell proliferation. Ki67 was used as a proliferation marker and labeled with an immunofluorescent stain. The cell proliferation index was calculated as the percent of Ki67-labeled cells in a culture. The highest three concentrations of PEG used here (2 mg/μL, 4 mg/μL, and 8 mg/μL) show a significant decline in cell proliferation compared to control *(p<0.05).
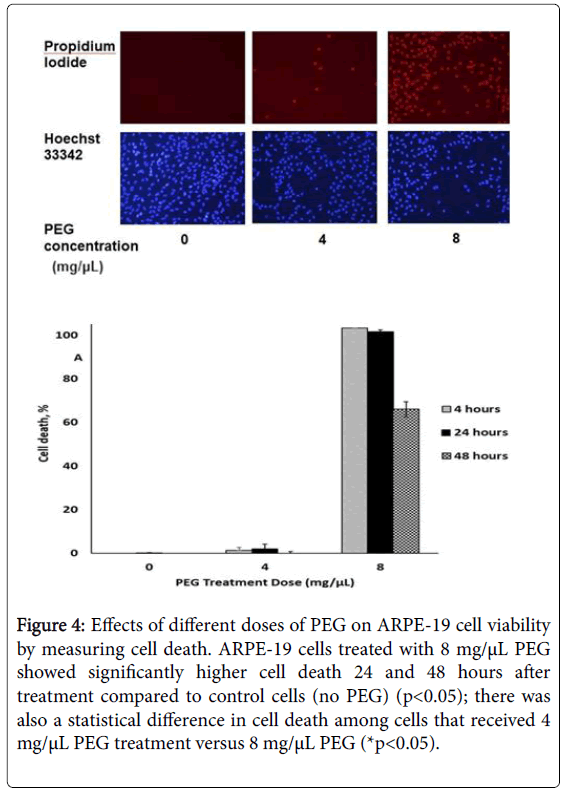
A statistically significant amount of cell death was also observed 24 and 48 hours after treatment with 8 mg/μL PEG (Figure 4).
Figure 4: Effects of different doses of PEG on ARPE-19 cell viability by measuring cell death. ARPE-19 cells treated with 8 mg/μL PEG showed significantly higher cell death 24 and 48 hours after treatment compared to control cells (no PEG) (p<0.05); there was also a statistical difference in cell death among cells that received 4 mg/μL PEG treatment versus 8 mg/μL PEG (*p<0.05).
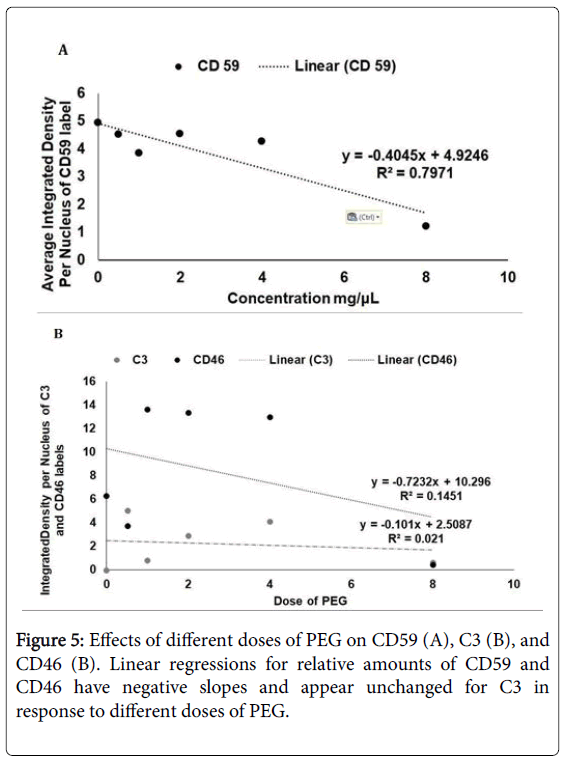
The initial analyses of CD59, CD46, and C3 in response to different doses of PEG indicated a decline in CD59 and CD46 as the PEG dose increased (Figure 5A and 5B). However, C3 seemed to be unaffected by the dose increase (Figure 5B). The availability of an inexpensive, controlled animal model of AMD is more relevant now than ever. The papers discussed above show a great potential for using PEG as a controlled way of inducing both wet and dry AMD. PEG injections into wild type mice induce RPE cell atrophy and proliferation, resulting in the loss of photoreceptors (Figure 2) [41]. One of the papers discussed above shows that a dose as small as 0.5 mg of PEG produces an effective model of dry AMD in mice [41]. In this paper, the results clearly demonstrate degenerative changes in RPE (reduction of RPE thickness, depigmentation, and deposition of drusen-like structures) and proliferation of RPE (expression of PCNA and an increase in the density of RPE cell nuclei) in PEG-treated mouse eyes compared to PBS controls. Morphological changes and gene expression patterns, involved in autophagy and/or phagocytosis, also provide evidence that PEG-induced pathological changes in mouse eyes are similar to the pathological changes seen in human dry AMD [41].
A dose of 1 mg of PEG had a drastic effect on mouse eyes, leading to the development of CNV (Figure 1) [42]. The early stages of CNV are less well understood than later stages due to the timing of the diagnosis. In the above studies, a single injection of PEG allowed researchers to observe the early events of CNV: RPE cell enlargement and vacuolization; formation of extensions from present choroidal capillaries; and vessel penetration into Bruch’s membrane [2]. Not only did the morphology of the impacted area change after PEG injection, but the composition of complement components was altered. For example, C3 levels and C3 split products increased in RPE choroid tissue on day 1 post-injection, indicating the activation of inflammatory processes [43]. C9 deposition also increased in the eye relative to systemic circulation 1 day after PEG injection. Both C9 and C3 deposition was followed by the previously described morphological changes.
Recently, Cammalleri and Bagnoli et al. published a paper that further bolsters the use of a PEG-induced dry AMD model [44]. They studied whether an orally fed compound based on fatty acids was able to limit acute inflammatory reactions, including macrophage infiltration triggered by the drusen-like insult, in a PEG-induced dry AMD model. Although other AMD models have been described, these authors used the PEG model (because it is a fast and less invasive model) specifically to examine the effect of fatty acids on geographical atrophy [44] With the PEG model, they reproduced the formation of drusen-like deposits between the RPE and choroid, and PEG activated the complement system [44]. This turn of events started a cascade of inflammatory reactions that ultimately degenerated the retina, RPE, and choroid interface [44].
Since laser induced model is very often used to study pathogenesis of AMD, here we will compare PEG induced model of AMD with laser induced model of AMD. The differences in PEG induced AMD model when compared to laser induced AMD model are that the PEG induced model is less invasive and easy to induce AMD. PEG model is also economical; no Argon or Krypton laser is necessary to induce PEG induced AMD model [41,42]. The similarities are that the PEG induced AMD model generates same level of CNV complex as generated by laser induced model. The days needed to generate desired CNV complex are same in both models [41,42].
The mechanism of both PEG induced AMD model and laser induced AMD model is the same i.e. both models activate complement system, MAC is accumulated in RPE and choroid which activates growth factor like VEGF and FGF and other inflammatory proteins. This process generates new vessels from choroid to form CNV complex [41,42].
Conclusions
In conclusion, there are several CNV and dry AMD models described in the literature, including genetic models, but many of these models are invasive or do not resemble human CNV or AMD [1-3,18-31,37-42]. As mentioned, we have focused this review article only on the PEG-induced models of AMD. However, the applications of the PEG models described here will be endless, ranging from in vivo and in vitro studies of cellular responses to AMD development in response to complement activation by PEG to the testing of potential drug candidates for AMD treatment and prevention. To our knowledge, this is the first review article to describe the role of PEG in the development and study of CNV and dry AMD.
Acknowledgement
Funding was provided by the Jones Eye Institute Research Foundation, University of Arkansas for Medical Sciences, Little Rock, Arkansas, 72205. Thanks to Ms. Kerry Evans (Science Communication Group at the University of Arkansas for Medical Sciences) for editing this manuscript.
Author Contributions
Puran S. Bora conceived of the idea. PSB and Nalini S. Bora wrote in parts and edited the contents of the manuscript. Xeniya V. Rudolph and Valeriy V. Lyzogubov performed some of the experiments and wrote parts of the manuscript.
Conflicts of Interest
None of the authors have any financial/conflicting interests to disclose.
References
- Finger RP, Fimmers R, Holz FG, Scholl HP (2011) Incidence of blindness and severe visual impairment in Germany: projections for 2030. Invest Ophthalmol Vis Sci 52: 4381-4389.
- Campochiaro PA (2000) Retinal and choroidal neovascularization. J Cell Physiol 184: 301-310.
- Coleman HR, Chan CC, Ferris FL 3rd, Chew EY (2008) Age-related macular degeneration Lancet 372: 1835-1845.
- la Cour M, Kiilgaard JF, Nissen MH (2002) Age-related macular degeneration: epidemiology and optimal treatment. Drugs Aging 19: 101-133.
- Gehrs KM, Anderson DH, Johnson LV, Hageman GS (2006) Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med 38: 450-471.
- Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, et al. (2004) Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 122: 564-572.
- Schnichels S, Hagemann U, Januschowski K, Hofmann J, Bartz-Schmidt KU, et al. (2013) Comparative toxicity and proliferation testing of aflibercept, bevacizumab and ranibizumab on different ocular cells. Br J Ophthalmol. 97: 917-923.
- Kim JH, Lee DW, Chang YS, Kim JW, Kim CG (2016) Twelve-month outcomes of treatment using ranibizumab or aflibercept for neovascular age-related macular degeneration: a comparative study. Graefes Arch Clin Exp Ophthalmol. 254: 210-217.
- Wiesinger K, Reinelt P, Ennemoser A, Edelmayr M, Schönherr U (2016) [What can anti-VEGF therapy achieve in clinical routine? : Effectiveness of anti-VEGF therapy in patients with macular diseases in clinical routine on 1492 eyes in Austria]. Ophthalmologe 22: 330-335.
- Souied EH, Dugel PU, Ferreira A, Hashmonay R, Lu J, et al. (2016) Severe Ocular Inflammation Following Ranibizumab or Aflibercept Injections for Age-Related Macular Degeneration: A Retrospective Claims Database Analysis. Ophthalmic Epidemiol. 23: 71-79.
- Saenz-de-Viteri M, Fernández-Robredo P, Hernández M, Bezunartea J, Reiter N, et al. (2016) Single- and repeated-dose toxicity study of bevacizumab, ranibizumab, and aflibercept in ARPE-19 cells under normal and oxidative stress conditions. Biochem Pharmacol. 103: 129-139.
- Amoaku WM, Chakravarthy U, Gale R, Gavin M, Ghanchi F, et al. (2015) Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond.) 29 : 721-731.
- Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, et al. (2014) Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 98: 1144-1167
- Li E, Greenberg PB, Voruganti I, Krzystolik MG (2013) Cost and Selection of Ophthalmic Anti-Vascular Endothelial Growth Factor Agents. R I Med J 99: 15-17.
- The CATT Research Group (2011) Ranibizumab and Bevacizumab for Neovascular Age-Related Macular Degeneration. N Engl J Med. 364: 1897–1908.
- Hutton D, Newman-Casey PA, Tavag M, Zacks D, Stein J (2014) Switching to Less Expensive Blindness Drug Could Save Medicare Part B $18 Billion Over a Ten-Year Period. Health Aff 33: 931-939.
- Zarbin MA (2004) Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 122: 598-614.
- Edwards, AO, Malek G (2007) Molecular genetics of AMD and current animal models. 10: 119-132.
- Bora PS, Sohn JH, Crus JM, Jha P, Nishihori H, et al. (2005) Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol 174: 491-497.
- Bora NS, Kaliappan S, Jha P, Xu Q, Sohn JH, et al. (2006) Complement activation via alternative pathway is critical in the development of laser-induced choroidal neovascularization: role of factor B and factor H. J Immunol 177: 1872-1878.
- Lyzogubov VV, Tytarenko RG, Jha P, Liu J, Bora NS, et al. (2010) Role of ocular complement factor H in murine model of choroidal neovascularization. Am J Pathol 177: 1870-1880.
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, et al. (2010) The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 29: 95-112.
- Kaliappan S, Jha P, Lyzogubov VV, Tytarenko RG, Bora NS, et al. (2008) Alcohol and nicotine consumption exacerbates choroidal neovascularization by modulating the regulation of complement system. FEBS Lett 582: 3451–3458.
- Bora NS, Kaliappan S, Jha P, Xu Q, Sivasankar B, et al. (2007) CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol 178: 1783–1790.
- Li W, Chen S, Ma M, Qian J, Ma X (2010) Complement 5b-9 complex-induced alterations in human RPE cell physiology. Med Sci Monit 16: BR17-BR23.
- Atkinson JP, Frank MM (1980) Complement. Parker CW (editor), Clinical Immunology, Saunders, Philadelphia, USA 219-271.
- Ross GD (1986) Immunobiology of the Complement System. Academic Press, Florida, USA 211-222.
- Muller-Eberhard HJ (1988) Molecular organization and function of the complement system. Annu Rev Biochem 57: 321-347.
- Morgan BP, and Harris CL (1999) Complement Regulatory Proteins, Academic Press, San Diego. 1-13.
- Bora NS, Jha P, Bora PS (2008) The role of complement in ocular pathology. Semin Immunopathol 30: 85-95.
- Bora NS, Jha P, Lyzogubov VV, Bora PS (2011) Emerging role of complement in ocular diseases. Curr Immunol Rev 7: 360-33-370.
- Dzik JM (2010) The ancestry and cumulative evolution of immune reactions. Acta Biochim Pol 57: 443-466.
- Anderson DH, Mullins RF, Hageman GS, Johnson LV (2002) A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol 134: 411-431.
- Johnson LV, Leitner WP, Staples MK, Anderson DH (2001) Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res 73: 887-896.
- Hamad I, Hunter AC, Szebeni J, Moghimi SM (2008) Poly (ethylene glycol) s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol Immunol 46: 225-232.
- Moghimi Moein S, Hamad I, Bunger R, Andersen TL, Jorgensen K, Hunter AC, et al. (2006) J Liposome Res 16: 167-174.
- Sarks SH, Arnod JJ, Killingsworth MC, Sarks JP (1999) Early drusen formation in the normal and aging eye and their relation to age relates maculopathy: a clinicopathological study. Br J Ophthalmol 83: 358-368.
- Goyal S, Lyzogubov V, Ali O, Bora P, Bora N, et al. (2016) Effect of aspirin on models of RPE pathology. Clinical and experimental ophthalmology 610-617.
- de Jong PT, Chakravarthy U, Rahu M (2013) Association between aspirin use and aging macula disorder: The European Eye Study. Ophthalmology 119: 112-118.
- Roth GJ, Stanford N, Majerus PW (1975) Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci USA 72: 3073-3076.
- Lyzogubov V, Tytarenko R, Liu J, Bora N, Bora P (2014) Polyethylene glycol induced mouse model of retinal degeneration. Experimental Eye Research 127: 143-152.
- Lyzogubov V, Tytarenko R, Liu J, Bora N, Bora P (2011) Polyethylene glycol (PEG)-induced mouse model of choroidal neovascularization. J Bio chem 286: 16229-16237.
- Palarasah Y, Skjodt K, Brandt J, Teisner B, Koch C, et al. (2010) Generation of a C3c specific monoclonal antibody and assessment of C3c as a putative inflammatory marker derived from complement factor C3. J Immunol Methods 362: 142-150.
- Cammalleri M, Dal Monte M, Locri F, Lardner E, Kvanta A, et al. (2017) Efficacy of a Fatty Acids Dietary Supplement in a Polyethylene Glycol-Induced Mouse Model of Retinal Degeneration. Nutrients 9: 1079-1088.
Citation: Rudolf XV, Lyzogubov VV, Bora NS, Bora PS (2018) Understanding the Polyethylene Glycol-Induced Mouse Model of Retinal Degeneration and Choroidal Neovascularization. J Cell Mol Pharmacol 1: 101.
Copyright: © 2018 Rudolf XV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2885
- [From(publication date): 0-2018 - Feb 22, 2025]
- Breakdown by view type
- HTML page views: 2193
- PDF downloads: 692