Understanding the Pharmacological Mechanisms of Antidepressants
Received: 01-May-2023 / Manuscript No. cpb-23-97847 / Editor assigned: 04-May-2023 / PreQC No. cpb-23-97847 / Reviewed: 19-May-2023 / QC No. cpb-23-97847 / Revised: 23-May-2023 / Manuscript No. cpb-23-97847 / Published Date: 31-May-2023 DOI: 10.4172/2167-065X.1000334
Abstract
Pharmacological research aimed at revolutionizing drug delivery methods through the utilization of nanotechnology. This emerging field holds immense potential for enhancing therapeutic outcomes, minimizing side effects, and optimizing treatment regimens. Nanotechnology involves manipulating materials at the nanoscale, typically between 1 and 100 nanometers, to create innovative drug delivery systems. One significant area of focus within this field is the development of nanocarriers, such as liposomes, polymeric nanoparticles, and Dendrimers, which can encapsulate drugs and transport them to target sites with enhanced precision. These Nano carriers offer several advantages, including increased drug stability, prolonged circulation time, and improved bioavailability. Furthermore, their ability to passively or actively target specific tissues or cells can significantly enhance drug accumulation at the desired site, while minimizing exposure to healthy tissues.
Moreover, researchers are exploring the integration of stimuli-responsive nanomaterials that can release drugs in response to specific triggers, such as changes in pH, temperature, or the presence of certain enzymes. This "smart" drug delivery approach holds great promise for achieving controlled and on-demand drug release, thereby improving therapeutic efficacy and reducing the frequency of administration. Additionally, nanotechnology-based drug delivery systems are being designed to overcome biological barriers, such as the blood-brain barrier, which restricts the entry of therapeutic agents into the central nervous system. By leveraging nanoscale carriers, researchers are exploring strategies to enhance drug penetration and deliver treatments for neurological disorders more effectively.
Keywords
Diabetes; Pharmacy; Health service; Diabetes prevention programs
Introduction
Antidepressants are a class of drugs commonly used to treat depression, anxiety, and other mood disorders. They work by altering the levels of certain chemicals in the brain, such as serotonin, norepinephrine, and dopamine. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed type of antidepressants. They work by blocking the reuptake of serotonin, which increases the amount of serotonin available in the brain. This increase in serotonin helps regulate mood and reduce symptoms of depression. Tricyclic antidepressants (TCAs) also increase the levels of serotonin and norepinephrine in the brain by blocking their reuptake. However, TCAs have more side effects than SSRIs, and are generally used as a secondline treatment [1,2].
Monoamine oxidase inhibitors (MAOIs) are another class of antidepressants that work by inhibiting the breakdown of neurotransmitters such as serotonin, norepinephrine, and dopamine. MAOIs have fallen out of favor in recent years due to their potential for dangerous interactions with certain foods and medications. A typical antidepressants, such as bupropion and mirtazapine, work by different mechanisms. Bupropion increases the levels of dopamine and norepinephrine in the brain, while mirtazapine enhances the release of both serotonin and norepinephrine.
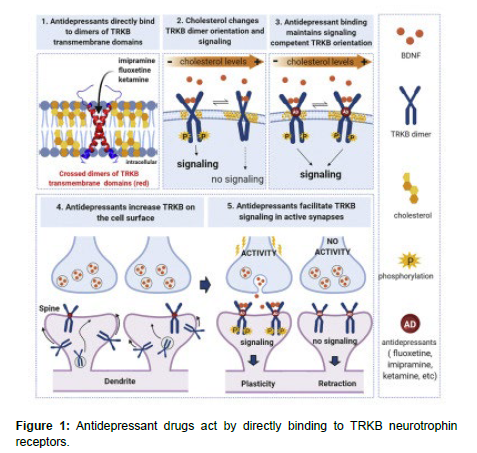
In conclusion, antidepressants work by altering the levels of certain neurotransmitters in the brain, and different classes of antidepressants work by different mechanisms. Understanding these mechanisms is important for selecting the most appropriate medication for an individual's specific symptoms and medical history. Pharmacology plays a crucial role in modern medicine, as it is the science that studies the effects of drugs on living organisms. This field is essential in the development of new treatments, as it provides a framework for understanding how drugs interact with the body and how they can be used to manage or cure diseases (Figure 1). Furthermore, nanotechnology is facilitating the development of personalized medicine by enabling targeted drug delivery based on an individual's genetic profile, disease stage, or specific biomarkers [3,4].
This approach has the potential to optimize treatment outcomes while minimizing adverse reactions. In conclusion, the integration of nanotechnology into pharmacological research is opening up new avenues for drug delivery, revolutionizing the field and transforming the way we approach therapeutic interventions. With continued advancements in nanomaterial synthesis, characterization techniques, and understanding of biological interactions, the future holds great promise for the development of more effective and patient-tailored pharmacological interventions (Table 1).
| Antidepressant Class | Pharmacological Mechanism |
|---|---|
| Selective Serotonin Reuptake | Inhibit the reuptake of serotonin, leading to increased serotonin levels in the brain. |
| Serotonin-Norepinephrine | Inhibit the reuptake of both serotonin and norepinephrine, increasing their availability in the brain. |
| Tricyclic Antidepressants (TCAs) | Block the reuptake of serotonin and norepinephrine, similar to SNRIs, but also have additional effects on other neurotransmitters. |
| Monoamine Oxidase | Inhibit the activity of the enzyme monoamine oxidase, which breaks down serotonin, norepinephrine, and dopamine, thereby increasing their levels. |
| Atypical Antidepressants | Various mechanisms of action, including modulation of serotonin and norepinephrine receptors, as well as other neurotransmitter systems. Examples include bupropion and mirtazapine. |
Pharmacologists study the pharmacokinetics and pharmacodynamics of drugs, which refer to the absorption, distribution, metabolism, and excretion of drugs in the body, as well as their mechanisms of action and therapeutic effects. By understanding these factors, pharmacologists can determine the optimal dosages, routes of administration, and treatment regimens for different drugs and conditions. Furthermore, pharmacology is involved in the development and testing of new drugs, as well as in the evaluation of their safety and efficacy. This process involves preclinical studies in animal models, clinical trials in human subjects, and post-marketing surveillance to monitor the long-term effects of drugs. Pharmacogenomics, the study of how an individual's genetic makeup influences their response to drugs, has emerged as a groundbreaking field within the realm of personalized medicine. This discipline combines pharmacology and genomics to optimize drug therapies, enhance patient outcomes, and minimize adverse drug reactions.
In recent years, significant advancements in genomic technologies have allowed researchers to identify genetic variations that impact drug metabolism, efficacy, and toxicity. This knowledge is invaluable in tailoring medication regimens to individual patients, considering their unique genetic profiles. By analyzing genetic markers, such as single nucleotide polymorphisms (SNPs), healthcare professionals can predict an individual's response to specific drugs and adjust dosages accordingly. One notable example is the field of oncology, where pharmacogenomics plays a crucial role in optimizing cancer treatments. Genetic testing can identify specific gene mutations or variations that influence the effectiveness of chemotherapy drugs or targeted therapies. This information allows oncologists to select the most suitable treatment options, improving patient outcomes and reducing unnecessary exposure to potentially ineffective drugs [5,6].
Material and Methods
Furthermore, pharmacogenomics has the potential to revolutionize the field of psychiatry. Genetic variations can significantly impact an individual's response to psychiatric medications, such as antidepressants or antipsychotics. By incorporating pharmacogenomics testing into clinical practice, psychiatrists can personalize treatment plans and optimize drug selection based on a patient's genetic profile. This approach improves medication efficacy, minimizes adverse effects, and enhances patient adherence to treatment regimens. Despite the promising advancements in pharmacogenomics, challenges remain in integrating this technology into routine clinical practice. There is a need for standardized guidelines on genetic testing, data interpretation, and implementation of results in treatment decision-making. Additionally, ethical considerations, patient privacy concerns, and cost-effectiveness need to be addressed to ensure widespread adoption of pharmacogenomics approaches. In conclusion, pharmacogenomics holds immense potential for personalized medicine by leveraging genetic information to optimize drug therapies. With further research and development, pharmacogenomics can pave the way for a new era of tailored treatments, improving patient outcomes, and reducing healthcare costs.
Results
In addition to drug development, pharmacology also plays a role in the management of drug interactions and adverse effects. Pharmacologists work with healthcare professionals to ensure that patients receive the appropriate medications and dosages, and that any potential risks or side effects are minimized. Overall, pharmacology is a critical component of modern medicine, as it provides the foundation for the development and use of drugs to improve health outcomes. As our understanding of the body and drugs continues to evolve, the role of pharmacology in medicine will only become more important. Pharmacology plays a crucial role in modern medicine by providing the necessary tools to treat a wide range of diseases and conditions. This field of study involves the study of drugs and their effects on the human body, including how they are absorbed, metabolized, and excreted.
Pharmacologists work to understand the mechanisms of action of different drugs and how they interact with the body's cells and tissues. This knowledge allows them to develop new drugs or improve existing ones to provide better treatment options for patients. In recent years, pharmacology has played a significant role in the development of drugs to treat chronic diseases such as cancer, diabetes, and cardiovascular disease. These drugs have revolutionized the treatment of these diseases, allowing patients to live longer and healthier lives.
Discussion
Pharmacology also plays an essential role in the development of new drugs to combat emerging infectious diseases, such as COVID-19. By understanding how viruses and bacteria interact with the body, pharmacologists can develop drugs that target these pathogens specifically. Overall, pharmacology is a critical field of study that has enabled the development of life-saving medications and treatments for a wide range of diseases and conditions. Its continued research and development are essential for the continued improvement of modern medicine.
Chronic pain is a complex medical condition that affects millions of people worldwide. The use of antidepressants in the treatment of chronic pain has gained attention in recent years. While antidepressants are primarily used to treat depression, they can also be effective in reducing chronic pain symptoms, such as neuropathic pain and fibromyalgia. One type of antidepressant that has been found to be effective in treating chronic pain is the tricyclic antidepressant (TCA) class of drugs. TCAs work by increasing the levels of certain neurotransmitters in the brain, which can reduce pain sensitivity. These drugs have been used for decades to treat chronic pain conditions, and are still considered an effective treatment option [7,8].
Another type of antidepressant that has shown promise in treating chronic pain is the selective serotonin reuptake inhibitor (SSRI) class of drugs. While SSRIs are primarily used to treat depression, they have been found to be effective in treating chronic pain conditions such as fibromyalgia and chronic headaches. SSRIs work by increasing the levels of serotonin in the brain, which can help reduce pain sensitivity. Antidepressants can be an effective treatment option for chronic pain, but it is important to note that they may take several weeks or months to take effect. Additionally, like all medications, they can have side effects. Common side effects of antidepressants include nausea, dizziness, and dry mouth. It is important to discuss the risks and benefits of using antidepressants to treat chronic pain with a healthcare provider before starting treatment [9-11].
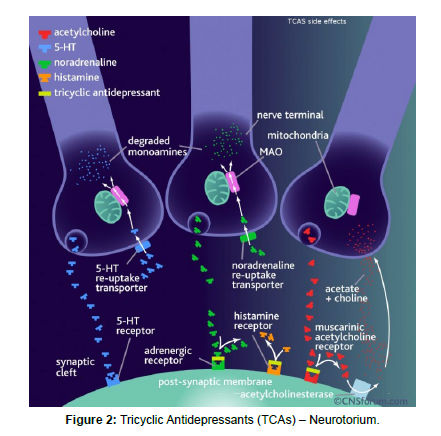
Pharmacology is a field of study that focuses on the effects of drugs on living organisms, including humans. It plays a vital role in modern medicine, as it helps researchers and healthcare professionals understand how drugs work and how they can be used to treat diseases. One of the key areas of pharmacology is drug development. Pharmaceutical companies invest heavily in research and development to identify new drugs that can treat a variety of conditions. Once a potential drug is identified, it goes through a rigorous testing process to ensure its safety and effectiveness. Pharmacology also plays a crucial role in the treatment of diseases. Healthcare professionals use drugs to manage symptoms and treat underlying conditions. They carefully consider factors such as a patient's age, weight, and medical history when selecting the most appropriate drug (Figure 2).
Additionally, pharmacology helps healthcare professionals understand the potential risks and side effects associated with drugs. It is essential to monitor patients carefully to ensure that they are not experiencing any adverse reactions to the drugs they are taking. In conclusion, pharmacology plays an essential role in modern medicine. It helps researchers develop new drugs, enables healthcare professionals to manage and treat diseases effectively, and ensures that patients receive safe and appropriate treatments. As the field of pharmacology continues to advance, it will undoubtedly lead to new and innovative treatments for a wide range of conditions.
Antibiotic resistance is a growing concern in pharmacology and healthcare. Antibiotics are a type of medication that can kill or slow down the growth of bacteria, and they have been an important tool in the fight against bacterial infections for decades. However, the overuse and misuse of antibiotics have led to the development of antibiotic-resistant bacteria, which can no longer be effectively treated with antibiotics. Antibiotic resistance is a complex issue that requires a multifaceted approach to address. The misuse of antibiotics is one of the primary factors contributing to the development of antibiotic-resistant bacteria. This can occur when antibiotics are prescribed unnecessarily, such as for viral infections like the common cold or when patients fail to complete a full course of antibiotics as prescribed. Another contributing factor is the use of antibiotics in agriculture. Antibiotics are commonly used in livestock farming to promote growth and prevent disease, but this can lead to the development of antibiotic-resistant bacteria in animals that can then be transmitted to humans [12-15].
These breakthroughs have been made possible by a deeper understanding of molecular mechanisms, advancements in drug discovery techniques, and the advent of innovative technologies. One area of immense progress is the development of targeted therapies that aim to selectively disrupt specific disease pathways. By identifying key molecular targets involved in the pathogenesis of diseases such as cancer, autoimmune disorders, and neurological conditions, researchers have been able to design drugs that precisely modulate these targets, minimizing off-target effects and enhancing therapeutic outcomes. This approach has resulted in remarkable improvements in patient survival rates and quality of life. Furthermore, the application of pharmacogenomics has emerged as a promising approach to tailor drug treatments to individual patients. Pharmacogenomics investigates the genetic variations that influence an individual's response to drugs, allowing healthcare professionals to predict the efficacy and potential adverse effects of medications. By utilizing this information, physicians can optimize drug selection and dosage, leading to more effective treatments with reduced risks of adverse events [16-18].
Another notable development is the rise of precision drug delivery systems. Nanotechnology has enabled the design and fabrication of nanocarriers capable of delivering drugs to specific tissues or cells, enhancing drug bioavailability and minimizing systemic side effects. These nanocarriers can be engineered to respond to specific stimuli, such as pH changes or enzymatic activity, enabling controlled and targeted release of therapeutic agents. This approach holds great promise for the treatment of diseases where localized drug delivery is crucial, such as tumors or inflammatory conditions. Additionally, pharmacological research has witnessed remarkable progress in the field of immunotherapy. Novel immunomodulatory drugs, including immune checkpoint inhibitors and CAR-T cell therapies, have revolutionized cancer treatment by harnessing the body's immune system to recognize and eliminate cancer cells. These breakthroughs have resulted in unprecedented response rates and durable remissions in previously untreatable cancers. In conclusion, recent advancements in pharmacological interventions have ushered in a new era of precision medicine. By harnessing the power of targeted therapies, pharmacogenomics, precision drug delivery systems, and immunotherapy, healthcare professionals can provide personalized treatments with enhanced efficacy and reduced adverse effects. These developments hold tremendous potential for improving patient outcomes and transforming the landscape of modern medicine [19,20].
Conclusion
The consequences of antibiotic resistance are significant. It can lead to longer hospital stays, higher healthcare costs, and an increased risk of death. It also limits our ability to treat bacterial infections, making routine surgeries and procedures, such as organ transplants and chemotherapy, more dangerous. To address antibiotic resistance, it is important to reduce the unnecessary use of antibiotics in both healthcare and agriculture. This can be done through education, regulation, and the development of new antibiotics and alternative treatments. It is also important for healthcare providers and patients to work together to ensure that antibiotics are used appropriately and responsibly. By taking these steps, we can help slow the development of antibiotic resistance and ensure that antibiotics remain an effective tool in the fight against bacterial infections.
Acknowledgement
None
Conflict of Interest
None
References
- Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL (2017) Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res Ther.
- Alzheimer’s A (2020) Alzheimer’s disease facts and figures. Alzheimer’s & Dement 16: 391-460.
- Terry RD, Davies P (1980) Dementia of the Alzheimer type. Annu Rev Neurosci 3: 77-95.
- Rathmann KL, Conner CS (1984) Alzheimer's disease: clinical features, pathogenesis, and treatment. Drug Intell Clin Pharm 18: 684-691.
- De Falco A, Cukierman DS, Hauser-Davis RA, Rey NA (2016) Alzheimer’s disease: etiological hypotheses and treatment perspectives. Quim Nova 39: 63-80.
- Racchi M, Mazzucchelli M, Porrello E, Lanni C, Govoni S (2004) Acetylcholinesterase inhibitors: novel activities of old molecules. Pharmacol Res 50: 441-451.
- Stancu IC, Vasconcelos B, Terwel D, Dewachter I (2014) Models of β-amyloid induced Tau-pathology: the long and “folded” road to understand the mechanism. Mol Neurodegener.
- Cabezas-Opazo FA, Vergara-Pulgar K, Pérez M J, Jara C, Fuentealba C, et al. (2015) Mitochondrial dysfunction contributes to the pathogenesis of Alzheimer’s Disease. Oxid Med Cell Longev.
- Benevento C E (2011) Mitochondrial dysfunction induced by beta-amyloid peptides. Universidade Estadual de Campinas.
- Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (2011) Interaction between Pyridostigmine Bromide and Oxidative Stress. Farmacologia 7th ed. Elsevier: Rio de Janeiro.
- Aguera Morales E, Tunez Finana I (2011) Demencia Medicine 10: 5123-5128.
- Ghanemi A (2015) Alzheimer ’s disease Therapies: Selected advances and future perspectives. Alexandria J Med 51: 1-3.
- Diamond J (2005) Report on Alzheimer’s disease and current research; Alzheimer Society of Canada. Toronto.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 34: 939-944.
- Folstein MF, Folstein SE, McHugh PR (975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189-198.
- Nitrini R, Caramelli P, Bottino CM, Damasceno BP, Brucki SM, et al. (2005) Diagnosis of Alzheimer’s disease in Brazil: Diagnostic criteria and auxiliary tests. Recommendations of the scientific department of cognitive neurology and aging of the brazilian academy of neurology. Arq. Neuropsiquiatr 63: 713-719.
- Grasso M, Piscopo P, Confaloni A, Denti MA (2014) Circulating miRNAs as biomarkers for neurodegenerative disorders. Molecules 19: 6891-6910.
- Delay C, Mandemakers W, Hébert SS (2012) MicroRNAs in Alzheimer ’s disease. Neurobiol Dis 46: 285-290.
- Cassani R, Estarellas M, San-Martin R, Fraga FJ, Falk TH (2018) Systematic review on resting-state EEG for alzheimer's disease diagnosis and progression assessment.Dis Markers.
- Jeong J (2004) EEG dynamics in patients with Alzheimer's disease.Clin Neurophysiol 115: 1490-505.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Wiedermann J (2023) Understanding the Pharmacological Mechanisms of Antidepressants. Clin Pharmacol Biopharm, 12: 334. DOI: 10.4172/2167-065X.1000334
Copyright: © 2023 Wiedermann J. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1423
- [From(publication date): 0-2023 - Mar 28, 2025]
- Breakdown by view type
- HTML page views: 1207
- PDF downloads: 216