Understanding Nipah Virus Disease
Received: 15-Dec-2021 / Manuscript No. JCPHN-21-49880 / Editor assigned: 17-Dec-2021 / PreQC No. JCPHN-21-49880(PQ) / Reviewed: 01-Jan-1970 / QC No. JCPHN-21-49880 / Revised: 05-Jan-2022 / Manuscript No. JCPHN-21-49880(R) / Accepted Date: 05-Jan-2022 / Published Date: 12-Jan-2022 DOI: 10.4172/2471-9846.1000327
Abstract
The outbreak of acute encephalitis among pig handlers with high mortality rates in 1988 in Malaysia led to the discovery of a new zoontic infection caused by a paramyxoviridae virus called Nipah virus belonging to the genus henipa virus, a biosafety level-4 pathogen, is transmitted by Pteropus spp. of fruit bats [1-2]. The disease was first reported in the village named Kampung Sungai Nipah village in Malaysia besides infecting human’s virus also infected local pigs simultaneously. These pigs exhibited marked respiratory and neurological disease called “barking pig syndrome” though with lesser morbidity and mortality [3]. Human cases were mostly found in adult men who had been in close proximity to pigs, implying that pigs acted as an amplifying, intermediary host, allowing the virus to spread from bats to humans. The infection had spread to humans and pigs in other parts of Malaysia by February 1999, and this expansion was linked to pig mobility [4-6]. In March 1999, the infection extended to abattoir workers in Singapore because to the migration of pigs [7].
Keywords
Encephalitis; Paramyxoviridae virus; Neurological disease; Barking pig syndrome
Introduction
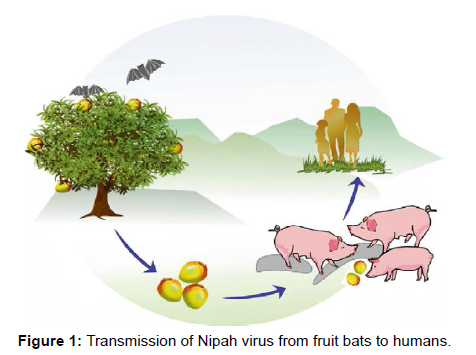
The outbreak in Malaysia and Singapore was initially thought to be caused by the Japanese encephalitis virus, but this was ruled out when it was discovered that the majority of cases were adult men rather than children, and when mosquito control and vaccination programmes were found to be ineffective in controlling the outbreak. Following that, a hitherto unknown paramyxovirus was recovered from the CSF of multiple fatal encephalitis cases. This virus was given the name Nipah virus and was later included in the Henipa virus genus alongside Hendra virus [8] (Figure 1).
During the 1998–1999 outbreaks, there were 276 human cases of encephalitis (265 cases in Malaysia; 11 cases in Singapore), with 106 deaths; more than 1,000,000 pigs were killed from nearly 900 farms to contain the outbreak [3,8]. Other than four probable cases of nosocomial transmission to health care workers, no human-to-human transmission of the Nipah virus has been reported in Malaysia or Singapore [9-10].
Nipah Virus
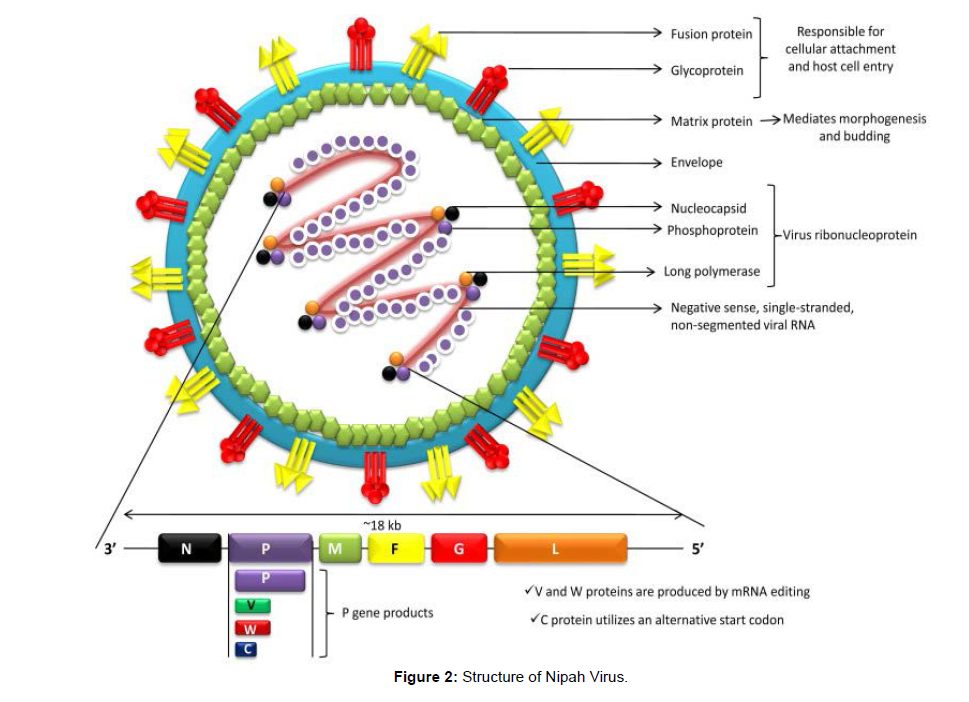
Nipah virus (NiV) is an emerging paramyxovirus (Henipavirus genus, paramyxovirinae subfamily, Paramyxoviridae family, orders Mononegavirales) that could cause severe respiratory disease and lethal encephalitis in people. It's a single-stranded, non segmented, enveloped RNA virus with helical symmetry in the negative sense. The nucleocapsid (N), phosphoprotein (P), matrix (M), fusion glycoprotein (F), attachment glycoprotein (G), and long polymerase (L) are the six genes that make up the RNA genome from 3' to 5'. (L) The virus ribo nucleoprotein is formed when the N, P, and L connect to the viral RNA (vRNP). The virion's cellular attachment and subsequent host cell invasion are controlled by the F and G proteins [11]. Host protease cleaves the newly generated precursor F-protein (F0) into two subunits, F1 and F2. The virus's fusion peptide, which is found in the F1 subunit, causes the viral and host cell membranes to fuse, allowing the virus to enter. Morphogenesis and budding are mediated by the viral M protein. Antibody against the G protein is required for NiV infectivity to be neutralized. After binding by the enveloped Henipaviruses, including NiV, the target cell (i.e. host cell) is invaded through the concerted efforts of the fusion (F) (class I) and attachment (G) glycoproteins. Interactions between host cell Class B ephrins (viral receptors) and NiV glycoprotein (G) cause conformational changes in the latter, resulting in F glycoprotein activation and membrane fusion. The increased pathogenicity of these viruses is thought to be due to their replication tactics as well as the fusion of the ephrin receptors. Henipaviruses encode several accessory proteins that help them evade the immune system of their hosts.
NiV infects its host cells by two glycoproteins, G and F. The G glycoprotein regulates virus attachment to host cell surface receptors, whereas the fusion (F) protein mediates entry into the cell by fusing virus-cell membranes. NiV's G protein interacts with ephrin B2/3 receptors in the host and causes conformational changes in the G protein, causing the F protein to refold. Monomeric ephrinB2 binding causes allosteric alterations in NiV G protein, allowing for complete activation and receptor-activated virus entry into host cells. Recently, it's found that viral modulation of host cell machinery targets the nucleolar DNA-damage response (DDR) pathway by inhibiting nucleolar Treacle protein, which enhances Nipah virus production (Figure 2).
Nipah virus can survive in some fruit juices or mango fruit for up to three days, and at least seven days in artificial date palm sap (13 percent sucrose and 0.21 percent BSA in water, pH 7.0) stored at 22°C. In the urine of fruit bats, the virus has an 18-hour half-life. NiV is relatively stable in the environment, and it can survive for 1 hour at 70°C. It can be entirely deactivated by heating it for more than 15 minutes at 100 degrees Celsius [12]. However, NiV can be quickly inactivated by commercially available disinfectants like sodium hypochlorite, soap and detergents [13].
In comparison to humans, NiV infection causes severe respiratory symptoms in pigs. Human airway epithelia that express high amounts of the NiV entry receptor ephrin-B2 show fast spread of NiV [14]. IFN-λ is upregulated in human respiratory epithelial cells after NiV infection. Pretreatment with IFN-λ may effectively display antiviral efficacy by inhibiting NiV replication, and hence differences in its receptor expression may play a role in NiV replication kinetics in different people [15]. In experimental hamster infection, the NiV V protein, one of three accessory proteins expressed by the viral P gene, plays a critical role in virus pathogenesis. NiV V protein has been found to regulate (suppress) activation of innate interferons by increasing the quantity of a host protein UBXN1 (UBX domain-containing protein 1) by restricting protein breakdown [16].
Transmission of the Disease
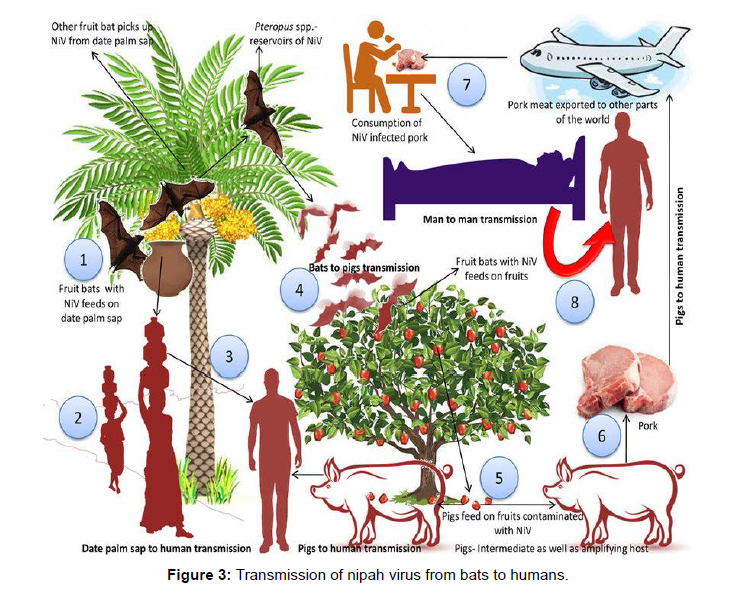
Several high-risk infections, such as Nipah, rabies, and Marburg viruses, employ bats as reservoir hosts. Viruses like these aren't linked to any significant pathogenic alterations in bat populations. Fruit bats serve as a natural reservoir for Nipah viruses, and in numerous outbreaks documented from throughout the world; these bats have been linked to the virus's transmission and infection in one way or another. The virus has spread from bats to various other species, including humans, via spilled over transmission, but only with limited transmission from person to person after that. NiV is spread through eating virus-contaminated foods and coming into touch with infected animals or human bodily fluids and proximity, such as touching, feeding, or attending a virus-infected person, increases the risk of droplet NiV transmission (Figure 3).
According to research conducted in Bangladesh between May and December 2004 based on comprehensive interviews with NiV infection survivors, medical practitioners, and careers, transmission occurred via both bats-to-man and man-to-man routes.
Pathogenesis of the disease
Evidence also suggests that viral proteins decrease MHC-I expression in immune cells, resulting in antigen presentation by antigen-presenting cells and stimulus for generating adaptive responses, ultimately leading to viral propagation and persistence in additional target organs [17,18]. Aside from these factors, virus-induced immune evasion accounts for the virus's long-term persistence in brain tissues and the following relapsed and late-onset lethal encephalitis in humans.
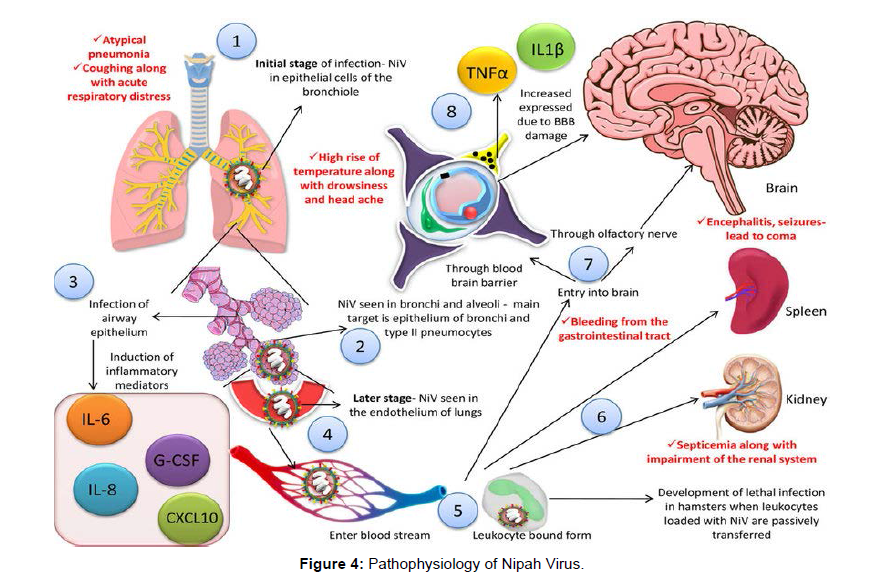
In the primary stages of infection, NiV can be detected in the epithelial cells of the bronchiole and antigens can be detected in bronchi and alveoli of the experimental animals primarily targeting the epithelium of bronchi and Type 2 pneumocytes [19]. When the airway epithelium (smaller ones) becomes infected, significant inflammatory mediators such as interleukin (IL)-1, IL-6, IL-8; granulocyte-colony stimulating factor (G-CSF), C-X-C motif chemokine 10 (CXCL10), etc. are produced [20]. In the latter stages of the disease, the virus spreads from the respiratory epithelium to the lungs' endothelial cells. Following that, the virus can enter the bloodstream and spread freely or in host leukocyte-bound form. Aside from the lungs, the spleen, kidneys, and brain may all be target organs, resulting in multiple organ failures [19,20]. When NiV-loaded leukocytes are passively transmitted to hamsters, they develop a fatal infection [21]. Monocytes, natural killer (NK) cells, and CD6+ CD8+T lymphocytes are all productively infected in pigs [22].
In the process of viral entrance into the central nervous system (CNS), two separate paths are involved: the hematogenous route (through the choroid plexus or blood vessels of the cerebrum) and/or the anterograde route (by the olfactory neurons) [23]. The blood-brain barrier (BBB) is damaged, and IL-1 and tumour necrosis factor (TNF)- are produced as a result of the virus infecting the CNS, resulting in the development of neurological symptoms [19](Figure 4).
A series of 32 fatal human instances of Nipah virus infection yielded clinical and postmortem data. Immunohistochemistry (IHC) and serology were used for diagnosis in all patients. Autopsy tissues were examined using routine histological stains, IHC, and electron microscopy. Systemic vasculitis with widespread thrombosis and parenchymal destruction, notably in the central nervous system, was the predominant histological findings. In the afflicted vessels, endothelial cell injury, necrosis, and syncytial giant cell development were seen. Light microscopy and electron microscopy exhibited characteristic viral inclusions. Antigens of the Nipah virus were found in endothelium and smooth muscle cells of blood arteries, according to IHC analyses. Virus antigens were also found in abundance in many parenchymal cells, particularly neurons. The pathophysiology of NiV appears to be influenced by endothelial cell and neuron infection, as well as vasculitis and thrombosis.
References
- Wong KT, Shieh WJ, Kumar S, Norain K, Abdullah W et al (2002) Nipah virus infection: pathology and pathogenesis of an emerging paramyxoviral zoonosis. Am. J. Pathol 1:161(6):2153-67.
- Singh RK, Dhama K, Chakraborty S, Tiwari R, Natesan S et al (2019) Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies–a comprehensive review. Vet Q Jan 1:39(1):26-55.
- Nor M, Gan CH, Ong BL (2000) Nipah virus infection of pigs in peninsular Malaysia. Rev Sci Tech 1:19(1):160-5.
- Centers for Disease Control and Prevention (1999) Outbreak of Hendra-Like Virus — Malaysia and Singapore, 1998–1999. MMWR 9:48(13):265-9.
- Parashar UD, Sunn LM, Ong F, Mounts AW, Arif MT (2000) Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998–1999 outbreak of severe encephalitis in Malaysia. J Infect Dis 1:181(5):1755-9.
- Tan KS, Tan CT, Goh KJ (1999) Epidemiological aspects of Nipah virus infection. Neurol J Southeast Asia 4(1):77-81.
- Paton NI, Leo YS, Zaki SR, Auchus AP, Lee KE et al (1999) Outbreak of Nipah-virus infection among abattoir workers in Singapore. The Lancet 9:354(9186):1253-6.
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A et al (2000) Nipah virus: a recently emergent deadly paramyxovirus. Science 26:288(5470):1432-5.
- Mounts AW, Kaur H, Parashar UD, Ksiazek TG, Cannon D, et al (2001) A cohort study of health care workers to assess nosocomial transmissibility of Nipah virus, Malaysia, 1999. J Infect Dis 1:183(5):810-3.
- Tan CT, Tan KS (2001) Nosocomial transmissibility of Nipah virus. J Infect Dis Nov 15:184(10):1367.
- Bossart KN, McEachern JA, Hickey AC, Choudhry V, Dimitrov DS et al (2007) Neutralization assays for differential henipavirus serology using Bio-Plex protein array systems. J Virol Methods Jun 1:142(1-2):29-40.
- de Wit E, Prescott J, Falzarano D, Bushmaker T, Scott D et al (2014) Foodborne transmission of Nipah virus in Syrian hamsters. PLoS pathogens 13:10(3):e1004001.
- Hassan MZ, Sazzad HM, Luby SP, Sturm-Ramirez K, Bhuiyan MU et al (2018) Nipah virus contamination of hospital surfaces during outbreaks, Bangladesh, 2013–2014. Emerg Infect Dis 1:24(1):15.
- Sauerhering L, Zickler M, Elvert M, Behner L, Matrosovich T et al (2016) Species-specific and individual differences in Nipah virus replication in porcine and human airway epithelial cells. J Gen Virol 1:97(7):1511-9.
- Sauerhering L, Müller H, Behner L, Elvert M, Fehling SK et al (2017) Variability of interferon-λ induction and antiviral activity in Nipah virus infected differentiated human bronchial epithelial cells of two human donors. J. Gen. Virol 1:98(10):2447-53.
- Uchida S, Horie R, Sato H, Kai C, Yoneda M (2018) Possible role of the Nipah virus V protein in the regulation of the interferon beta induction by interacting with UBX domain-containing protein1. Nature 16:8(1):1-3.
- Dasgupta A, Hammarlund E, Slifka MK, Früh K (2007) Cowpox virus evades CTL recognition and inhibits the intracellular transport of MHC class I molecules. J Immunol 1:178(3):1654-61.
- Seto J, Qiao L, Guenzel CA, Xiao S, Shaw ML et al (2010) Novel Nipah virus immune-antagonism strategy revealed by experimental and computational study. J Virol 1:84(21):10965-73.
- Rockx B, Brining D, Kramer J, Callison J, Ebihara H et al (2011) Clinical outcome of henipavirus infection in hamsters is determined by the route and dose of infection. Journal of virology 1:85(15):7658-71.
- Escaffre O, Borisevich V, Rockx B (2013) Pathogenesis of Hendra and Nipah virus infection in humans. J Infect Dev Ctries 17:7(04):308-11.
- Mathieu C, Pohl C, Szecsi J, Trajkovic-Bodennec S, Devergnas S et al (2011) Nipah virus uses leukocytes for efficient dissemination within a host. J Virol 1:85(15):7863-71.
- Stachowiak B, Weingartl HM (2012) Nipah virus infects specific subsets of porcine peripheral blood mononuclear cells. PloS one 27:7(1):e30855.
- Weingartl H, Czub S, Copps J, Berhane Y, Middleton D et al (2005) Invasion of the central nervous system in a porcine host by Nipah virus. J Virol 15:79(12):7528-34.
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Indexedat Google Scholar Crossref
Citation: Tiwari VK, Akhil KV (2022) Understanding Nipah Virus Disease. J Comm Pub Health Nursing, 8: 327. DOI: 10.4172/2471-9846.1000327
Copyright: © 2022 Tiwari VK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2192
- [From(publication date): 0-2022 - Dec 20, 2024]
- Breakdown by view type
- HTML page views: 1812
- PDF downloads: 380